Implications of a Soy-Based Diet for Animal Models
Abstract
:1. Introduction
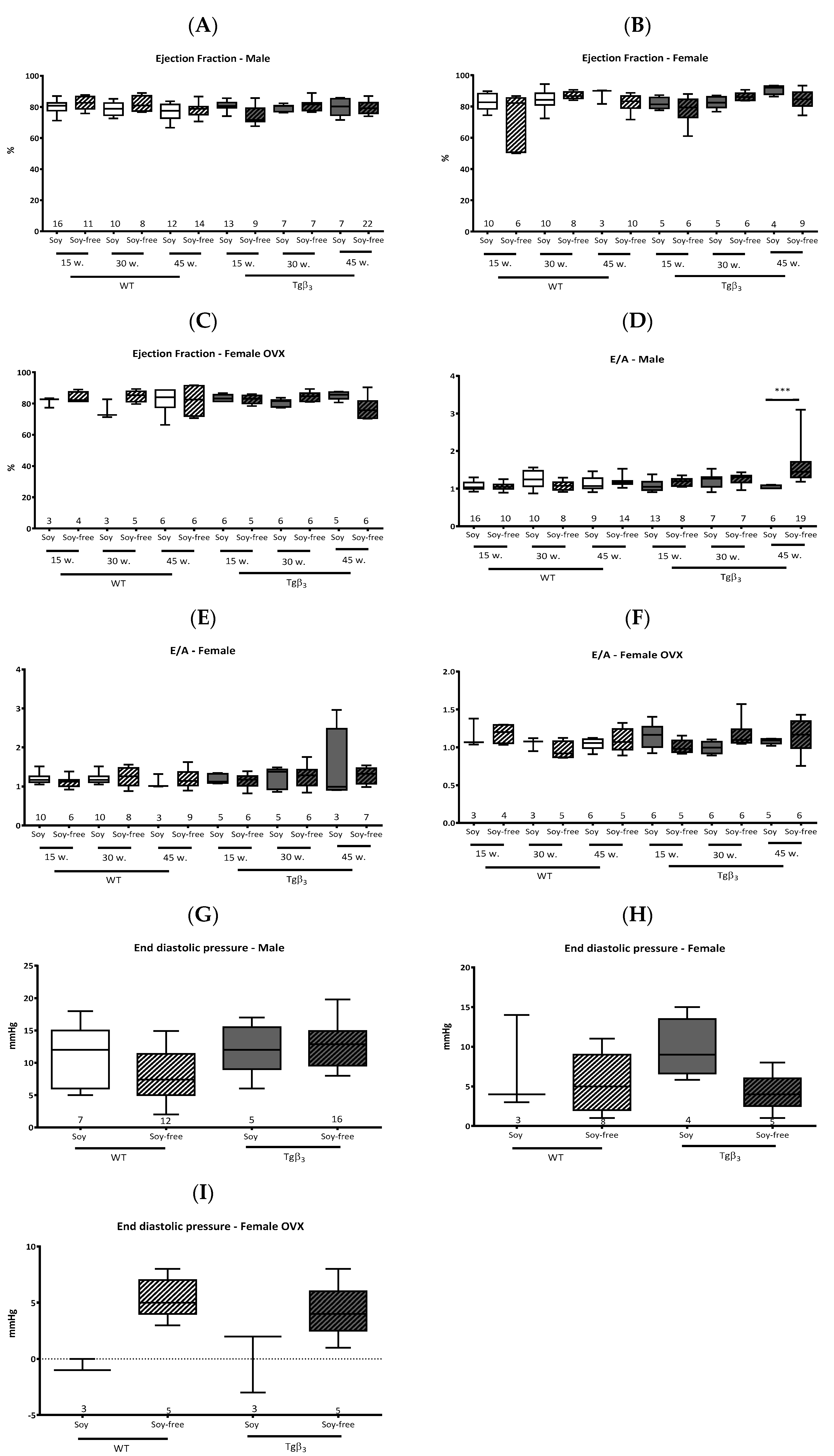
2. Results and Discussion
3. Materials and Methods
3.1. Animal Model
3.2. Echocardiography
3.3. Pressure Measurement
3.4. Western Blots
3.5. Statistical Analyses
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AFE | Atwater Fuel Energy |
| TGE | Total Genistein Equivalent |
| ER | Estrogen receptors |
| •NO | Nitric Oxide |
| NOS | •NO-synthases |
| eNOS | endothelial •NO-synthase |
| iNOS | inducible •NO-synthase |
| ICAM-2 | intercellular adhesion molecule-2 |
| nNOS | neuronal •NO-synthase |
| OVX | Ovariectomized |
| NIH | National Institutes of Health |
| LV | Left Ventricle |
| LVEDP | Left Ventricle End Diastolic Pressure |
| ANOVA | ANalyses Of VAriance |
| SEM | Standard Error of the Mean |
References
- Global, Regional, and National Age-Sex Specific All-Cause and Cause-Specific Mortality for 240 Causes of Death, 1990–2013: A Systematic Analysis for the Global Burden of Disease Study 2013. Lancet 2015, 385, 117–171. [CrossRef]
- Mauvais-Jarvis, F.; Arnold, A.P.; Reue, K. A Guide for the Design of Pre-Clinical Studies on Sex Differences in Metabolism. Cell Metab. 2017, 25, 1216–1230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jackson, S.J.; Andrews, N.; Ball, D.; Bellantuono, I.; Gray, J.; Hachoumi, L.; Holmes, A.; Latcham, J.; Petrie, A.; Potter, P.; et al. Does Age Matter? The Impact of Rodent Age on Study Outcomes. Lab. Anim. 2017, 51, 160–169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaillant, F.; Lauzier, B.; Poirier, I.; Gélinas, R.; Rivard, M.-E.; Robillard Frayne, I.; Thorin, E.; Des Rosiers, C. Mouse Strain Differences in Metabolic Fluxes and Function of Ex Vivo Working Hearts. Am. J. Physiol. Heart Circ. Physiol. 2013, 306, H78–H87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- How Isoflavone Levels in Common Rodent Diets Can Interfere with the Value of Animal Models and with Experimental Results. Lab. Anim. 2007, 41, 1–18. [CrossRef] [PubMed] [Green Version]
