Hijacking Sexual Immuno-Privilege in GBM—An Immuno-Evasion Strategy
Abstract
:1. Introduction
2. Results
2.1. HiF and RORC-Treg Gene Cross Correlation, Tumor Microenvironment and Impacts GBM Patient Outcome
Validation of HiF and RORC-Treg Gene Markers
2.2. HiF and Tregs Impacts on Survival and Tumor Microenvironment
2.2.1. Survival and HiF/RORC-Treg Status
2.2.2. CMP Subtypes
2.2.3. Stratification Reportage and Microglial Phenotypes
2.2.4. MDSCs
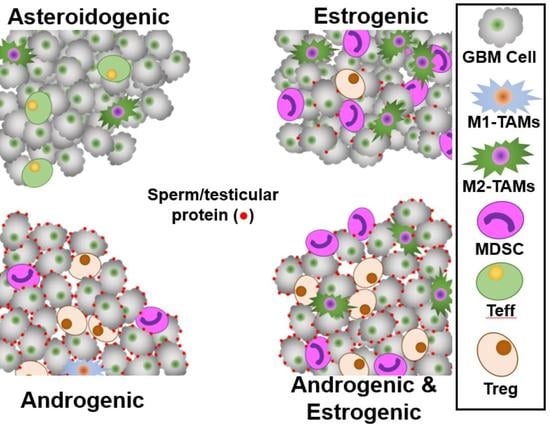
2.2.5. Sperm/Testicular Proteins
2.2.6. Androgen and Estrogen Reporters and Macrophage Status
- (a)
- Keratin 37, our androgen reporter, correlates with Tregs.
- (b)
- Aromatase (CYP19A1), which converts testosterone to estrogen is elevated with HiF.
- (c)
- Sperm/testicular-specific gene transcripts ACRBP, CATSPER1, LY6K, SPATA12 and THEG all correlate with Tregs.
2.3. Steroidogenic Status Impacts GBM Patient Outcome
2.3.1. Steroidogenesis in GMB
2.3.2. Overall Survival and Status
2.3.3. CMP Subtypes
2.3.4. HiF and Tregs
2.3.5. Microglial Phenotypes
2.3.6. MDSCs
2.3.7. Sperm/Testicular Proteins
2.3.8. GBM Steroidogenesis Validation, Macrophage Infiltration and Polarization
2.4. Elevation of Sperm/Testicular Specific Antibodies in GBM Patients
3. Discussion
4. Materials and Methods
4.1. Stratification of GBM Phenotype by Warburg and Treg Infiltration Status
4.1.1. Stratification of GBM Tumors by HiF
4.1.2. Stratification of GBM Tumors by Tregs
4.1.3. Selection of Treg&HiF and Neither Groups
4.1.4. Assaying Microglia Levels and Status
4.2. Stratification of GBM Phenotype by Steroidogenesis
4.2.1. Choice of Genes for Stratification of Tumors by Steroidogenesis
4.2.2. Canonical Steroidogenesis Groups A&P and E&P
4.2.3. Non-Canonical Steroidogenesis, the E&A Group
4.2.4. Selection of Androgen/Estrogen in the Four Groups
4.2.5. Visualization of Anti-Sperm Antibodies from Patient Sera
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

References
- Stupp, R.; Mason, W.P.; van den Bent, M.J.; Weller, M.; Fisher, B.; Taphoorn, M.J.; Belanger, K.; Brandes, A.A.; Marosi, C.; Bogdahn, U.; et al. Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N. Engl. J. Med. 2005, 352, 987–996. [Google Scholar] [CrossRef] [PubMed]
- Marenco-Hillembrand, L.; Wijesekera, O.; Suarez-Meade, P.; Mampre, D.; Jackson, C.; Peterson, J.; Trifiletti, D.; Hammack, J.; Ortiz, K.; Lesser, E.; et al. Trends in glioblastoma: Outcomes over time and type of intervention: A systematic evidence based analysis. J. Neurooncol. 2020, 147, 297–307. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, M.R.; Dignam, J.J.; Armstrong, T.S.; Wefel, J.S.; Blumenthal, D.T.; Vogelbaum, M.A.; Colman, H.; Chakravarti, A.; Pugh, S.; Won, M.; et al. A randomized trial of bevacizumab for newly diagnosed glioblastoma. N. Engl. J. Med. 2014, 370, 699–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Louis, D.N.; Perry, A.; Reifenberger, G.; von Deimling, A.; Figarella-Branger, D.; Cavenee, W.K.; Ohgaki, H.; Wiestler, O.D.; Kleihues, P.; Ellison, D.W. The 2016 World Health Organization Classification of Tumors of the Central Nervous System: A summary. Acta Neuropathol. 2016, 131, 803–820. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stringfield, O.; Arrington, J.A.; Johnston, S.K.; Rognin, N.G.; Peeri, N.C.; Balagurunathan, Y.; Jackson, P.R.; Clark-Swanson, K.R.; Swanson, K.R.; Egan, K.M.; et al. Multiparameter MRI Predictors of Long-Term Survival in Glioblastoma Multiforme. Tomography 2019, 5, 135–144. [Google Scholar] [CrossRef]
- Villanueva-Meyer, J.E.; Mabray, M.C.; Cha, S. Current Clinical Brain Tumor Imaging. Neurosurgery 2017, 81, 397–415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, S.; Michalek, J.E.; Reardon, D.A.; Wen, P.Y.; Floyd, J.R.; Fox, P.T.; Clarke, G.D.; Jerabek, P.A.; Schmainda, K.M.; Muzi, M.; et al. Assessment of tumor hypoxia and perfusion in recurrent glioblastoma following bevacizumab failure using MRI and 18F-FMISO PET. Sci. Rep. 2021, 11, 7632. [Google Scholar] [CrossRef] [PubMed]
- Sharpe, M.A.; Ismail, N.; Baskin, D.S. Metabolic sculpting of the mitochondria, cell signaling and the cancer phenotype. Transl. Cancer Res. 2017, 6, S182–S188. [Google Scholar] [CrossRef]
- Krzeslak, A.; Wojcik-Krowiranda, K.; Forma, E.; Jozwiak, P.; Romanowicz, H.; Bienkiewicz, A.; Brys, M. Expression of GLUT1 and GLUT3 glucose transporters in endometrial and breast cancers. Pathol. Oncol. Res. 2012, 18, 721–728. [Google Scholar] [CrossRef] [Green Version]
- Flavahan, W.A.; Wu, Q.; Hitomi, M.; Rahim, N.; Kim, Y.; Sloan, A.E.; Weil, R.J.; Nakano, I.; Sarkaria, J.N.; Stringer, B.W.; et al. Brain Tumor Initiating Cells Adapt to Restricted Nutrition through Preferential Glucose Uptake. Nat. Neurosci. 2013, 16, 1373–1382. [Google Scholar] [CrossRef]
- Masin, M.; Vazquez, J.; Rossi, S.; Groeneveld, S.; Samson, N.; Schwalie, P.C.; Deplancke, B.; Frawley, L.E.; Gouttenoire, J.; Moradpour, D.; et al. GLUT3 is induced during epithelial-mesenchymal transition and promotes tumor cell proliferation in non-small cell lung cancer. Cancer Metab. 2014, 2, 11. [Google Scholar] [CrossRef] [Green Version]
- Luo, X.; Xu, S.; Zhong, Y.; Tu, T.; Xu, Y.; Li, X.; Wang, B.; Yang, F. High gene expression levels of VEGFA and CXCL8 in the peritumoral brain zone are associated with the recurrence of glioblastoma: A bioinformatics analysis. Oncol. Lett. 2019, 18, 6171–6179. [Google Scholar] [CrossRef] [Green Version]
- Shen, G.M.; Zhao, Y.Z.; Chen, M.T.; Zhang, F.L.; Liu, X.L.; Wang, Y.; Liu, C.Z.; Yu, J.; Zhang, J.W. Hypoxia-inducible factor-1 (HIF-1) promotes LDL and VLDL uptake through inducing VLDLR under hypoxia. Biochem. J. 2012, 441, 675–683. [Google Scholar] [CrossRef] [PubMed]
- Masoud, G.N.; Li, W. HIF-1α pathway: Role, regulation and intervention for cancer therapy. Acta Pharm. Sin. B 2015, 5, 378–389. [Google Scholar] [CrossRef] [Green Version]
