Natural Polyhydroxy Flavonoids, Curcuminoids, and Synthetic Curcumin Analogs as α7 nAChRs Positive Allosteric Modulators
Abstract
:1. Introduction
2. Results and Discussion
2.1. Evaluation of Polyhydroxy Natural Products (NPs)
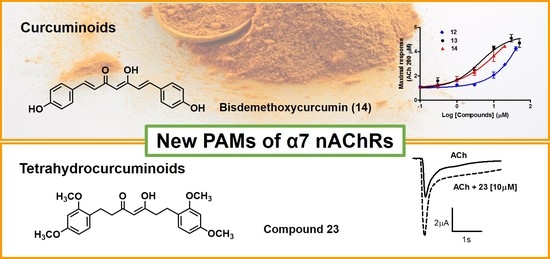
2.2. Design and Synthesis of New Curcumin Derivatives
2.3. Biological Evaluation of New Curcumin Derivatives
3. Materials and Methods
3.1. Chemistry
General Methods
3.2. Biological Evaluation
Oocyte Expression and Electrophysiological Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bouzat, C.; Lasala, M.; Nielsen, B.E.; Corradi, J.; del C Esandi, M. Molecular function of α7 nicotinic receptors as drug targets. J. Physiol. 2018, 596, 1847–1861. [Google Scholar] [CrossRef] [PubMed]
- Bagdas, D.; Gurun, M.S.; Flood, P.; Papke, R.L.; Damaj, M.I. New Insights on Neuronal Nicotinic Acetylcholine Receptors as Targets for Pain and Inflammation: A Focus on α7 nAChRs. Curr. Neuropharmacol. 2018, 16, 415–425. [Google Scholar] [CrossRef] [PubMed]
- Titus, D.J.; Johnstone, T.; Johnson, N.H.; London, S.H.; Chapalamadugu, M.; Hogenkamp, D.; Gee, K.W.; Atkins, C.M. Positive allosteric modulation of the α7 nicotinic acetylcholine receptor as a treatment for cognitive deficits after traumatic brain injury. PLoS ONE 2019, 14, e0223180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, K.G.; Qian, Y.H. Alpha 7 nicotinic acetylcholine receptor and its effects on Alzheimer’s disease. Neuropeptides 2019, 73, 96–106. [Google Scholar] [CrossRef]
- Sinha, N.; Karche, N.P.; Verma, M.K.; Walunj, S.S.; Nigade, P.B.; Jana, G.; Kurhade, S.P.; Hajare, A.K.; Tilekar, A.R.; Jadhav, G.R.; et al. Discovery of Novel, Potent, Brain-Permeable, and Orally Efficacious Positive Allosteric Modulator of α7 Nicotinic Acetylcholine Receptor [4-(5-(4-Chlorophenyl)-4-methyl-2-propionylthiophen-3-yl)benzenesulfonamide]: Structure-Activity Relationship and Preclinical Characterization. J. Med. Chem. 2020, 63, 944–960. [Google Scholar] [CrossRef] [PubMed]
- Antonio-Tolentino, K.; Hopkins, C.R. Selective α7 nicotinic receptor agonists and positive allosteric modulators for the treatment of schizophrenia—A review. Expert Opin. Investig. Drugs 2020, 29, 603–610. [Google Scholar] [CrossRef]
- Terry, A.V.; Callahan, P.M. α7 nicotinic acetylcholine receptors as therapeutic targets in schizophrenia: Update on animal and clinical studies and strategies for the future. Neuropharmacology 2020, 170, 108053. [Google Scholar] [CrossRef]
- Bertrand, D.; Terry, A.V. The wonderland of neuronal nicotinic acetylcholine receptors. Biochem. Pharmacol. 2018, 151, 214–225. [Google Scholar] [CrossRef]
- Mitra, S.; Khatri, S.N.; Maulik, M.; Bult-Ito, A.; Schulte, M. Allosterism of Nicotinic Acetylcholine Receptors: Therapeutic Potential for Neuroinflammation Underlying Brain Trauma and Degenerative Disorders. Int. J. Mol. Sci. 2020, 21, 4918. [Google Scholar] [CrossRef] [PubMed]
- Williams, D.K.; Wang, J.; Papke, R.L. Positive allosteric modulators as an approach to nicotinic acetylcholine receptor-targeted therapeutics: Advantages and limitations. Biochem. Pharmacol. 2011, 82, 915–930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chatzidaki, A.; Millar, N.S. Allosteric modulation of nicotinic acetylcholine receptors. Biochem. Pharmacol. 2015, 97, 408–417. [Google Scholar] [CrossRef] [PubMed]