- Patisaul, H.B.; Jefferson, W. The Pros and Cons of Phytoestrogens. Front. Neuroendocr. 2010, 31, 400–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dhot, J.; Ferron, M.; Prat, V.; Persello, A.; Roul, D.; Stévant, D.; Guijarro, D.; Piriou, N.; Aillerie, V.; Erraud, A.; et al. Overexpression of Endothelial Β3-Adrenergic Receptor Induces Diastolic Dysfunction in Rats. ESC Heart Fail. 2020. [Google Scholar] [CrossRef] [PubMed]
- Morito, K.; Hirose, T.; Kinjo, J.; Hirakawa, T.; Okawa, M.; Nohara, T.; Ogawa, S.; Inoue, S.; Muramatsu, M.; Masamune, Y. Interaction of Phytoestrogens with Estrogen Receptors α and β. Biol. Pharm. Bull. 2001, 24, 351–356. [Google Scholar] [CrossRef] [PubMed]
- Qin, W.; Du, N.; Zhang, L.; Wu, X.; Hu, Y.; Li, X.; Shen, N.; Li, Y.; Yang, B.; Xu, C.; et al. Genistein Alleviates Pressure Overload-induced Cardiac Dysfunction and Interstitial Fibrosis in Mice. Br. J. Pharm. 2015, 172, 5559–5572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matori, H.; Umar, S.; Nadadur, R.D.; Sharma, S.; Partow-Navid, R.; Afkhami, M.; Amjedi, M.; Eghbali, M. Genistein, a Soy Phytoestrogen, Reverses Severe Pulmonary Hypertension and Prevents Right Heart Failure in Rats. Hypertension 2012, 60, 425–430. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Zhang, S.; Tong, H.; Shi, S. Comprehensive Evaluation of the Role of Soy and Isoflavone Supplementation in Humans and Animals over the Past Two Decades. Phytother. Res. 2018, 32, 384–394. [Google Scholar] [CrossRef] [PubMed]
- Merlet, N.; Piriou, N.; Rozec, B.; Grabherr, A.; Lauzier, B.; Trochu, J.-N.; Gauthier, C. Increased Beta2-Adrenoceptors in Doxorubicin-Induced Cardiomyopathy in Rat. PLoS ONE 2013, 8, e64711. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferron, M.; Cadiet, J.; Persello, A.; Prat, V.; Denis, M.; Erraud, A.; Aillerie, V.; Mevel, M.; Bigot, E.; Chatham, J.C.; et al. O-GlcNAc Stimulation: A New Metabolic Approach to Treat Septic Shock. Sci. Rep. 2019, 9, 18751. [Google Scholar] [CrossRef] [PubMed]

| RM1 | 2914C | |
|---|---|---|
| Wheat/barley (g/kg) | 885 | 630 |
| Soybean (g/kg) | 60 | X |
| Whey (g/kg) | 25 | X |
| Soy oil (g/kg) | 5 | X |