- Krzywinska, E.; Stockmann, C. Hypoxia, Metabolism and Immune Cell Function. Biomedicines 2018, 6, 56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mi, Y.; Guo, N.; Luan, J.; Cheng, J.; Hu, Z.; Jiang, P.; Jin, W.; Gao, X. The Emerging Role of Myeloid-Derived Suppressor Cells in the Glioma Immune Suppressive Microenvironment. Front. Immunol. 2020, 11, 737. [Google Scholar] [CrossRef] [PubMed]
- Bayik, D.; Zhou, Y.; Park, C.; Hong, C.; Vail, D.; Silver, D.J.; Lauko, A.; Roversi, G.; Watson, D.C.; Lo, A.; et al. Myeloid-Derived Suppressor Cell Subsets Drive Glioblastoma Growth in a Sex-Specific Manner. Cancer Discov. 2020, 10, 1210–1225. [Google Scholar] [CrossRef] [Green Version]
- Landry, A.P.; Balas, M.; Alli, S.; Spears, J.; Zador, Z. Distinct regional ontogeny and activation of tumor associated macrophages in human glioblastoma. Sci. Rep. 2020, 10, 19542. [Google Scholar] [CrossRef]
- Takenaka, M.C.; Gabriely, G.; Rothhammer, V.; Mascanfroni, I.D.; Wheeler, M.A.; Chao, C.-C.; Gutiérrez-Vázquez, C.; Kenison, J.; Tjon, E.C.; Barroso, A.; et al. Control of tumor-associated macrophages and T cells in glioblastoma via AHR and CD39. Nat. Neurosci. 2019, 22, 729–740. [Google Scholar] [CrossRef]
- Miska, J.; Rashidi, A.; Chang, A.L.; Muroski, M.E.; Han, Y.; Zhang, L.; Lesniak, M.S. Anti-GITR therapy promotes immunity against malignant glioma in a murine model. Cancer Immunol. Immunother. 2016, 65, 1555–1567. [Google Scholar] [CrossRef]
- Sayour, E.J.; McLendon, P.; McLendon, R.; De Leon, G.; Reynolds, R.; Kresak, J.; Sampson, J.H.; Mitchell, D.A. Increased proportion of FoxP3+ regulatory T cells in tumor infiltrating lymphocytes is associated with tumor recurrence and reduced survival in patients with glioblastoma. Cancer Immunol. Immunother. 2015, 64, 419–427. [Google Scholar] [CrossRef] [PubMed]
- Sieow, J.L.; Gun, S.Y.; Wong, S.C. The Sweet Surrender: How Myeloid Cell Metabolic Plasticity Shapes the Tumor Microenvironment. Front. Cell Dev. Biol. 2018, 6, 168. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, X.Y.; Zhang, G.H.; Wang, Z.N.; Duan, H.; Xie, T.; Liang, L.; Cui, R.; Hu, H.R.; Wu, Y.; Dong, J.J.; et al. A novel Foxp3-related immune prognostic signature for glioblastoma multiforme based on immunogenomic profiling. Aging 2021, 13, 3501–3517. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Huang, J.; Liu, X.; Cheng, Q.; Luo, C.; Liu, Z. CTLA-4 correlates with immune and clinical characteristics of glioma. Cancer Cell Int. 2020, 20, 7. [Google Scholar] [CrossRef]
- Aslan, K.; Turco, V.; Blobner, J.; Sonner, J.K.; Liuzzi, A.R.; Núñez, N.G.; De Feo, D.; Kickingereder, P.; Fischer, M.; Green, E.; et al. Heterogeneity of response to immune checkpoint blockade in hypermutated experimental gliomas. Nat. Commun. 2020, 11, 931. [Google Scholar] [CrossRef] [Green Version]
- Patel, M.A.; Kim, J.E.; Theodros, D.; Tam, A.; Velarde, E.; Kochel, C.M.; Francica, B.; Nirschl, T.R.; Ghasemzadeh, A.; Mathios, D.; et al. Agonist anti-GITR monoclonal antibody and stereotactic radiation induce immune-mediated survival advantage in murine intracranial glioma. J. Immunother. Cancer 2016, 4, 28. [Google Scholar] [CrossRef] [Green Version]
- Takashima, Y.; Kawaguchi, A.; Hayano, A.; Yamanaka, R. CD276 and the gene signature composed of GATA3 and LGALS3 enable prognosis prediction of glioblastoma multiforme. PLoS ONE 2019, 14, e0216825. [Google Scholar] [CrossRef]
- Ayyoub, M.; Deknuydt, F.; Raimbaud, I.; Dousset, C.; Leveque, L.; Bioley, G.; Valmori, D. Human memory FOXP3+ Tregs secrete IL-17 ex vivo and constitutively express the T(H)17 lineage-specific transcription factor RORgamma t. Proc. Natl. Acad. Sci. USA 2009, 106, 8635–8640. [Google Scholar] [CrossRef] [Green Version]
- Yang, B.H.; Hagemann, S.; Mamareli, P.; Lauer, U.; Hoffmann, U.; Beckstette, M.; Föhse, L.; Prinz, I.; Pezoldt, J.; Suerbaum, S.; et al. Foxp3+ T cells expressing RORγt represent a stable regulatory T-cell effector lineage with enhanced suppressive capacity during intestinal inflammation. Mucosal Immunol. 2016, 9, 444–457. [Google Scholar] [CrossRef]
- Weaver, C.T.; Hatton, R.D. Interplay between the TH17 and TReg cell lineages: A (co-)evolutionary perspective. Nat. Rev. Immunol. 2009, 9, 883–889. [Google Scholar] [CrossRef]
- Herrnstadt, G.R.; Steinmetz, O.M. The role of Treg subtypes in glomerulonephritis. Cell Tissue Res. 2020. [Google Scholar] [CrossRef]
- Kluger, M.A.; Meyer, M.C.; Nosko, A.; Goerke, B.; Luig, M.; Wegscheid, C.; Tiegs, G.; Stahl, R.A.; Panzer, U.; Steinmetz, O.M. RORγt(+)Foxp3(+) Cells are an Independent Bifunctional Regulatory T Cell Lineage and Mediate Crescentic GN. J. Am. Soc. Nephrol. 2016, 27, 454–465. [Google Scholar] [CrossRef] [Green Version]
- Le Tortorec, A.; Matusali, G.; Mahé, D.; Aubry, F.; Mazaud-Guittot, S.; Houzet, L.; Dejucq-Rainsford, N. From Ancient to Emerging Infections: The Odyssey of Viruses in the Male Genital Tract. Physiol. Rev. 2020, 100, 1349–1414. [Google Scholar] [CrossRef]
- Teles, A.; Schumacher, A.; Kühnle, M.; Linzke, N.; Thuere, C.; Reichardt, P.; Tadokoro, C.; Hämmerling, G.; Zenclussen, A. Control of Uterine Microenvironment by Foxp3+ Cells Facilitates Embryo Implantation. Front. Immunol. 2013, 4, 158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, S.M.; Xing, F.Q.; Sui, H.; Wang, Y.L.; Mai, X.F.; Luo, Z.Q.; Chen, X.Q.; Chen, G.H.; Kong, Z.J. [CA 125 expression in cervical and vaginal secretions in women in normal reproductive period]. Nan Fang Yi Ke Da Xue Xue Bao 2010, 30, 173–175. [Google Scholar] [PubMed]
- Tavmergen, E.; Sendag, F.; Goker, E.N.T.; Levi, R. Value of serum CA-125 concentrations as predictors of pregnancy in assisted reproduction cycles. Hum. Reprod. 2001, 16, 1129–1134. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Geva, E.; Arava, J.; Lerner-Geva, L.; Hareuveni, M.; Lessing, J.B.; Ballas, S.; Amit, A. The ratio between carcinoma antigen-125 levels in the seminal plasma and serum may be a marker for fertilization in intracytoplasmic sperm injection. Fertil. Steril. 1997, 68, 1120–1124. [Google Scholar] [CrossRef]
