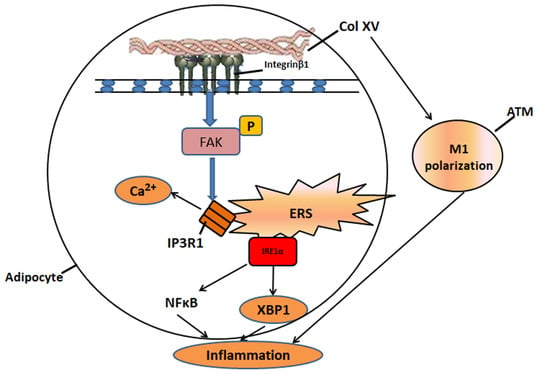
Collagen XV Promotes ER Stress-Induced Inflammation through Activating Integrin β1/FAK Signaling Pathway and M1 Macrophage Polarization in Adipose Tissue
Abstract
:1. Introduction
2. Results
2.1. Col XV Aggravates Adipose Tissue Endoplasmic Reticulum Stress
2.2. Col XV Induces Adipose Tissue Inflammation by Enhancing Adipocyte ERS
2.3. Interaction between Col XV and Integrin β1 Is Necessary for Activation of FAK
2.4. Col XV Triggers Adipocyte ERS by Disrupting Intracellular Ca2+ Homeostasis through IP3R1
2.5. IRE1α/XBP1 Branch Pathway of ERS is Predominantly Activated during Col XV-Induced Adipocyte Inflammation
2.6. Col XV Aggravates Adipose Tissue Inflammation Depending on M1 Macrophage Polarization
2.7. IFNβ Secretion from Adipose Tissue Induced by ERS Plays a Role in M1 Macrophage Polarization
3. Research Highlights
- Col XV aggravates adipose tissue ERS;
- Interaction between Col XV and integrin β1 is necessary for activation of FAK;
- Col XV triggers adipocyte ERS by disrupting intracellular Ca2+ homeostasis through IP3R1;
- IFNβ secretion from adipose tissue induced by ERS plays a role in M1 macrophage polarization;
- Col XV promotes ERS induced adipose inflammation through FAK/integrin β1 signaling pathway and M1 macrophage polarization in adipose tissue.
4. Discussion
5. Materials and Methods
5.1. Experimental Animals
5.2. Isolation and Culture of Adipocytes
5.3. RNA-Seq Analysis
5.4. Drug Treatments
5.5. Cultivation of Macrophage with Conditioned Media
5.6. Immunohistochemistry (IHC)
5.7. Immunofluorescence
5.8. Quantitative Real-Time PCR (qRT–PCR)
5.9. Western Blot Analysis
5.10. Co-Immunoprecipitation (IP) and Immunoblotting
5.11. Enzyme-Linked Immunosorbent Assay (ELISA)
5.12. Intracellular Ca2+ Measurement
5.13. ER-Tracker Red Staining
- (1)
- The ER-Tracker Red (Beyotime, Beijing, China) stock solution was diluted with PBS into a working solution in accordance with the instructions for use.
- (2)
- Dyeing
- ash the cells carefully with an appropriate amount of buffer such as PBS. For the staining of suspension cells, please refer to the staining method of adherent cells.
- Mix the prepared staining solution with the cells, and incubate for 30 min in the incubator.
- Remove the ER-Tracker Red staining working solution, and carefully wash the cells 1–2 times with PBS.
- Observe the cells through a fluorescence microscope and analyze changes in fluorescence intensity.