- Yang, T.; Xiao, T.; Sun, Q.; Wang, K. The current agonists and positive allosteric modulators of α 7 nAChR for CNS indications in clinical trials. Acta Pharm. Sin. B 2017, 7, 611–622. [Google Scholar] [CrossRef] [PubMed]
- Tregellas, J.R.; Wylie, K.P. Alpha7 Nicotinic Receptors as Therapeutic Targets in Schizophrenia. Nicotine Tob. Res. 2019, 21, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kantrowitz, J.T.; Javitt, D.C.; Freedman, R.; Sehatpour, P.; Kegeles, L.S.; Carlson, M.; Sobeih, T.; Wall, M.M.; Choo, T.-H.; Vail, B.; et al. Double blind, two dose, randomized, placebo-controlled, cross-over clinical trial of the positive allosteric modulator at the alpha7 nicotinic cholinergic receptor AVL-3288 in schizophrenia patients. Neuropsychopharmacology 2020, 45, 1339–1345. [Google Scholar] [CrossRef]
- Clinical Trial of AVL-3288 in Schizophrenia Patients—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT02978599 (accessed on 1 October 2020).
- Deardorff, W.J.; Shobassy, A.; Grossberg, G.T. Safety and clinical effects of EVP-6124 in subjects with Alzheimer’s disease currently or previously receiving an acetylcholinesterase inhibitor medication. Expert Rev. Neurother. 2014, 15, 7–17. [Google Scholar] [CrossRef]
- A Safety and Cognitive Function Study of EVP-6124 versus Placebo in Subjects with Nicotine Dependence—Full Text View—ClinicalTrials.gov. Available online: https://clinicaltrials.gov/ct2/show/NCT01480232 (accessed on 21 October 2020).
- Balsera, B.; Mulet, J.; Fernández-Carvajal, A.; de la Torre-Martínez, R.; Ferrer-Montiel, A.; Hernández-Jiménez, J.G.; Estévez-Herrera, J.; Borges, R.; Freitas, A.E.; López, M.G.; et al. Chalcones as positive allosteric modulators of α7 nicotinic acetylcholine receptors: A new target for a privileged structure. Eur. J. Med. Chem. 2014, 86, 724–739. [Google Scholar] [CrossRef]
- Criado, M.; Balsera, B.; Mulet, J.; Sala, S.; Sala, F.; de la Torre-Martínez, R.; Fernández-Carvajal, A.; Ferrer-Montiel, A.; Moreno-Fernández, S.; Miguel, M.; et al. 1,3-diphenylpropan-1-ones as allosteric modulators of α7 nACh receptors with analgesic and antioxidant properties. Future Med. Chem. 2016, 8, 731–749. [Google Scholar] [CrossRef] [Green Version]
- Balsera, B.; Mulet, J.; Sala, S.; Sala, F.; de la Torre-Martínez, R.; González-Rodríguez, S.; Plata, A.; Naesens, L.; Fernández-Carvajal, A.; Ferrer-Montiel, A.; et al. Amino acid and peptide prodrugs of diphenylpropanones positive allosteric modulators of α7 nicotinic receptors with analgesic activity. Eur. J. Med. Chem. 2018, 143, 157–165. [Google Scholar] [CrossRef]
- Pérez de Vega, M.J.; Fernandez-Mendivil, C.; de la Torre Martínez, R.; González-Rodríguez, S.; Mullet, J.; Sala, F.; Sala, S.; Criado, M.; Moreno-Fernández, S.; Miguel, M.; et al. 1-(2′,5′-Dihydroxyphenyl)-3-(2-fluoro-4-hydroxyphenyl)-1-propanone (RGM079): A Positive Allosteric Modulator of α7 Nicotinic Receptors with Analgesic and Neuroprotective Activity. ACS Chem. Neurosci. 2019, 10, 3900–3909. [Google Scholar] [CrossRef]