- Reinartz, S.R.; Pfisterer, J.; du Bois, A.; Jackisch, C.; Baumann, K.H.; Wagner, U. Suppressive activity rather than frequency of FoxP3(+) regulatory T cells is essential for CA-125-specific T-cell activation after abagovomab treatment. Hum. Immunol. 2010, 71, 36–44. [Google Scholar] [CrossRef]
- Pinget, G.V.; Corpuz, T.M.; Stolp, J.; Lousberg, E.L.; Diener, K.R.; Robertson, S.A.; Sprent, J.; Webster, K.E. The majority of murine γδ T cells at the maternal–fetal interface in pregnancy produce IL-17. Immunol. Cell Biol. 2016, 94, 623–630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallino, L.; Hauk, V.; Fernández, L.; Soczewski, E.; Gori, S.; Grasso, E.; Calo, G.; Saraco, N.; Berensztein, E.; Waschek, J.A.; et al. VIP Promotes Recruitment of Tregs to the Uterine–Placental Interface During the Peri-Implantation Period to Sustain a Tolerogenic Microenvironment. Front. Immunol. 2020, 10, 2907. [Google Scholar] [CrossRef]
- Robertson, S.A.; Care, A.S.; Moldenhauer, L.M. Regulatory T cells in embryo implantation and the immune response to pregnancy. J. Clin. Investig. 2018, 128, 4224–4235. [Google Scholar] [CrossRef] [Green Version]
- Wilharm, A.; Brigas, H.C.; Sandrock, I.; Ribeiro, M.; Amado, T.; Reinhardt, A.; Demera, A.; Hoenicke, L.; Strowig, T.; Carvalho, T.; et al. Microbiota-dependent expansion of testicular IL-17-producing Vγ6+ γδ T cells upon puberty promotes local tissue immune surveillance. Mucosal Immunol. 2021, 14, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Franke, F.; Kraus, S.; Pauls, K.; Lalani, E.-N.; Bergmann, M. MUC1 in normal and impaied spermatogenesis. Mol. Hum. Reprod. 2001, 7, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Russo, C.L.; Spurr-Michaud, S.; Tisdale, A.; Pudney, J.; Anderson, D.; Gipson, I.K. Mucin gene expression in human male urogenital tract epithelia. Hum. Reprod. 2006, 21, 2783–2793. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, B.; Benavides, G.A.; Geng, J.; Koralov, S.B.; Hu, H.; Darley-Usmar, V.M.; Harrington, L.E. Mitochondrial Oxidative Phosphorylation Regulates the Fate Decision between Pathogenic Th17 and Regulatory T Cells. Cell Rep. 2020, 30, 1898–1909.e4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheng, L.; Kelly, C.J.; Colgan, S.P. Physiologic hypoxia and oxygen homeostasis in the healthy intestine. A Review in the Theme: Cellular Responses to Hypoxia. Am. J. Physiol. Cell Physiol. 2015, 309, C350–C360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bohlen, H.G. Intestinal mucosal oxygenation influences absorptive hyperemia. Am. J. Physiol. 1980, 239, H489–H493. [Google Scholar] [CrossRef]
- Dominguez, J.M.; Davis, R.T.; McCullough, D.J.; Stabley, J.N.; Behnke, B.J. Aging and exercise training reduce testes microvascular PO2 and alter vasoconstrictor responsiveness in testicular arterioles. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2011, 301, R801–R810. [Google Scholar] [CrossRef] [Green Version]
- Movsas, B.; Chapman, J.D.; Hanlon, A.L.; Horwitz, E.M.; Greenberg, R.E.; Stobbe, C.; Hanks, G.E.; Pollack, A. Hypoxic prostate/muscle po2 ratio predicts for biochemical failure in patients with prostate cancer: Preliminary findings. Urology 2002, 60, 634–639. [Google Scholar] [CrossRef]
- Fukuda, M.; Fukuda, K.; Ranoux, C. Unexpected low oxygen tension of intravaginal culture. Hum. Reprod. 1996, 11, 1293–1295. [Google Scholar] [CrossRef] [Green Version]
- Rashad, A.L.; Toffler, W.L.; Wolf, N.; Thornburg, K.; Kirk, E.P.; Ellis, G.; Whitehead, W.E. Vaginal PO2 in healthy women and in women infected with Trichomonas vaginalis: Potential implications for metronidazole therapy. Am. J. Obs. Gynecol. 1992, 166, 620–624. [Google Scholar] [CrossRef]
- Hill, D.R.; Brunner, M.E.; Schmitz, D.C.; Davis, C.C.; Flood, J.A.; Schlievert, P.M.; Wang-Weigand, S.Z.; Osborn, T.W. In vivo assessment of human vaginal oxygen and carbon dioxide levels during and post menses. J. Appl. Physiol. 2005, 99, 1582–1591. [Google Scholar] [CrossRef] [PubMed]
- McKeown, S.R. Defining normoxia, physoxia and hypoxia in tumours-implications for treatment response. Br. J. Radiol. 2014, 87, 20130676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaupel, P.; Höckel, M.; Mayer, A. Detection and characterization of tumor hypoxia using pO2 histography. Antioxid. Redox Signal. 2007, 9, 1221–1235. [Google Scholar] [CrossRef] [PubMed]
- Pinacho-Garcia, L.M.; Valdez, R.A.; Navarrete, A.; Cabeza, M.; Segovia, J.; Romano, M.C. The effect of finasteride and dutasteride on the synthesis of neurosteroids by glioblastoma cells. Steroids 2020, 155, 108556. [Google Scholar] [CrossRef] [PubMed]
- Orozco, M.; Valdez, R.A.; Ramos, L.; Cabeza, M.; Segovia, J.; Romano, M.C. Dutasteride combined with androgen receptor antagonists inhibit glioblastoma U87 cell metabolism, proliferation, and invasion capacity: Androgen regulation. Steroids 2020, 164, 108733. [Google Scholar] [CrossRef]
- Chuang, J.Y.; Lo, W.L.; Ko, C.Y.; Chou, S.Y.; Chen, R.M.; Chang, K.Y.; Hung, J.J.; Su, W.C.; Chang, W.C.; Hsu, T.I. Upregulation of CYP17A1 by Sp1-mediated DNA demethylation confers temozolomide resistance through DHEA-mediated protection in glioma. Oncogenesis 2017, 6, e339. [Google Scholar] [CrossRef] [Green Version]
- Lin, H.Y.; Ko, C.Y.; Kao, T.J.; Yang, W.B.; Tsai, Y.T.; Chuang, J.Y.; Hu, S.L.; Yang, P.Y.; Lo, W.L.; Hsu, T.I. CYP17A1 Maintains the Survival of Glioblastomas by Regulating SAR1-Mediated Endoplasmic Reticulum Health and Redox Homeostasis. Cancers 2019, 11, 1378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rižner, T.L.; Penning, T.M. Role of aldo-keto reductase family 1 (AKR1) enzymes in human steroid metabolism. Steroids 2014, 79, 49–63. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Vega, A.M.; Del Moral-Morales, A.; Zamora-Sánchez, C.J.; Piña-Medina, A.G.; González-Arenas, A.; Camacho-Arroyo, I. Estradiol Induces Epithelial to Mesenchymal Transition of Human Glioblastoma Cells. Cells 2020, 9, 1930. [Google Scholar] [CrossRef]