5.14. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
Abbreviations
| Extracellular Matrix | ECM |
| Collagen XV | Col XV |
| Endoplasmic Reticulum | ER |
| Endoplasmic Reticulum Stress | ERS |
| focal adhesion kinase | FAK |
| Inositol-Requiring Enzyme-1α | IRE1α |
| X-Box Binding Protein 1 | XBP1 |
| C/EBP-Homologous Protein | CHOP |
| 78kDa Glucose-Regulated Protein | GRP78 |
| Interleukin 6 | IL-6 |
| Tumor Necrosis Factor α | TNFα |
| Recombinant Activating Transcription Factor 6 | TAF6 |
| Protein kinase R-like ER kinase | PERK |
| TNF Receptor Associated Factor 2 | TRAF2 |
| Nuclear Factor kappa-B | NF-Κb |
| NLR Family Pyrin domain containing 3 | NLRP3 |
| Interferon beta | IFNβ |
| Adipose Tissue | AT |
| Inguinal White Adipose Tissue | iWAT |
| Basement Membrane | BM |
| Unfolded Protein Response | UPR |
| InsP3R | IP3R1 |
| 4-Phenylbutyrate | 4-PBA |
| Gene Ontology | GO |
| Tunicamycin | TM |
| Crown-Like Structure | CLS |
| Adipose Tissue Macrophage | ATM |
| Integrin β1 | Itg β1 |
| 2-Aminoethoxydiphenyl Borate | 2-APB |
| Conditioned Medium | CM |
| Flow Cytometry | FCM |
Appendix A
| Antibody Information Sheet | ||
| Antibody | Model | Company |
| anti-GAPDH | AB2000 | Abways |
| anti-Col XV | ab202554 | Abcam |
| anti-CHOP | YM3668 | Immuno Way |
| anti-GRP78 | ab21685 | Abcam |
| anti-IRE1α | ab124945 | Abcam |
| anti-IL-6 | ab100712 | Abcam |
| anti-MCP1 | ab100712 | Abcam |
| anti-IL-1β | ab100712 | Abcam |
| anti-CD206 | b125028 | Abcam |
| anti-CD163 | YM6146 | Immuno Way |
| anti-TNFα | 11948P | Cell signaling |
| anti-pFAK | ab81298 | Abcam |
| anti-FAK | ab40794 | Abcam |
| anti-Integrinβ1 | ab24693 | Abcam |
| Horseradish peroxidase anti-rabbit | ATM | Sigma-Aldrich |
| Horseradish peroxidase anti-goat | Itg β1 | Sigma-Aldrich |
References
- Li, C.-J.; Fang, Q.-H.; Liu, M.-L.; Lin, J.-N. Current understanding of the role of Adipose-derived Extracellular Vesicles in Metabolic Homeostasis and Diseases: Communication from the distance between cells/tissues. Theranostics 2020, 10, 7422–7435. [Google Scholar] [CrossRef]
- Xia, Y.; He, F.; Wu, X.; Tan, B.; Chen, S.; Liao, Y.; Qi, M.; Chen, S.; Peng, Y.; Yin, Y.; et al. GABA transporter sustains IL-1β production in macrophages. Sci. Adv. 2021, 7, eabe9274. [Google Scholar] [CrossRef]
- Zhang, C.; Yang, M.; Ericsson, A.C. Function of Macrophages in Disease: Current Understanding on Molecular Mechanisms. Front. Immunol. 2021, 12, 620510. [Google Scholar] [CrossRef] [PubMed]
- Kiran, S.; Kumar, V.; Kumar, S.; Price, R.; Singh, U. Adipocyte, Immune Cells, and miRNA Crosstalk: A Novel Regulator of Metabolic Dysfunction and Obesity. Cells 2021, 10, 1004. [Google Scholar] [CrossRef]
- Wang, P.-Y.; Feng, J.-Y.; Zhang, Z.; Chen, Y.; Qin, Z.; Dai, X.-M.; Wei, J.; Hu, B.-H.; Zhang, W.-D.; Sun, Y.; et al. The adipokine orosomucoid alleviates adipose tissue fibrosis via the AMPK pathway. Acta Pharmacol. Sin. 2021, 1–9. [Google Scholar] [CrossRef]
- Ling, L.; Mulligan, J.A.; Ouyang, Y.; Shimpi, A.A.; Williams, R.M.; Beeghly, G.F.; Hopkins, B.D.; Spector, J.A.; Adie, S.G.; Fischbach, C. Obesity-Associated Adipose Stromal Cells Promote Breast Cancer Invasion through Direct Cell Contact and ECM Remodeling. Adv. Funct. Mater. 2020, 30. [Google Scholar] [CrossRef] [PubMed]