- Zhan, C.; Yang, J. Protective effects of isoliquiritigenin in transient middle cerebral artery occlusion-induced focal cerebral ischemia in rats. Pharmacol. Res. 2006, 53, 303–309. [Google Scholar] [CrossRef]
- Min, J.L.; Chae, H.Y.; Jeon, J.P.; Hwang, M. Protective effects of isoliquiritigenin against methamphetamine-induced neurotoxicity in mice. J. Pharmacol. Sci. 2009, 111, 216–220. [Google Scholar] [CrossRef] [Green Version]
- Saudagar, R.B.; Saokar, S. Anti-inflammatory Natural Compounds from Herbal and Marine Origin. J. Drug Deliv. Ther. 2019, 9, 669–672. [Google Scholar] [CrossRef]
- Sung, S.; Kwon, D.; Um, E.; Kim, B. Could Polyphenols Help in the Control of Rheumatoid Arthritis? Molecules 2019, 24, 1589. [Google Scholar] [CrossRef] [Green Version]
- Uddin, M.S.; Kabir, M.T.; Niaz, K.; Jeandet, P.; Clément, C.; Mathew, B.; Rauf, A.; Rengasamy, K.R.R.; Sobarzo-Sánchez, E.; Ashraf, G.M.; et al. Molecular Insight into the Therapeutic Promise of Flavonoids against Alzheimer’s Disease. Molecules 2020, 25, 1267. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maan, G.; Sikdar, B.; Kumar, A.; Shukla, R.; Mishra, A. Role of Flavonoids in Neurodegenerative Diseases: Limitations and Future Perspectives. Curr. Top. Med. Chem. 2020, 20, 1169–1194. [Google Scholar] [CrossRef]
- Dajas, F.; Juan Andres, A.-C.; Florencia, A.; Carolina, E.; Felicia, R.-M. Neuroprotective Actions of Flavones and Flavonols: Mechanisms and Relationship to Flavonoid Structural Features. Cent. Nerv. Syst. Agents Med. Chem. 2013, 13, 30–35. [Google Scholar] [CrossRef]
- Fusi, F.; Spiga, O.; Trezza, A.; Sgaragli, G.; Saponara, S. The surge of flavonoids as novel, fine regulators of cardiovascular Cav channels. Eur. J. Pharmacol. 2017, 796, 158–174. [Google Scholar] [CrossRef]
- Montané, X.; Kowalczyk, O.; Reig-Vano, B.; Bajek, A.; Roszkowski, K.; Tomczyk, R.; Pawliszak, W.; Giamberini, M.; Mocek-Płóciniak, A.; Tylkowski, B. Current Perspectives of the Applications of Polyphenols and Flavonoids in Cancer Therapy. Molecules 2020, 25, 3342. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel-Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A. Therapeutic Potential of Flavonoids in Pain and Inflammation: Mechanisms of Action, Pre-Clinical and Clinical Data, and Pharmaceutical Development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef] [Green Version]
- Basu, P.; Basu, A. In Vitro and In Vivo Effects of Flavonoids on Peripheral Neuropathic Pain. Molecules 2020, 25, 1171. [Google Scholar] [CrossRef] [Green Version]
- Dey, R.; Chen, L. In search of allosteric modulators of α7-nAChR by solvent density guided virtual screening. J. Biomol. Struct. Dyn. 2011, 28, 695–715. [Google Scholar] [CrossRef] [PubMed]