- Zhao, N.; Wang, F.; Ahmed, S.; Liu, K.; Zhang, C.; Cathcart, S.J.; DiMaio, D.J.; Punsoni, M.; Guan, B.; Zhou, P.; et al. Androgen Receptor, Although Not a Specific Marker For, Is a Novel Target to Suppress Glioma Stem Cells as a Therapeutic Strategy for Glioblastoma. Front. Oncol. 2021, 11, 1696. [Google Scholar]
- Adurthi, S.; Kumar, M.M.; Vinodkumar, H.S.; Mukherjee, G.; Krishnamurthy, H.; Acharya, K.K.; Bafna, U.D.; Uma, D.K.; Abhishekh, B.; Krishna, S.; et al. Oestrogen Receptor-α binds the FOXP3 promoter and modulates regulatory T-cell function in human cervical cancer. Sci. Rep. 2017, 7, 17289. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, A.; Wada, T.; Nishimura, S.; Ito, T.; Okekawa, A.; Onogi, Y.; Watanabe, E.; Sameshima, A.; Tanaka, T.; Tsuneki, H.; et al. Estrogen regulates sex-specific localization of regulatory T cells in adipose tissue of obese female mice. PLoS ONE 2020, 15, e0230885. [Google Scholar] [CrossRef] [PubMed]
- Mjösberg, J.; Svensson, J.; Johansson, E.; Hellström, L.; Casas, R.; Jenmalm, M.C.; Boij, R.; Matthiesen, L.; Jönsson, J.I.; Berg, G.; et al. Systemic reduction of functionally suppressive CD4dimCD25highFoxp3+ Tregs in human second trimester pregnancy is induced by progesterone and 17beta-estradiol. J. Immunol. 2009, 183, 759–769. [Google Scholar] [CrossRef] [Green Version]
- Zhu, M.L.; Bakhru, P.; Conley, B.; Nelson, J.S.; Free, M.; Martin, A.; Starmer, J.; Wilson, E.M.; Su, M.A. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat. Commun. 2016, 7, 11350. [Google Scholar] [CrossRef] [PubMed]
- Walecki, M.; Eisel, F.; Klug, J.; Baal, N.; Paradowska-Dogan, A.; Wahle, E.; Hackstein, H.; Meinhardt, A.; Fijak, M. Androgen receptor modulates Foxp3 expression in CD4+CD25+Foxp3+ regulatory T-cells. Mol. Biol. Cell 2015, 26, 2845–2857. [Google Scholar] [CrossRef]
- Consiglio, C.R.; Udartseva, O.; Ramsey, K.D.; Bush, C.; Gollnick, S.O. Enzalutamide, an Androgen Receptor Antagonist, Enhances Myeloid Cell-Mediated Immune Suppression and Tumor Progression. Cancer Immunol. Res. 2020, 8, 1215–1227. [Google Scholar] [CrossRef]
- Sharpe, M.A.; Ijare, O.B.; Baskin, D.S.; Baskin, A.M.; Baskin, B.N.; Pichumani, K. The Leloir Cycle in Glioblastoma: Galactose Scavenging and Metabolic Remodeling. Cancers 2021, 13, 1815. [Google Scholar] [CrossRef]
- Shraibman, B.; Barnea, E.; Kadosh, D.M.; Haimovich, Y.; Slobodin, G.; Rosner, I.; López-Larrea, C.; Hilf, N.; Kuttruff, S.; Song, C.; et al. Identification of Tumor Antigens Among the HLA Peptidomes of Glioblastoma Tumors and Plasma. Mol. Cell. Proteom. 2018, 17, 2132–2145. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, Y.; Komiyama, M.; Miyata, H.; Yagoto, M.; Ashizawa, T.; Iizuka, A.; Oshita, C.; Kume, A.; Nogami, M.; Ito, I.; et al. Novel cancer-testis antigen expression on glioma cell lines derived from high-grade glioma patients. Oncol. Rep. 2014, 31, 1683–1690. [Google Scholar] [CrossRef]
- Freitas, M.; Malheiros, S.; Stávale, J.N.; Biassi, T.P.; Zamunér, F.T.; de Souza Begnami, M.; Soares, F.A.; Vettore, A.L. Expression of cancer/testis antigens is correlated with improved survival in glioblastoma. Oncotarget 2013, 4, 636–646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sreekanthreddy, P.; Srinivasan, H.; Kumar, D.M.; Nijaguna, M.B.; Sridevi, S.; Vrinda, M.; Arivazhagan, A.; Balasubramaniam, A.; Hegde, A.S.; Chandramouli, B.A.; et al. Identification of potential serum biomarkers of glioblastoma: Serum osteopontin levels correlate with poor prognosis. Cancer Epidemiol. Biomark. Prev. 2010, 19, 1409–1422. [Google Scholar] [CrossRef] [Green Version]
- Jones, K.; Ballesteros, A.; Mentink-Kane, M.; Warren, J.; Rattila, S.; Malech, H.; Kang, E.; Dveksler, G. PSG9 Stimulates Increase in FoxP3+ Regulatory T-Cells through the TGF-β1 Pathway. PLoS ONE 2016, 11, e0158050. [Google Scholar] [CrossRef] [PubMed]
- Timganova, V.P.; Zamorina, S.A.; Litvinova, L.S.; Todosenko, N.M.; Bochkova, M.S.; Khramtsov, P.V.; Rayev, M.B. The effects of human pregnancy-specific β1-glycoprotein preparation on Th17 polarization of CD4+ cells and their cytokine profile. BMC Immunol. 2020, 21, 56. [Google Scholar] [CrossRef]
- Zheng, B. Expression and clinical importance of a newly discovered alternative splice variant of the gene for acrosin binding protein found in human brain tumors. Asian Biomed. 2020, 14, 243–252. [Google Scholar] [CrossRef]
- Li, X.; Yan, J.; Fan, R.; Luo, B.; Zhang, Q.; Lin, Y.; Zhou, S.; Luo, G.; Xie, X.; Xiao, S. Serum immunoreactivity of cancer/testis antigen OY-TES-1 and its tissues expression in glioma. Oncol. Lett. 2017, 13, 3080–3086. [Google Scholar] [CrossRef] [Green Version]
- Redzovic, A.; Laskarin, G.; Dominovic, M.; Haller, H.; Rukavina, D. Mucins Help to Avoid Alloreactivity at the Maternal Fetal Interface. Clin. Dev. Immunol. 2013, 2013, 542152. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xu, H.; Kufe, D. MUC1 Oncoprotein Stabilizes and Activates Estrogen Receptor α. Mol. Cell 2006, 21, 295–305. [Google Scholar] [CrossRef]
- Meseguer, M.; Aplin, J.D.; Caballero-Campo, P.; O’Connor, J.E.; Martín, J.C.; Remohí, J.; Pellicer, A.; Simón, C. Human Endometrial Mucin MUC1 Is Up-Regulated by Progesterone and Down-Regulated In Vitro by the Human Blastocyst1. Biol. Reprod. 2001, 64, 590–601. [Google Scholar] [CrossRef] [Green Version]
- Aubert, S.; Fauquette, V.; Hémon, B.; Lepoivre, R.; Briez, N.; Bernard, D.; Van Seuningen, I.; Leroy, X.; Perrais, M. MUC1, a New Hypoxia Inducible Factor Target Gene, Is an Actor in Clear Renal Cell Carcinoma Tumor Progression. Cancer Res. 2009, 69, 5707. [Google Scholar] [CrossRef] [Green Version]