- Logie, C.; van Schaik, T.; Pompe, T.; Pietsch, K. Fibronectin-functionalization of 3D collagen networks supports immune tolerance and inflammation suppression in human monocyte-derived macrophages. Biomaterials 2021, 268, 120498. [Google Scholar] [CrossRef] [PubMed]
- Henegar, C.; Tordjman, J.; Achard, V.; Lacasa, D.; Cremer, I.; Guerre-Millo, M.; Poitou, C.; Basdevant, A.; Stich, V.; Viguerie, N.; et al. Adipose tissue transcriptomic signature highlights the pathological relevance of extracellular matrix in human obesity. Genome Biol. 2008, 9, R14. [Google Scholar] [CrossRef]
- Myers, J.C.; Amenta, P.S.; Dion, A.S.; Sciancalepore, J.P.; Nagaswami, C.; Weisel, J.W.; Yurchenco, P.D. The molecular structure of human tissue type XV presents a unique conformation among the collagens. Biochem. J. 2007, 404, 535–544. [Google Scholar] [CrossRef] [Green Version]
- Dhungana, H.; Huuskonen, M.T.; Pihlajaniemi, T.; Heljasvaara, R.; Vivien, D.; Kanninen, K.M.; Malm, T.; Koistinaho, J.; Lemarchant, S. Lack of collagen XV is protective after ischemic stroke in mice. Cell Death Dis. 2017, 8, e2541. [Google Scholar] [CrossRef] [Green Version]
- Lemoinne, S.; Cadoret, A.; Rautou, P.-E.; El Mourabit, H.; Ratziu, V.; Corpechot, C.; Rey, C.; Bosselut, N.; Barbu, V.; Wendum, D.; et al. Portal myofibroblasts promote vascular remodeling underlying cirrhosis formation through the release of microparticles. Hepatology 2015, 61, 1041–1055. [Google Scholar] [CrossRef]
- Liu, G.; Li, M.; Xu, Y.; Wu, S.; Saeed, M.; Sun, C. ColXV promotes adipocyte differentiation via inhibiting DNA methylation and cAMP/PKA pathway in mice. Oncotarget 2017, 8, 60135–60148. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lisignoli, G.; Lambertini, E.; Manferdini, C.; Gabusi, E.; Penolazzi, L.; Paolella, F.; Angelozzi, M.; Casagranda, V.; Piva, R. Collagen type XV and the ‘osteogenic status’. J. Cell. Mol. Med. 2017, 21, 2236–2244. [Google Scholar] [CrossRef] [Green Version]
- Gabusi, E.; Manferdini, C.; Grassi, F.; Piacentini, A.; Cattini, L.; Filardo, G.; Lambertini, E.; Piva, R.; Zini, N.; Facchini, A.; et al. Extracellular calcium chronically induced human osteoblasts effects: Specific modulation of osteocalcin and collagen type XV. J. Cell. Physiol. 2012, 227, 3151–3161. [Google Scholar] [CrossRef] [PubMed]
- Xia, T.; Shen, Z.; Cai, J.; Pan, M.; Sun, C. ColXV Aggravates Adipocyte Apoptosis by Facilitating Abnormal Extracellular Matrix Remodeling in Mice. Int. J. Mol. Sci. 2020, 21, 959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mori, S.; Kiuchi, S.; Ouchi, A.; Hase, T.; Murase, T. Characteristic Expression of Extracellular Matrix in Subcutaneous Adipose Tissue Development and Adipogenesis; Comparison with Visceral Adipose Tissue. Int. J. Biol. Sci. 2014, 10, 825–833. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ojansivu, M.; Wang, X.; Hyväri, L.; Kellomäki, M.; Hupa, L.; Vanhatupa, S.; Miettinen, S. Bioactive glass induced osteogenic differentiation of human adipose stem cells is dependent on cell attachment mechanism and mitogen-activated protein kinases. Eur. Cells Mater. 2018, 35, 54–72. [Google Scholar] [CrossRef] [PubMed]