- Cho, C.-H. Rapid Upregulation of 7 Nicotinic Acetylcholine Receptors by Tyrosine Dephosphorylation. J. Neurosci. 2005, 25, 3712–3723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gronlien, J.H.; Hakerud, M.; Ween, H.; Thorin-Hagene, K.; Briggs, C.A.; Gopalakrishnan, M.; Malysz, J. Distinct Profiles of 7 nAChR Positive Allosteric Modulation Revealed by Structurally Diverse Chemotypes. Mol. Pharmacol. 2007, 72, 715–724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grønlien, J.H.; Ween, H.; Thorin-Hagene, K.; Cassar, S.; Li, J.; Briggs, C.A.; Gopalakrishnan, M.; Malysz, J. Importance of M2-M3 loop in governing properties of genistein at the α7 nicotinic acetylcholine receptor inferred from α7/5-HT3A chimera. Eur. J. Pharmacol. 2010, 647, 37–47. [Google Scholar] [CrossRef] [PubMed]
- Cancello, R.; Zulian, A.; Maestrini, S.; Mencarelli, M.; Della Barba, A.; Invitti, C.; Liuzzi, A.; Di Blasio, A.M. The nicotinic acetylcholine receptor a7 in subcutaneous mature adipocytes: Downregulation in human obesity and modulation by diet-induced weight loss. Int. J. Obes. 2012, 36, 1552–1557. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, B.E.; Bermudez, I.; Bouzat, C. Flavonoids as positive allosteric modulators of α7 nicotinic receptors. Neuropharmacology 2019, 160, 107794. [Google Scholar] [CrossRef]
- Pulido-Moran, M.; Moreno-Fernandez, J.; Ramirez-Tortosa, C.; Ramirez-Tortosa, M. Curcumin and Health. Molecules 2016, 21, 264. [Google Scholar] [CrossRef]
- Ashrafizadeh, M.; Zarrabi, A.; Hashemi, F.; Moghadam, E.R.; Hashemi, F.; Entezari, M.; Hushmandi, K.; Mohammadinejad, R.; Najafi, M. Curcumin in cancer therapy: A novel adjunct for combination chemotherapy with paclitaxel and alleviation of its adverse effects. Life Sci. 2020, 256, 117984. [Google Scholar] [CrossRef]
- Fidelis, G.K.; Louis, H.; Fidelis Tizhe, T.; Solomon, O. Curcumin and Curcumin-based derivatives as anti-cancer agents: Recent Nano-Synthetic Methodologies and Anti-cancer Therapeutic Mechanisms. J. Med. Chem. Sci. 2019, 2, 59–63. [Google Scholar] [CrossRef]
- Mohammadian Haftcheshmeh, S.; Momtazi-Borojeni, A.A. Immunomodulatory therapeutic effects of curcumin in rheumatoid arthritis. Autoimmun. Rev. 2020, 19, 102593. [Google Scholar] [CrossRef]
- Bhat, A.; Mahalakshmi, A.M.; Ray, B.; Tuladhar, S.; Hediyal, T.A.; Manthiannem, E.; Padamati, J.; Chandra, R.; Chidambaram, S.B.; Sakharkar, M.K. Benefits of curcumin in brain disorders. BioFactors 2019, 45, 666–689. [Google Scholar] [CrossRef] [PubMed]
- Rabiei, Z.; Solati, K.; Amini-Khoei, H. Phytotherapy in treatment of Parkinson’s disease: A review. Pharm. Biol. 2019, 57, 355–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calfio, C.; Gonzalez, A.; Singh, S.K.; Rojo, L.E.; MacCioni, R.B. The Emerging Role of Nutraceuticals and Phytochemicals in the Prevention and Treatment of Alzheimer’s Disease. J. Alzheimer’s Dis. 2020, 77, 33–51. [Google Scholar] [CrossRef]
- Sun, J.; Chen, F.; Braun, C.; Zhou, Y.-Q.; Rittner, H.; Tian, Y.-K.; Cai, X.-Y.; Ye, D.-W. Role of curcumin in the management of pathological pain. Phytomedicine 2018, 48, 129–140. [Google Scholar] [CrossRef] [PubMed]
- Tabeshpour, J.; Banaeeyeh, S.; Eisvand, F.; Sathyapalan, T.; Hashemzaei, M.; Sahebkar, A. Effects of Curcumin on Ion Channels and Pumps: A Review. IUBMB Life 2019, 71, 812–820. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Chen, Q.; Wang, Y.; Peng, W.; Cai, H. Effects of curcumin on ion channels and transporters. Front. Physiol. 2014, 5, 94. [Google Scholar] [CrossRef] [Green Version]