- Pyzer, A.R.; Stroopinsky, D.; Rajabi, H.; Washington, A.; Tagde, A.; Coll, M.; Fung, J.; Bryant, M.P.; Cole, L.; Palmer, K.; et al. MUC1-mediated induction of myeloid-derived suppressor cells in patients with acute myeloid leukemia. Blood 2017, 129, 1791–1801. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Seo, Y.; Chowdhury, T.; Yu, H.J.; Lee, C.E.; Kim, K.-M.; Kang, H.; Kim, H.J.; Park, S.-J.; Kim, K.; et al. Inhibition of MUC1 exerts cell-cycle arrest and telomerase suppression in glioblastoma cells. Sci. Rep. 2020, 10, 18238. [Google Scholar] [CrossRef]
- Phillips, H.S.; Kharbanda, S.; Chen, R.; Forrest, W.F.; Soriano, R.H.; Wu, T.D.; Misra, A.; Nigro, J.M.; Colman, H.; Soroceanu, L.; et al. Molecular subclasses of high-grade glioma predict prognosis, delineate a pattern of disease progression, and resemble stages in neurogenesis. Cancer Cell 2006, 9, 157–173. [Google Scholar] [CrossRef] [Green Version]
- Verhaak, R.G.; Hoadley, K.A.; Purdom, E.; Wang, V.; Qi, Y.; Wilkerson, M.D.; Miller, C.R.; Ding, L.; Golub, T.; Mesirov, J.P.; et al. Integrated genomic analysis identifies clinically relevant subtypes of glioblastoma characterized by abnormalities in PDGFRA, IDH1, EGFR, and NF1. Cancer Cell 2010, 17, 98–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Teo, W.-Y.; Sekar, K.; Seshachalam, P.; Shen, J.; Chow, W.-Y.; Lau, C.C.; Yang, H.; Park, J.; Kang, S.-G.; Li, X.; et al. Relevance of a TCGA-derived Glioblastoma Subtype Gene-Classifier among Patient Populations. Sci. Rep. 2019, 9, 7442. [Google Scholar] [CrossRef]
- Bowman, R.L.; Wang, Q.; Carro, A.; Verhaak, R.G.W.; Squatrito, M. GlioVis data portal for visualization and analysis of brain tumor expression datasets. Neuro Oncol. 2017, 19, 139–141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ohue, Y.; Nishikawa, H. Regulatory T (Treg) cells in cancer: Can Treg cells be a new therapeutic target? Cancer Sci. 2019, 110, 2080–2089. [Google Scholar] [CrossRef] [PubMed]
- Knee, D.A.; Hewes, B.; Brogdon, J.L. Rationale for anti-GITR cancer immunotherapy. Eur. J. Cancer 2016, 67, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kim, B.-S.; Lu, H.; Ichiyama, K.; Chen, X.; Zhang, Y.-B.; Mistry, N.A.; Tanaka, K.; Lee, Y.-H.; Nurieva, R.; Zhang, L.; et al. Generation of RORγt(+) Antigen-Specific T Regulatory 17 Cells from Foxp3(+) Precursors in Autoimmunity. Cell Rep. 2017, 21, 195–207. [Google Scholar] [CrossRef] [Green Version]
- Wohlfert, E.A.; Grainger, J.R.; Bouladoux, N.; Konkel, J.E.; Oldenhove, G.; Ribeiro, C.H.; Hall, J.A.; Yagi, R.; Naik, S.; Bhairavabhotla, R.; et al. GATA3 controls Foxp3⁺ regulatory T cell fate during inflammation in mice. J. Clin. Investig. 2011, 121, 4503–4515. [Google Scholar] [CrossRef]
- Ho, I.C.; Tai, T.-S.; Pai, S.-Y. GATA3 and the T-cell lineage: Essential functions before and after T-helper-2-cell differentiation. Nat. Rev. Immunol. 2009, 9, 125–135. [Google Scholar] [CrossRef] [Green Version]
- Sousa, C.; Golebiewska, A.; Poovathingal, S.K.; Kaoma, T.; Pires-Afonso, Y.; Martina, S.; Coowar, D.; Azuaje, F.; Skupin, A.; Balling, R.; et al. Single-cell transcriptomics reveals distinct inflammation-induced microglia signatures. EMBO Rep. 2018, 19, e46171. [Google Scholar] [CrossRef]
- Kuo, L.-C.; Cheng, L.-C.; Lee, C.-H.; Lin, C.-J.; Chen, P.-Y.; Li, L.-A. Estrogen and cigarette sidestream smoke particulate matter exhibit ERα-dependent tumor-promoting effects in lung adenocarcinoma cells. Am. J. Physiol. Lung Cell. Mol. Physiol. 2017, 313, L477–L490. [Google Scholar] [CrossRef]
- Dassen, H.; Punyadeera, C.; Kamps, R.; Klomp, J.; Dunselman, G.; Dijcks, F.; de Goeij, A.; Ederveen, A.; Groothuis, P. Progesterone regulation of implantation-related genes: New insights into the role of oestrogen. Cell. Mol. Life Sci. 2007, 64, 1009–1032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Droog, M.; Nevedomskaya, E.; Kim, Y.; Severson, T.; Flach, K.D.; Opdam, M.; Schuurman, K.; Gradowska, P.; Hauptmann, M.; Dackus, G.; et al. Comparative Cistromics Reveals Genomic Cross-talk between FOXA1 and ERα in Tamoxifen-Associated Endometrial Carcinomas. Cancer Res. 2016, 76, 3773–3784. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chan, H.J.; Li, H.; Liu, Z.; Yuan, Y.C.; Mortimer, J.; Chen, S. SERPINA1 is a direct estrogen receptor target gene and a predictor of survival in breast cancer patients. Oncotarget 2015, 6, 25815–25827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Q.; He, H.; Zhang, Y.-L.; Li, X.-M.; Guo, X.; Huo, R.; Bi, Y.; Li, J.; Fan, H.-Y.; Sha, J. Phosphoinositide 3-kinase p110δ mediates estrogen- and FSH-stimulated ovarian follicle growth. Mol. Endocrinol. 2013, 27, 1468–1482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, M.J.; Chen, X.D.; Zhou, X.L.; Wan, Y.J.; Lan, B.; Zhang, C.Z.; Cao, Y. Estrogen induces Vav1 expression in human breast cancer cells. PLoS ONE 2014, 9, e99052. [Google Scholar] [CrossRef]
- Zhao, Z.; Zhang, K.-N.; Wang, Q.; Li, G.; Zeng, F.; Zhang, Y.; Wu, F.; Chai, R.; Wang, Z.; Zhang, C.; et al. Chinese Glioma Genome Atlas (CGGA): A Comprehensive Resource with Functional Genomic Data from Chinese Gliomas. Genom. Proteom. Bioinform. 2021, 9, 1–12. [Google Scholar] [CrossRef]
- Gravendeel, L.A.M.; Kouwenhoven, M.C.M.; Gevaert, O.; de Rooi, J.J.; Stubbs, A.P.; Duijm, J.E.; Daemen, A.; Bleeker, F.E.; Bralten, L.B.C.; Kloosterhof, N.K.; et al. Intrinsic Gene Expression Profiles of Gliomas Are a Better Predictor of Survival than Histology. Cancer Res. 2009, 69, 9065. [Google Scholar] [CrossRef] [Green Version]
- Cassetta, L.; Baekkevold, E.S.; Brandau, S.; Bujko, A.; Cassatella, M.A.; Dorhoi, A.; Krieg, C.; Lin, A.; Loré, K.; Marini, O.; et al. Deciphering myeloid-derived suppressor cells: Isolation and markers in humans, mice and non-human primates. Cancer Immunol. Immunother. CII 2019, 68, 687–697. [Google Scholar] [CrossRef] [Green Version]
- Jave-Suarez, L.F.; Langbein, L.; Winter, H.; Praetzel, S.; Rogers, M.A.; Schweizer, J. Androgen regulation of the human hair follicle: The type I hair keratin hHa7 is a direct target gene in trichocytes. J. Investig. Derm. 2004, 122, 555–564. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burkart, M.F.; Wren, J.D.; Herschkowitz, J.I.; Perou, C.M.; Garner, H.R. Clustering microarray-derived gene lists through implicit literature relationships. Bioinformatics 2007, 23, 1995–2003. [Google Scholar] [CrossRef] [Green Version]