- Yoshimoto, T.; Fujita, T.; Kajiya, M.; Ouhara, K.; Matsuda, S.; Komatsuzawa, H.; Shiba, H.; Kurihara, H. Aggregatibacter actinomycetemcomitansouter membrane protein 29 (Omp29) induces TGF-β-regulated apoptosis signal in human gingival epithelial cells via fibronectin/integrinβ1/FAK cascade. Cell. Microbiol. 2016, 18, 1723–1738. [Google Scholar] [CrossRef] [Green Version]
- Lin, T.H.; Aplin, A.E.; Shen, Y.; Chen, Q.; Schaller, M.; Romer, L.; Aukhil, I.; Juliano, R.L. Integrin-mediated Activation of MAP Kinase Is Independent of FAK: Evidence for Dual Integrin Signaling Pathways in Fibroblasts. J. Cell Biol. 1997, 136, 1385–1395. [Google Scholar] [CrossRef]
- Zhang, Z.; Wu, S.; Muhammad, S.; Ren, Q.; Sun, C. miR-103/107 promote ER stress-mediated apoptosis via targeting the Wnt3a/β-catenin/ATF6 pathway in preadipocytes. J. Lipid Res. 2018, 59, 843–853. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Deng, X.; Mancarella, S.; Hendron, E.; Eguchi, S.; Soboloff, J.; Tang, X.D.; Gill, D.L. The Calcium Store Sensor, STIM1, Reciprocally Controls Orai and CaV1.2 Channels. Science 2010, 330, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Seidel, E.; Dassa, L.; Kahlon, S.; Tirosh, B.; Halenius, A.; Malkinson, T.S.; Mandelboim, O. A slowly cleaved viral signal peptide acts as a protein-integral immune evasion domain. Nat. Commun. 2021, 12, 1–19. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.-T.; Lin, M.-H.; Liu, K.-Y.; Chiou, S.-S.; Wang, S.-N.; Chai, C.-Y.; Tseng, L.-W.; Chiou, H.-Y.C.; Wang, H.-C.; Yokoyama, K.K.; et al. WLS/wntless is essential in controlling dendritic cell homeostasis via a WNT signaling-independent mechanism. Autophagy 2021, 1–16. [Google Scholar]
- Ron, D.; Walter, P. Signal integration in the endoplasmic reticulum unfolded protein response. Nat. Rev. Mol. Cell Biol. 2007, 8, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Govaere, O.; Cockell, S.; Tiniakos, D.; Queen, R.; Younes, R.; Vacca, M.; Alexander, L.; Ravaioli, F.; Palmer, J.; Petta, S.; et al. Transcriptomic profiling across the nonalcoholic fatty liver disease spectrum reveals gene signatures for steatohepatitis and fibrosis. Sci. Transl. Med. 2020, 12, eaba4448. [Google Scholar] [CrossRef]
- Hotamisligil, G.S. Endoplasmic Reticulum Stress and the Inflammatory Basis of Metabolic Disease. Cell 2010, 140, 900–917. [Google Scholar] [CrossRef] [Green Version]
- Nakajima, S.; Kato, H.; Gu, L.; Takahashi, S.; Johno, H.; Umezawa, K.; Kitamura, M. Pleiotropic Potential of Dehydroxymethylepoxyquinomicin for NF-κB Suppression via Reactive Oxygen Species and Unfolded Protein Response. J. Immunol. 2013, 190, 6559–6569. [Google Scholar] [CrossRef] [Green Version]
- Katsnelson, M.A.; Rucker, L.G.; Russo, H.M.; Dubyak, G.R. K+ Efflux Agonists Induce NLRP3 Inflammasome Activation Independently of Ca2+ Signaling. J. Immunol. 2015, 194, 3937–3952. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Gan, L.; Zhang, T.; Ren, Q.; Sun, C. Melatonin alleviates adipose inflammation through elevating α-ketoglutarate and diverting adipose-derived exosomes to macrophages in mice. J. Pineal Res. 2018, 64, e12455. [Google Scholar] [CrossRef] [PubMed]