| Mineral (g/kg) | 25 | 20 |
| Corn-derived (g/kg) | X | 350 |
| Ash (%) | 6 | 4.5 |
| Crude Fibre | 4.65 | 4.1 |
| Neutral Detergent Fibre | 16.17 | 18 |
| Total Saturated Fatty Acids (%) | 0.51 | 0.6 |
| Total Monounsaturated Fatty Acids (%) | 0.88 | 0.7 |
| Total Polyunsaturated Fatty Acids (%) | 0.88 | 2.1 |
| Metabolism energy (kJ/g) | 10 | 12 |
| AFE (kJ/g) | 13.75 | 14.47 |
| AFE from Oil | 7.42 | 8.77 |
| AFE from Protein | 17.49 | 16.52 |
| AFE from Carbohydrates | 75.09 | 76.26 |
| Calcium (%) | 0.73 | 0.7 |
| Total Phosphorus (%) | 0.52 | 0.6 |
| Sodium (%) | 0.25 | 0.1 |
| Chloride (%) | 0.38 | 0.3 |
| Potassium (%) | 0.67 | 0.6 |
| Magnesium (%) | 0.23 | 0.2 |
| TGE (mg/kg) | 100–200 | <20 |
| Vitamin A (IU/g) | 8.5 | 6 |
| Vitamin D3 (IU/g) | 0.6 | 0.6 |
| Vitamin E (IU/g) | 84.1 | 120 |
| Vitamin K3 (mg/kg) | 10.17 | 20 |
| Vitamin B1 (mg/kg) | 8.58 | 12 |
| Vitamin B2 (mg/kg) | 4.33 | 6 |
| Niacin (mg/kg) | 61.32 | 54 |
| Vitamin B6 (mg/kg) | 4.81 | 10 |
| Pantothenic Acid (mg/kg) | 20.17 | 17 |
| Vitamin B12 (mg/kg) | 0.007 | 0.003 |
| Biotin (mg/kg) | 0.27 | 0.26 |
| Folate (mg/kg) | 0.79 | 2 |
| Choline (mg/kg) | 1080 | 1030 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dhot, J.; Prat, V.; Ferron, M.; Aillerie, V.; Erraud, A.; Rozec, B.; Waard, M.D.; Gauthier, C.; Lauzier, B. Implications of a Soy-Based Diet for Animal Models. Int. J. Mol. Sci. 2021, 22, 774. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020774
Dhot J, Prat V, Ferron M, Aillerie V, Erraud A, Rozec B, Waard MD, Gauthier C, Lauzier B. Implications of a Soy-Based Diet for Animal Models. International Journal of Molecular Sciences. 2021; 22(2):774. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020774
Chicago/Turabian StyleDhot, Justine, Valentine Prat, Marine Ferron, Virginie Aillerie, Angélique Erraud, Bertrand Rozec, Michel De Waard, Chantal Gauthier, and Benjamin Lauzier. 2021. "Implications of a Soy-Based Diet for Animal Models" International Journal of Molecular Sciences 22, no. 2: 774. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020774