- Chu, F.; Mason, K.E.; Anex, D.S.; Jones, A.D.; Hart, B.R. Hair Proteome Variation at Different Body Locations on Genetically Variant Peptide Detection for Protein-Based Human Identification. Sci. Rep. 2019, 9, 7641. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Taguchi, H.; Kitahara, T.; Takema, Y.; Visscher, M.O.; Schweizer, J.; Langbein, L. Keratins of the human occipital hair medulla: Androgenic regulation of in vitro hair keratin K37 expression. Br. J. Derm. 2013, 169, 218–221. [Google Scholar] [CrossRef] [PubMed]
- Ke, X.; Chen, C.; Song, Y.; Cai, Q.; Li, J.; Tang, Y.; Han, X.; Qu, W.; Chen, A.; Wang, H.; et al. Hypoxia modifies the polarization of macrophages and their inflammatory microenvironment, and inhibits malignant behavior in cancer cells. Oncol. Lett. 2019, 18, 5871–5878. [Google Scholar] [CrossRef] [Green Version]
- Canciello, A.; Russo, V.; Berardinelli, P.; Bernabò, N.; Muttini, A.; Mattioli, M.; Barboni, B. Progesterone prevents epithelial-mesenchymal transition of ovine amniotic epithelial cells and enhances their immunomodulatory properties. Sci. Rep. 2017, 7, 3761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Zhang, H.; Ding, S.; Qi, S.; Liu, S.; Sun, D.; Dong, W.; Yin, L.; Li, M.; Zhao, X.; et al. βKlotho inhibits androgen/androgen receptor-associated epithelial-mesenchymal transition in prostate cancer through inactivation of ERK1/2 signaling. Oncol. Rep. 2018, 40, 217–225. [Google Scholar] [CrossRef] [Green Version]
- Velez-Perez, A.; Holder, M.K.; Fountain, S.; Blaustein, J.D. Estradiol Increases Microglial Response to Lipopolysaccharide in the Ventromedial Hypothalamus during the Peripubertal Sensitive Period in Female Mice. Eneuro 2020, 7, 0505-19. [Google Scholar] [CrossRef]
- Contreras-Zárate, M.J.; Cittelly, D.M. Sex steroid hormone function in the brain niche: Implications for brain metastatic colonization and progression. Cancer Rep. 2020, e1241. [Google Scholar] [CrossRef]
- Aryanpour, R.; Pasbakhsh, P.; Zibara, K.; Namjoo, Z.; Beigi Boroujeni, F.; Shahbeigi, S.; Kashani, I.R.; Beyer, C.; Zendehdel, A. Progesterone therapy induces an M1 to M2 switch in microglia phenotype and suppresses NLRP3 inflammasome in a cuprizone-induced demyelination mouse model. Int. Immunopharmacol. 2017, 51, 131–139. [Google Scholar] [CrossRef]
- Yao, P.-L.; Zhuo, S.; Mei, H.; Chen, X.-F.; Li, N.; Zhu, T.-F.; Chen, S.-T.; Wang, J.-M.; Hou, R.-X.; Le, Y.-Y. Androgen alleviates neurotoxicity of β-amyloid peptide (Aβ) by promoting microglial clearance of Aβ and inhibiting microglial inflammatory response to Aβ. CNS Neurosci. Ther. 2017, 23, 855–865. [Google Scholar] [CrossRef] [PubMed]
- Su, E.J.; Ernst, L.; Abdallah, N.; Chatterton, R.; Xin, H.; Monsivais, D.; Coon, J.; Bulun, S.E. Estrogen receptor-β and fetoplacental endothelial prostanoid biosynthesis: A link to clinically demonstrated fetal growth restriction. J. Clin. Endocrinol. Metab. 2011, 96, E1558–E1567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, M.; Deb, S.; Sebastian, S.; Okamura, K.; Bulun, S.E. Estrogen up-regulates cyclooxygenase-2 via estrogen receptor in human uterine microvascular endothelial cells. Fertil. Steril. 2004, 81, 1351–1356. [Google Scholar] [CrossRef] [PubMed]
- Cianciaruso, C.; Beltraminelli, T.; Duval, F.; Nassiri, S.; Hamelin, R.; Mozes, A.; Gallart-Ayala, H.; Ceada Torres, G.; Torchia, B.; Ries, C.H.; et al. Molecular Profiling and Functional Analysis of Macrophage-Derived Tumor Extracellular Vesicles. Cell Rep. 2019, 27, 3062–3080.e11. [Google Scholar] [CrossRef] [Green Version]
- Dou, C.; Ding, N.; Zhao, C.; Hou, T.; Kang, F.; Cao, Z.; Liu, C.; Bai, Y.; Dai, Q.; Ma, Q.; et al. Estrogen Deficiency-Mediated M2 Macrophage Osteoclastogenesis Contributes to M1/M2 Ratio Alteration in Ovariectomized Osteoporotic Mice. J. Bone Miner. Res. 2018, 33, 899–908. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsai, Y.C.; Tseng, J.T.; Wang, C.Y.; Su, M.T.; Huang, J.Y.; Kuo, P.L. Medroxyprogesterone acetate drives M2 macrophage differentiation toward a phenotype of decidual macrophage. Mol. Cell. Endocrinol. 2017, 452, 74–83. [Google Scholar] [CrossRef] [PubMed]
- Lang, Q.; Yidong, X.; Xueguang, Z.; Sixian, W.; Wenming, X.; Tao, Z. ETA-mediated anti-TNF-α therapy ameliorates the phenotype of PCOS model induced by letrozole. PLoS ONE 2019, 14, e0217495. [Google Scholar] [CrossRef]
- Korbecki, J.; Olbromski, M.; Dzięgiel, P. CCL18 in the Progression of Cancer. Int. J. Mol. Sci. 2020, 21, 7955. [Google Scholar] [CrossRef]
- Tung, K.S.K.; Harakal, J.; Qiao, H.; Rival, C.; Li, J.C.H.; Paul, A.G.A.; Wheeler, K.; Pramoonjago, P.; Grafer, C.M.; Sun, W.; et al. Egress of sperm autoantigen from seminiferous tubules maintains systemic tolerance. J. Clin. Investig. 2017, 127, 1046–1060. [Google Scholar] [CrossRef] [Green Version]
- Rival, C.; Wheeler, K.; Jeffrey, S.; Qiao, H.; Luu, B.; Tewalt, E.F.; Engelhard, V.H.; Tardif, S.; Hardy, D.; del Rio, R.; et al. Regulatory T cells and vasectomy. J. Reprod. Immunol. 2013, 100, 66–75. [Google Scholar] [CrossRef] [Green Version]
- Wheeler, K.; Tardif, S.; Rival, C.; Luu, B.; Bui, E.; Del Rio, R.; Teuscher, C.; Sparwasser, T.; Hardy, D.; Tung, K.S. Regulatory T cells control tolerogenic versus autoimmune response to sperm in vasectomy. Proc. Natl. Acad. Sci. USA 2011, 108, 7511. [Google Scholar] [CrossRef] [Green Version]
- Kessler, D.L.; Smith, W.D.; Hamilton, M.S.; Berger, R.E. Infertility in mice after unilateral vasectomy. Fertil. Steril. 1985, 43, 308–312. [Google Scholar] [CrossRef]
- Tung, K.S.; Cooke, W.D., Jr.; McCarty, T.A.; Robitaille, P. Human sperm antigens and antisperm antibodies. II. Age-related incidence of antisperm antibodies. Clin. Exp. Immunol. 1976, 25, 73–79. [Google Scholar]