- Clementz, A.G.; Mutolo, M.J.; Leir, S.-H.; Morris, K.J.; Kucybala, K.; Harris, H.; Harris, A. Collagen XV Inhibits Epithelial to Mesenchymal Transition in Pancreatic Adenocarcinoma Cells. PLoS ONE 2013, 8, e72250. [Google Scholar] [CrossRef] [Green Version]
- Wei, H.; Malik, M.; Sheikh, A.M.; Merz, G.; Brown, W.T.; Li, X. Abnormal Cell Properties and Down-Regulated FAK-Src Complex Signaling in B Lymphoblasts of Autistic Subjects. Am. J. Pathol. 2011, 179, 66–74. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhao, M.; Jin, X.; Ney, G.; Yang, K.B.; Peng, F.; Cao, J.; Iwawaki, T.; Del Valle, J.; Chen, X.; et al. Adaptive endoplasmic reticulum stress signalling via IRE1α–XBP1 preserves self-renewal of haematopoietic and pre-leukaemic stem cells. Nat. Cell Biol. 2019, 21, 328–337. [Google Scholar] [CrossRef] [PubMed]
- Asanović, I.; Strandback, E.; Kroupova, A.; Pasajlic, D.; Meinhart, A.; Tsung-Pin, P.; Djokovic, N.; Anrather, D.; Schuetz, T.; Suskiewicz, M.J.; et al. The oxidoreductase PYROXD1 uses NAD(P)+ as an antioxidant to sustain tRNA ligase activity in pre-tRNA splicing and unfolded protein response. Mol. Cell 2021, 81, 2520–2532. [Google Scholar] [CrossRef]
- Tan, H.-Y.; Wang, N.; Tsao, S.-W.; Che, C.-M.; Yuen, M.-F.; Feng, Y. IRE1α inhibition by natural compound genipin on tumour associated macrophages reduces growth of hepatocellular carcinoma. Oncotarget 2016, 7, 43792–43804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Foley, K.P.; Chen, Y.; Barra, N.G.; Heal, M.; Kwok, K.; Tamrakar, A.K.; Chi, W.; Duggan, B.M.; Henriksbo, B.D.; Liu, Y.; et al. Inflammation promotes adipocyte lipolysis via IRE1 kinase. J. Biol. Chem. 2021, 296, 100440. [Google Scholar] [CrossRef] [PubMed]
- Yin, S.; Li, L.; Tao, Y.; Yu, J.; Wei, S.; Liu, M.; Li, J. The Inhibitory Effect of Artesunate on Excessive Endoplasmic Reticulum Stress Alleviates Experimental Colitis in Mice. Front. Pharmacol. 2021, 12, 629798. [Google Scholar] [CrossRef]
- Hu, F.; Yu, X.; Wang, H.; Zuo, D.; Guo, C.; Yi, H.; Tirosh, B.; Subjeck, J.R.; Qiu, X.; Wang, X.-Y. ER stress and its regulator X-box-binding protein-1 enhance polyIC-induced innate immune response in dendritic cells. Eur. J. Immunol. 2011, 41, 1086–1097. [Google Scholar] [CrossRef]
- Chou, X.; Ma, K.; Shen, Y.; Min, Z.; Wu, Q.; Sun, D. Dual role of inositol-requiring enzyme 1α (IRE-1α) in Cd-induced apoptosis in human renal tubular epithelial cells: Endoplasmic reticulum stress and STAT3 signaling activation. Toxicology 2021, 456, 152769. [Google Scholar] [CrossRef] [PubMed]
- Zeng, L.; Liu, Y.-P.; Sha, H.; Chen, H.; Qi, L.; Smith, J.A. XBP-1 Couples Endoplasmic Reticulum Stress to Augmented IFN-β Induction via a cis-Acting Enhancer in Macrophages. J. Immunol. 2010, 185, 2324–2330. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.A.; Turner, M.J.; DeLay, M.L.; Klenk, E.I.; Sowders, D.P.; Colbert, R.A. Endoplasmic reticulum stress and the unfolded protein response are linked to synergistic IFN-β inductionvia X-box binding protein 1. Eur. J. Immunol. 2008, 38, 1194–1203. [Google Scholar] [CrossRef] [Green Version]