- El Nebrisi, E.; Al Kury, L.T.; Yang, K.H.S.; Jayaprakash, P.; Howarth, F.C.; Kabbani, N.; Oz, M. Curcumin potentiates the function of human α 7 -nicotinic acetylcholine receptors expressed in SH-EP1 cells. Neurochem. Int. 2018, 114, 80–84. [Google Scholar] [CrossRef]
- El Nebrisi, E.G.; Bagdas, D.; Toma, W.; Al Samri, H.; Brodzik, A.; Alkhlaif, Y.; Yang, K.-H.S.; Howarth, F.C.; Damaj, I.M.; Oz, M. Curcumin Acts as a Positive Allosteric Modulator of α7 -Nicotinic Acetylcholine Receptors and Reverses Nociception in Mouse Models of Inflammatory Pain. J. Pharmacol. Exp. Ther. 2018, 365, 190–200. [Google Scholar] [CrossRef] [Green Version]
- Qiao, S.; Dou, Y.; Hu, H.; Dai, Y. Curcumin Activates Vagal Afferent Neurons Through the Modulation of Ion Channels via α7 nAChR. Nat. Prod. Commun. 2019, 14, 1–6. [Google Scholar] [CrossRef]
- Slika, L.; Patra, D. A short review on chemical properties, stability and nano-technological advances for curcumin delivery. Expert Opin. Drug Deliv. 2020, 17, 61–75. [Google Scholar] [CrossRef]
- Teymouri, M.; Barati, N.; Pirro, M.; Sahebkar, A. Biological and pharmacological evaluation of dimethoxycurcumin: A metabolically stable curcumin analogue with a promising therapeutic potential. J. Cell. Physiol. 2018, 233, 124–140. [Google Scholar] [CrossRef] [PubMed]
- Aggarwal, B.; Deb, L.; Prasad, S. Curcumin Differs from Tetrahydrocurcumin for Molecular Targets, Signaling Pathways and Cellular Responses. Molecules 2014, 20, 185–205. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wu, J.-C.; Tsai, M.-L.; Lai, C.-S.; Wang, Y.-J.; Ho, C.-T.; Pan, M.-H. Chemopreventative effects of tetrahydrocurcumin on human diseases. Food Funct. 2014, 5, 12–17. [Google Scholar] [CrossRef] [PubMed]
- Lai, C.-S.; Ho, C.-T.; Pan, M.-H. The Cancer Chemopreventive and Therapeutic Potential of Tetrahydrocurcumin. Biomolecules 2020, 10, 831. [Google Scholar] [CrossRef] [PubMed]
- Pabon, H.J.J. A synthesis of curcumin and related compounds. Recl. Trav. Chim. Pays Bas 1964, 83, 379–386. [Google Scholar] [CrossRef]
- Mazumder, A.; Neamati, N.; Sunder, S.; Schulz, J.; Pertz, H.; Eich, E.; Pommier, Y. Curcumin analogs with altered potencies against HIV-1 integrase as probes for biochemical mechanisms of drug action. J. Med. Chem. 1997, 40, 3057–3063. [Google Scholar] [CrossRef]
- Ferrari, E.; Pignedoli, F.; Imbriano, C.; Marverti, G.; Basile, V.; Venturi, E.; Saladini, M. Newly synthesized curcumin derivatives: Crosstalk between chemico-physical properties and biological activity. J. Med. Chem. 2011, 54, 8066–8077. [Google Scholar] [CrossRef]
- Gillis, E.P.; Eastman, K.J.; Hill, M.D.; Donnelly, D.J.; Meanwell, N.A. Applications of Fluorine in Medicinal Chemistry. J. Med. Chem. 2015, 58, 8315–8359. [Google Scholar] [CrossRef]
- Lin, L.; Shi, Q.; Nyarko, A.K.; Bastow, K.F.; Wu, C.-C.; Su, C.-Y.; Shih, C.C.-Y.; Lee, K.-H. Antitumor Agents. 250. † Design and Synthesis of New Curcumin Analogues as Potential Anti-Prostate Cancer Agents. J. Med. Chem. 2006, 49, 3963–3972. [Google Scholar] [CrossRef] [Green Version]