- Mancini, R.E.; Andrada, J.A.; Saraceni, D.; Bachmann, A.E.; Lavieri, J.C.; Nemirovsky, M. Immunological and testicular response in man sensitized with human testicular homogenate. J. Clin. Endocrinol. Metab. 1965, 25, 859–875. [Google Scholar] [CrossRef]
- Taguchi, O.; Nishizuka, Y. Experimental autoimmune orchitis after neonatal thymectomy in the mouse. Clin. Exp. Immunol. 1981, 46, 425–434. [Google Scholar]
- Wong, A. The Molecular Evolution of Animal Reproductive Tract Proteins: What Have We Learned from Mating-System Comparisons? Int. J. Evol. Biol. 2011, 2011, 908735. [Google Scholar] [CrossRef] [Green Version]
- Mahata, B.; Pramanik, J.; van der Weyden, L.; Polanski, K.; Kar, G.; Riedel, A.; Chen, X.; Fonseca, N.A.; Kundu, K.; Campos, L.S.; et al. Tumors induce de novo steroid biosynthesis in T cells to evade immunity. Nat. Commun. 2020, 11, 3588. [Google Scholar] [CrossRef] [PubMed]
- The Cancer Genome Atlas Research Network. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008, 455, 1061–1068. [Google Scholar] [CrossRef] [PubMed]
- Yanguas-Casás, N. Physiological sex differences in microglia and their relevance in neurological disorders. Neuroimmunol. Neuroinflamm. 2020, 7, 13–22. [Google Scholar] [CrossRef]
- Penning, T.M. Aldo-Keto Reductase (AKR) 1C3 inhibitors: A patent review. Expert Opin. Pat. 2017, 27, 1329–1340. [Google Scholar] [CrossRef]
- Shibuya, R.; Suzuki, T.; Miki, Y.; Yoshida, K.; Moriya, T.; Ono, K.; Akahira, J.; Ishida, T.; Hirakawa, H.; Evans, D.B.; et al. Intratumoral concentration of sex steroids and expression of sex steroid-producing enzymes in ductal carcinoma in situ of human breast. Endocr. Relat. Cancer 2008, 15, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Wang, Y.; Wu, D.; Wang, S.; Chen, Z.; Xiang, S.; Chan, F.L. Orphan nuclear receptors as regulators of intratumoral androgen biosynthesis in castration-resistant prostate cancer. Oncogene 2021, 40, 2625–2634. [Google Scholar] [CrossRef] [PubMed]
- Kamrath, C.; Hochberg, Z.; Hartmann, M.F.; Remer, T.; Wudy, S.A. Increased activation of the alternative “backdoor” pathway in patients with 21-hydroxylase deficiency: Evidence from urinary steroid hormone analysis. J. Clin. Endocrinol. Metab. 2012, 97, E367–E375. [Google Scholar] [CrossRef] [Green Version]
- Chang, K.-H.; Li, R.; Papari-Zareei, M.; Watumull, L.; Zhao, Y.D.; Auchus, R.J.; Sharifi, N. Dihydrotestosterone synthesis bypasses testosterone to drive castration-resistant prostate cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 13728–13733. [Google Scholar] [CrossRef] [Green Version]
- Cai, C.; Balk, S.P. Intratumoral androgen biosynthesis in prostate cancer pathogenesis and response to therapy. Endocr. Relat. Cancer 2011, 18, R175–R182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohler, J.L.; Titus, M.A.; Wilson, E.M. Potential prostate cancer drug target: Bioactivation of androstanediol by conversion to dihydrotestosterone. Clin. Cancer Res. 2011, 17, 5844–5849. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oksala, R.; Karimaa, M.; Simola, O.; Ramela, M.; Riikonen, R.; Vehmaan-Kreula, P.; Rummakko, P.; Wohlfahrt, G.; Kallio, P.; Mustonen, M.V.J. CYP11A1 inhibition as a therapeutic approach for the treatment of castration resistant prostate cancer. J. Clin. Oncol. 2018, 36, 340. [Google Scholar] [CrossRef]
- Dutt, S.S.; Gao, A.C. Molecular mechanisms of castration-resistant prostate cancer progression. Future Oncol. 2009, 5, 1403–1413. [Google Scholar] [CrossRef] [Green Version]
- O’Donnell, A.; Judson, I.; Dowsett, M.; Raynaud, F.; Dearnaley, D.; Mason, M.; Harland, S.; Robbins, A.; Halbert, G.; Nutley, B.; et al. Hormonal impact of the 17alpha-hydroxylase/C(17,20)-lyase inhibitor abiraterone acetate (CB7630) in patients with prostate cancer. Br. J. Cancer 2004, 90, 2317–2325. [Google Scholar] [CrossRef] [PubMed]
- Girish, V.; Vijayalakshmi, A. Affordable image analysis using NIH Image/ImageJ. Indian J. Cancer 2004, 41, 47. [Google Scholar]
- Sharpe, M.A.; Baskin, D.S. Monoamine oxidase B levels are highly expressed in human gliomas and are correlated with the expression of HiF-1α and with transcription factors Sp1 and Sp3. Oncotarget 2016, 7, 3379–3393. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carmona, L.M.; Schatz, D.G. New insights into the evolutionary origins of the recombination-activating gene proteins and V(D)J recombination. FEBS J. 2017, 284, 1590–1605. [Google Scholar] [CrossRef] [PubMed]
- Gutcher, I.; Becher, B. APC-derived cytokines and T cell polarization in autoimmune inflammation. J. Clin. Investig. 2007, 117, 1119–1127. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zarnitsyna, V.I.; Evavold, B.D.; Schoettle, L.N.; Blattman, J.N.; Antia, R. Estimating the diversity, completeness, and cross-reactivity of the T cell repertoire. Front. Immunol. 2013, 4, 485. [Google Scholar] [CrossRef] [Green Version]
- Mora, T.; Walczak, A.M. How many different clonotypes do immune repertoires contain? Curr. Opin. Syst. Biol. 2019, 18, 104–110. [Google Scholar] [CrossRef]
- Wang, W.; Thomas, R.; Sizova, O.; Su, D.M. Thymic Function Associated With Cancer Development, Relapse, and Antitumor Immunity—A Mini-Review. Front. Immunol. 2020, 11, 773. [Google Scholar] [CrossRef]
- Gotot, J.; Gottschalk, C.; Leopold, S.; Knolle, P.A.; Yagita, H.; Kurts, C.; Ludwig-Portugall, I. Regulatory T cells use programmed death 1 ligands to directly suppress autoreactive B cells in vivo. Proc. Natl. Acad. Sci. USA 2012, 109, 10468–10473. [Google Scholar] [CrossRef] [Green Version]
- Dal Secco, V.; Riccioli, A.; Padula, F.; Ziparo, E.; Filippini, A. Mouse Sertoli cells display phenotypical and functional traits of antigen-presenting cells in response to interferon gamma. Biol. Reprod. 2008, 78, 234–242. [Google Scholar] [CrossRef] [Green Version]