- Vera-Otarola, J.; Solis, L.; Lowy, F.; Olguín, V.; Angulo, J.; Pino, K.; Tischler, N.; Otth, C.; Padula, P.; López-Lastra, M. The Andes Orthohantavirus NSs Protein Antagonizes the Type I Interferon Response by Inhibiting MAVS Signaling. J. Virol. 2020, 94. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.-Q.; Zielinska, M.; Li, W.; Bernkopf, D.B.; Heilingloh, C.S.; Neurath, M.F.; Becker, C. PGAM5-MAVS interaction regulates TBK1/ IRF3 dependent antiviral responses. Sci. Rep. 2020, 10, 8323. [Google Scholar] [CrossRef]
- Zhang, L.; Cao, M.; Li, Q.; Yan, X.; Xue, T.; Song, L.; Su, B.; Li, C. Genome-wide identification of NOD-like receptors and their expression profiling in mucosal tissues of turbot (Scophthalmus maximus L.) upon bacteria challenge. Mol. Immunol. 2021, 134, 48–61. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Zhou, B.; Meng, M.; Zhao, W.; Wang, D.; Yuan, Y.; Zheng, Y.; Qiu, J.; Li, Y.; Li, G.; et al. FOXA3 induction under endoplasmic reticulum stress contributes to non-alcoholic fatty liver disease. J. Hepatol. 2021, 75, 150–162. [Google Scholar] [CrossRef] [PubMed]
- Eizirik, D.L.; Cardozo, A.K.; Cnop, M. The Role for Endoplasmic Reticulum Stress in Diabetes Mellitus. Endocr. Rev. 2008, 29, 42–61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.; Calay, E.S.; Fan, J.; Arduini, A.; Kunz, R.C.; Gygi, S.P.; Yalcin, A.; Fu, S.; Hotamisligil, G.S. S-Nitrosylation links obesity-associated inflammation to endoplasmic reticulum dysfunction. Science 2015, 349, 500–506. [Google Scholar] [CrossRef] [Green Version]
- Liu, Z.; Gan, L.; Wu, T.; Feng, F.; Luo, D.; Gu, H.; Liu, S.; Sun, C. Adiponectin reduces ER stress-induced apoptosis through PPARα transcriptional regulation of ATF2 in mouse adipose. Cell Death Dis. 2016, 7, e2487. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luk, C.T.; Shi, S.Y.; Cai, E.P.; Sivasubramaniyam, T.; Krishnamurthy, M.; Brunt, J.J.; Schroer, S.A.; Winer, D.A.; Woo, M. FAK signalling controls insulin sensitivity through regulation of adipocyte survival. Nat. Commun. 2017, 8, 14360. [Google Scholar] [CrossRef] [Green Version]
- Hussey, G.S.; Keane, T.J.; Badylak, S.F. The extracellular matrix of the gastrointestinal tract: A regenerative medicine platform. Nat. Rev. Gastroenterol. Hepatol. 2017, 14, 540–552. [Google Scholar] [CrossRef]
- Tang, J.; Wu, Y.-M.; Zhao, P.; Yang, X.-M.; Jiang, J.-L.; Chen, Z.-N. Overexpression of HAb18G/CD147 promotes invasion and metastasis via α3β1 integrin mediated FAK-paxillin and FAK-PI3K-Ca2+ pathways. Cell. Mol. Life Sci. 2008, 65, 2933–2942. [Google Scholar] [CrossRef]
- Son, S.M.; Byun, J.; Roh, S.-E.; Kim, S.-J.; Mook-Jung, I. Reduced IRE1α mediates apoptotic cell death by disrupting calcium homeostasis via the InsP3 receptor. Cell Death Dis. 2014, 5, e1188. [Google Scholar] [CrossRef] [Green Version]
- Bujisic, B.; Martinon, F. IRE1 gives weight to obesity-associated inflammation. Nat. Immunol. 2017, 18, 479–480. [Google Scholar] [CrossRef] [PubMed]