- Ryu, E.K.; Choe, Y.S.; Lee, K.-H.; Choi, Y.; Kim, B.-T. Curcumin and Dehydrozingerone Derivatives: Synthesis, Radiolabeling, and Evaluation for β-Amyloid Plaque Imaging. J. Med. Chem. 2006, 49, 6111–6119. [Google Scholar] [CrossRef]
- Elavarasan, S.; Bhakiaraj, D.; Chellakili, B.; Elavarasan, T.; Gopalakrishnan, M. One pot synthesis, structural and spectral analysis of some symmetrical curcumin analogues catalyzed by calcium oxide under microwave irradiation. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 97, 717–721. [Google Scholar] [CrossRef] [PubMed]
- Mori, A.; Miyakawa, Y.; Ohashi, E.; Haga, T.; Maegawa, T.; Sajiki, H. Pd/C-catalyzed chemoselective hydrogenation in the presence of diphenylsulfide. Org. Lett. 2006, 8, 3279–3281. [Google Scholar] [CrossRef] [PubMed]
- McOmie, J.F.W.; Watts, M.L.; West, D.E. Demethylation of aryl methyl ethers by boron tribromide. Tetrahedron 1968, 24, 2289–2292. [Google Scholar] [CrossRef]
- Caselli, M.; Ferrari, E.; Imbriano, C.; Pignedoli, F.; Saladini, M.; Ponterini, G. Probing solute–solvent hydrogen bonding with fluorescent water-soluble curcuminoids. J. Photochem. Photobiol. A Chem. 2010, 210, 115–124. [Google Scholar] [CrossRef]
- Sala, F.; Mulet, J.; Valor, L.M.; Criado, M.; Sala, S. Effects of benzothiazepines on human neuronal nicotinic receptors expressed in Xenopus oocytes. Br. J. Pharmacol. 2002, 136, 183–192. [Google Scholar] [CrossRef]




| Compound Number | Molecular Structure | % ± s.e. Current vs. ACh [200 µM] a |
|---|---|---|
| 1 Apigenin |  | 108 ± 4 |
| 2 Quercetin |  | 130 ± 10 |
| 3 Luteolin |  | 125 ± 5.5 |
| 4 Genistein |  | 154 ± 10 |
| 5 Gossypetin |  | 115 ± 2 |
| 6 Kaempferol |  | 155 ± 5 |
| 7 Myricetin |  | 97 ± 6 |
| 8 Eriodictyol |  | 108 ± 2.5 |
| 9 Naringenin |  | 105 ± 3 |
| 10 Taxifolin |  | 107 ± 5.5 |
| 11 Phloretin |  | 231 ± 0.5 |
| 12 Curcumin |  | 148 ± 43 |
| 13 Demethoxycurcumin (DMC) |  | 408 ± 18 |
| 14 Bisdemethoxycurcumin (BDMC) |  | 469 ± 42 |
| Compound Number | Molecular Structure | % ± s.e. Current Vs. ACh [200 µM] a |
|---|---|---|
| 17 |  | 143 ± 3.5 |
| 21 |  | 107 ± 4 |
| 22 |  | 153 ± 17 |
| 23 |  | 498 ± 7 |
| 24 |  | 53.5 ± 3.5 |
| 25 |  | 251 ± 24 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ximenis, M.; Mulet, J.; Sala, S.; Sala, F.; Criado, M.; González-Muñiz, R.; Pérez de Vega, M.J. Natural Polyhydroxy Flavonoids, Curcuminoids, and Synthetic Curcumin Analogs as α7 nAChRs Positive Allosteric Modulators. Int. J. Mol. Sci. 2021, 22, 973. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020973
Ximenis M, Mulet J, Sala S, Sala F, Criado M, González-Muñiz R, Pérez de Vega MJ. Natural Polyhydroxy Flavonoids, Curcuminoids, and Synthetic Curcumin Analogs as α7 nAChRs Positive Allosteric Modulators. International Journal of Molecular Sciences. 2021; 22(2):973. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020973
Chicago/Turabian StyleXimenis, Marta, José Mulet, Salvador Sala, Francisco Sala, Manuel Criado, Rosario González-Muñiz, and María Jesús Pérez de Vega. 2021. "Natural Polyhydroxy Flavonoids, Curcuminoids, and Synthetic Curcumin Analogs as α7 nAChRs Positive Allosteric Modulators" International Journal of Molecular Sciences 22, no. 2: 973. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22020973