- Kisand, K.; Peterson, P. Autoimmune polyendocrinopathy candidiasis ectodermal dystrophy: Known and novel aspects of the syndrome. Ann. N. Y. Acad. Sci. 2011, 1246, 77–91. [Google Scholar] [CrossRef]
- O’Donnell, L.; Rebourcet, D.; Dagley, L.F.; Sgaier, R.; Infusini, G.; O’Shaughnessy, P.J.; Chalmel, F.; Fietz, D.; Weidner, W.; Legrand, J.M.D.; et al. Sperm proteins and cancer-testis antigens are released by the seminiferous tubules in mice and men. FASEB J. 2021, 35, e21397. [Google Scholar] [PubMed]
- Fijak, M.; Pilatz, A.; Hedger, M.P.; Nicolas, N.; Bhushan, S.; Michel, V.; Tung, K.S.K.; Schuppe, H.-C.; Meinhardt, A. Infectious, inflammatory and ‘autoimmune’ male factor infertility: How do rodent models inform clinical practice? Hum. Reprod. Update 2018, 24, 416–441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, Z.Y.; Ming, W.; Li, L.D.; Louis, J.; Fei, W.Y. Adoptive transfer of murine autoimmune orchitis with sperm-specific T lymphoblasts. Arch. Androl. 1990, 24, 51–59. [Google Scholar] [CrossRef] [Green Version]
- Al-Fahham, A.A. Etiology of Antisperm Antibodies in the Serum of Virgins. Open J. Obstet. Gynecol. 2018, 8, 236–245. [Google Scholar] [CrossRef] [Green Version]
- Mahdi, B.M.; Salih, W.H.; Caitano, A.E.; Kadhum, B.M.; Ibrahim, D.S. Frequency of antisperm antibodies in infertile women. J. Reprod. Infertil. 2011, 12, 261–265. [Google Scholar]
- Robertson, S.A.; Prins, J.R.; Sharkey, D.J.; Moldenhauer, L.M. Seminal Fluid and the Generation of Regulatory T Cells for Embryo Implantation. Am. J. Reprod. Immunol. 2013, 69, 315–330. [Google Scholar] [CrossRef]
- Robertson, S.A.; Guerin, L.R.; Moldenhauer, L.M.; Hayball, J.D. Activating T regulatory cells for tolerance in early pregnancy—The contribution of seminal fluid. J. Reprod. Immunol. 2009, 83, 109–116. [Google Scholar] [CrossRef]
- Remes Lenicov, F.; Rodriguez Rodrigues, C.; Sabatté, J.; Cabrini, M.; Jancic, C.; Ostrowski, M.; Merlotti, A.; Gonzalez, H.; Alonso, A.; Pasqualini, R.A.; et al. Semen Promotes the Differentiation of Tolerogenic Dendritic Cells. J. Immunol. 2012, 189, 4777–4786. [Google Scholar] [CrossRef] [Green Version]
- Remes Lenicov, F.; Paletta, A.L.; Gonzalez Prinz, M.; Varese, A.; Pavillet, C.E.; Lopez Malizia, Á.; Sabatté, J.; Geffner, J.R.; Ceballos, A. Prostaglandin E2 Antagonizes TGF-β Actions During the Differentiation of Monocytes Into Dendritic Cells. Front. Immunol. 2018, 9, 1441. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bruno, A.; Mortara, L.; Baci, D.; Noonan, D.M.; Albini, A. Myeloid Derived Suppressor Cells Interactions With Natural Killer Cells and Pro-angiogenic Activities: Roles in Tumor Progression. Front. Immunol. 2019, 10, 771. [Google Scholar] [CrossRef]
- Obermajer, N.; Kalinski, P. Generation of myeloid-derived suppressor cells using prostaglandin E2. Transplant. Res. 2012, 1, 15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendvold, E.; Gottlieb, C.; Svanborg, K.; Bygdeman, M.; Eneroth, P. Concentration of prostaglandins in seminal fluid of fertile men. Int. J. Androl. 1987, 10, 463–469. [Google Scholar] [CrossRef] [PubMed]
- Sumida, K.; Ohno, Y.; Ohtake, J.; Kaneumi, S.; Kishikawa, T.; Takahashi, N.; Taketomi, A.; Kitamura, H. IL-11 induces differentiation of myeloid-derived suppressor cells through activation of STAT3 signalling pathway. Sci. Rep. 2015, 5, 13650. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Li, S.; Li, M.O. TGF-β Control of Adaptive Immune Tolerance: A Break from Treg Cells. BioEssays News Rev. Mol. Cell Dev. Biol. 2018, 40, e1800063. [Google Scholar] [CrossRef]
- Chen, J.C.; Johnson, B.A.; Erikson, D.W.; Piltonen, T.T.; Barragan, F.; Chu, S.; Kohgadai, N.; Irwin, J.C.; Greene, W.C.; Giudice, L.C.; et al. Seminal plasma induces global transcriptomic changes associated with cell migration, proliferation and viability in endometrial epithelial cells and stromal fibroblasts. Hum. Reprod. 2014, 29, 1255–1270. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, O.S.; Zheng, Y.; Nakamura, K.; Attridge, K.; Manzotti, C.; Schmidt, E.M.; Baker, J.; Jeffery, L.E.; Kaur, S.; Briggs, Z.; et al. Trans-endocytosis of CD80 and CD86: A molecular basis for the cell-extrinsic function of CTLA-4. Science 2011, 332, 600–603. [Google Scholar] [CrossRef] [Green Version]
- Kowalczyk, A.; D’Souza, C.A.; Zhang, L. Cell-extrinsic CTLA4-mediated regulation of dendritic cell maturation depends on STAT3. Eur. J. Immunol. 2013, 44, 1143–1155. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, N.; Persson, G.; Hviid, T.V.F. The Tolerogenic Function of Regulatory T Cells in Pregnancy and Cancer. Front. Immunol. 2019, 10, 911. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tsuda, S.; Nakashima, A.; Shima, T.; Saito, S. New Paradigm in the Role of Regulatory T Cells during Pregnancy. Front. Immunol. 2019, 10, 573. [Google Scholar] [CrossRef] [Green Version]
- Baskin, M.J. Temporary sterilization by the injection of human spermatozoa. A preliminary report. Am. J. Obstet. Gynecol. 1932, 24, 892–897. [Google Scholar] [CrossRef]
- Kaur, K.; Prabha, V. Immunocontraceptives: New approaches to fertility control. BioMed Res. Int. 2014, 2014, 868196. [Google Scholar] [CrossRef] [PubMed] [Green Version]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharpe, M.A.; Baskin, D.S.; Jenson, A.V.; Baskin, A.M. Hijacking Sexual Immuno-Privilege in GBM—An Immuno-Evasion Strategy. Int. J. Mol. Sci. 2021, 22, 10983. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222010983
Sharpe MA, Baskin DS, Jenson AV, Baskin AM. Hijacking Sexual Immuno-Privilege in GBM—An Immuno-Evasion Strategy. International Journal of Molecular Sciences. 2021; 22(20):10983. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222010983
Chicago/Turabian StyleSharpe, Martyn A., David S. Baskin, Amanda V. Jenson, and Alexandra M. Baskin. 2021. "Hijacking Sexual Immuno-Privilege in GBM—An Immuno-Evasion Strategy" International Journal of Molecular Sciences 22, no. 20: 10983. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222010983