- Lumeng, C.N.; Bodzin, J.L.; Saltiel, A.R. Obesity induces a phenotypic switch in adipose tissue macrophage polarization. J. Clin. Investig. 2007, 117, 175–184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shan, B.; Wang, X.; Wu, Y.; Xu, C.; Xia, Z.; Dai, J.; Shao, M.; Zhao, F.; He, S.; Yang, L.; et al. The metabolic ER stress sensor IRE1α suppresses alternative activation of macrophages and impairs energy expenditure in obesity. Nat. Immunol. 2017, 18, 519–529. [Google Scholar] [CrossRef]
- Qiu, Y.; Nguyen, K.; Odegaard, J.I.; Cui, X.; Tian, X.Y.; Locksley, R.M.; Palmiter, R.D.; Chawla, A. Eosinophils and Type 2 Cytokine Signaling in Macrophages Orchestrate Development of Functional Beige Fat. Cell 2014, 157, 1292–1308. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, M.; Ikeda, K.; Suganami, T.; Komiya, C.; Ochi, K.; Shirakawa, I.; Hamaguchi, M.; Nishimura, S.; Manabe, I.; Matsuda, T.; et al. Macrophage-inducible C-type lectin underlies obesity-induced adipose tissue fibrosis. Nat. Commun. 2014, 5, 4982. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Gan, L.; Luo, D.; Sun, C. Melatonin promotes circadian rhythm-induced proliferation through Clock/histone deacetylase 3/c-Myc interaction in mouse adipose tissue. J. Pineal Res. 2017, 62, e12383. [Google Scholar] [CrossRef] [PubMed]
- Cao, W.; Huang, H.; Xia, T.; Liu, C.; Muhammad, S.; Sun, C. Homeobox a5 Promotes White Adipose Tissue Browning Through Inhibition of the Tenascin C/Toll-Like Receptor 4/Nuclear Factor Kappa B Inflammatory Signaling in Mice. Front. Immunol. 2018, 9, 647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, R.; Fang, L.; Li, Y.; Xue, P.; Li, Y.; Guan, Y.; Chang, Y.; Chen, C.; Wang, N. Pro-inflammatory Macrophages suppress PPARγ activity in Adipocytes via S-nitrosylation. Free. Radic. Biol. Med. 2015, 89, 895–905. [Google Scholar] [CrossRef]
- Gan, L.; Liu, Z.; Luo, D.; Ren, Q.; Wu, H.; Li, C.; Sun, C. Reduced Endoplasmic Reticulum Stress-Mediated Autophagy Is Required for Leptin Alleviating Inflammation in Adipose Tissue. Front. Immunol. 2017, 8, 1507. [Google Scholar] [CrossRef] [Green Version]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, C.; Liu, Y.; Li, Y.; Tai, R.; Sun, Z.; Wu, Q.; Liu, Y.; Sun, C. Collagen XV Promotes ER Stress-Induced Inflammation through Activating Integrin β1/FAK Signaling Pathway and M1 Macrophage Polarization in Adipose Tissue. Int. J. Mol. Sci. 2021, 22, 9997. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22189997
Li C, Liu Y, Li Y, Tai R, Sun Z, Wu Q, Liu Y, Sun C. Collagen XV Promotes ER Stress-Induced Inflammation through Activating Integrin β1/FAK Signaling Pathway and M1 Macrophage Polarization in Adipose Tissue. International Journal of Molecular Sciences. 2021; 22(18):9997. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22189997
Chicago/Turabian StyleLi, Changxing, Yuexia Liu, Yizhou Li, Ruiqing Tai, Zhuwen Sun, Qiong Wu, Yongnian Liu, and Chao Sun. 2021. "Collagen XV Promotes ER Stress-Induced Inflammation through Activating Integrin β1/FAK Signaling Pathway and M1 Macrophage Polarization in Adipose Tissue" International Journal of Molecular Sciences 22, no. 18: 9997. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22189997