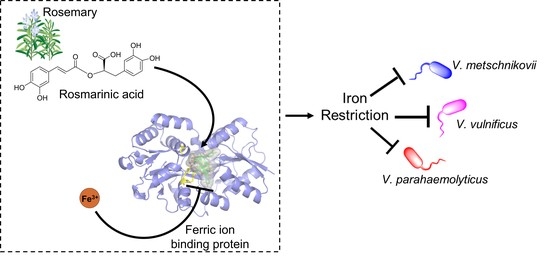
Rosmarinic Acid and Sodium Citrate Have a Synergistic Bacteriostatic Effect against Vibrio Species by Inhibiting Iron Uptake
Abstract
:1. Introduction
2. Results
2.1. Screening of VmFbpA Inhibitors from Aqueous Extracts of 20 Spices
2.2. Spectral Analysis of VmFbpA Interacting with RA
2.3. Quantitative Analysis of the Inhibition of Fe3+ Binding to VmFbpA by Rosemary Extracts and RA
2.4. Spectra Analysis of Fe3+ Interacting with RA
2.5. Inhibition of Bacterial Growth by RA
2.6. Iron Utilization in V. metschnikovii
2.7. Inhibition by RA and Sodium Citrate (SC) of the Growth of V. metschnikovii
2.8. Mechanism of RA Binding to VmFbpA
2.9. Inhibition by RA and SC of the Growth of V. vulnificus and V. parahaemolyticus
3. Discussion
3.1. Screening and Identification of RA as a VmFbpA Inhibitor
3.2. Interaction between RA and VmFbpA
3.3. Interaction between RA and Fe3+
3.4. Inhibition of V. metschnikovii Growth by RA
3.5. Effect of RA Combined with SC on Bacterial Growth
4. Materials and Methods
4.1. Chemicals and Materials
4.2. Aqueous Extracts of Spices
4.3. Expression and Purification of 6× His-tev-VmFbpA
4.4. Preparation of 6× His-tag-free VmFbpA
4.5. Preparation of Fe3+-VmFbpA and Apo VmFbpA
4.6. Six × His-based VmFbpA Inhibition Assay (Pull-Down Assay)
4.7. Determination of the 50% Inhibitory Concentration
4.8. Spectral Analysis of VmFbpA Interacting with Rosemary Extracts and RA
4.9. Spectral Analysis of RA Interacting with Fe3+
4.10. Analysis of RA on Fe3+ Reduction
4.11. ITC Analysis
4.12. Crystallization and X-ray Diffraction Data Collection
4.13. Docking Simulation
4.14. Inhibition by RA of V. metschnikovii Growth
4.15. Inhibition of E. coli Growth by RA
4.16. Measurement of Iron Utilization by V. metschnikovii
4.17. Recovery of V. metschnikovii by Supplementation of Fe3+
4.18. Inhibition by RA and SC of the Growth of V. metschnikovii
4.19. Inhibition by RA and SC of the Growth of E. coli
4.20. Inhibition by RA and SC of the Growth of V. vulnificus and V. parahaemolyticus
4.21. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Baker-Austin, C.; Oliver, J.D.; Alam, M.; Ali, A.; Waldor, M.K.; Qadri, F.; Martinez-Urtaza, J. Vibrio spp. infections. Nat. Rev. Dis. Prim. 2018, 4, 8. [Google Scholar] [CrossRef]
- Bonnin-Jusserand, M.; Copin, S.; Le Bris, C.; Brauge, T.; Gay, M.; Brisabois, A.; Grard, T.; Midelet-Bourdin, G. Vibrio species involved in seafood-borne outbreaks (Vibrio cholerae, V. parahaemolyticus and V. vulnificus): Review of microbiological versus recent molecular detection methods in seafood products. Crit. Rev. Food Sci. Nutr. 2019, 59, 597–610. [Google Scholar] [CrossRef]
- Pachauri, R.K.; Allen, M.R.; Barros, V.R.; Broome, J.; Cramer, W.; Christ, R.; Church, J.A.; Clarke, L.; Dahe, Q.; Dasgupta, P.; et al. Climate Change 2014: Synthesis Report. Contribution of Working Groups I, II and III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Pachauri, R.K., Meyer, L., Eds.; IPCC: Geneva, Switzerland, 2014. [Google Scholar]
- Maggiore, A.; Afonso, A.; Barrucci, F.; Sanctis, G. De Climate change as a driver of emerging risks for food and feed safety, plant, animal health and nutritional quality. EFSA Support. Publ. 2020, 17, 1881E. [Google Scholar] [CrossRef]
- Froelich, B.A.; Daines, D.A. In hot water: Effects of climate change on Vibrio–human interactions. Environ. Microbiol. 2020, 22, 4101–4111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Newton, A.; Kendall, M.; Vugia, D.J.; Henao, O.L.; Mahon, B.E. Increasing rates of vibriosis in the United States, 1996–2010: Review of surveillance data from 2 systems. Clin. Infect. Dis. 2012, 54 (Suppl. 5), S391–S395. [Google Scholar] [CrossRef]
- Vibrio Species Causing Vibriosis. Available online: https://www.cdc.gov/vibrio/faq.html (accessed on 1 November 2021).
- Matté, M.H.; Baldassi, L.; Barbosa, M.L.; Malucelli, M.I.C.; Nitrini, S.M.O.O.; Matté, G.R. Virulence factors of Vibrio metschnikovii strains isolated from fish in Brazil. Food Control 2007, 18, 747–751. [Google Scholar] [CrossRef]
- Miller, R.A.; Harbottle, H. Antimicrobial drug resistance in fish pathogens. Microbiol. Spectr. 2018, 6, 213–238. [Google Scholar] [CrossRef]
- Hameed, A.; Naveed, S.; Qamar, F.; Alam, T.; Abbas, S.S.; Sharif, N. Irrational use of antibiotics, in different age groups of karachi: A wakeup call for antibiotic resistance and future infections. J. Bioequiv. Availab. 2016, 8, 242–245. [Google Scholar] [CrossRef] [Green Version]
- Payne, S.M.; Mey, A.R.; Wyckoff, E.E. Vibrio iron transport: Evolutionary adaptation to life in multiple environments. Microbiol. Mol. Biol. Rev. 2016, 80, 69–90. [Google Scholar] [CrossRef] [Green Version]
- Wazen, R.M.; Kuroda, S.; Nishio, C.; Sellin, K.; Brunski, J.B.; Nanci, A. Iron in infection and immunity. Cell Host Microbe. 2014, 8, 1385–1395. [Google Scholar] [CrossRef]
- Schaible, U.E.; Kaufmann, S.H.E. Iron and microbial infection. Nat. Rev. Microbiol. 2004, 2, 946–953. [Google Scholar] [CrossRef]
- Parker Siburt, C.J.; Mietzner, T.A.; Crumbliss, A.L. FbpA—A bacterial transferrin with more to offer. Biochim. Biophys. Acta—Gen. Subj. 2012, 1820, 379–392. [Google Scholar] [CrossRef]
- Maleki, A.; Ghafourian, S.; Pakzad, I.; Badakhsh, B.; Sadeghifard, N. mazE antitoxin of toxin antitoxin system and fbpA as reliable targets to eradication of Neisseria meningitidis. Curr. Pharm. Des. 2018, 24, 1204–1210. [Google Scholar] [CrossRef] [PubMed]
- Klančnik, A.; Piskernik, S.; Jeršek, B.; Možina, S.S. Evaluation of diffusion and dilution methods to determine the antibacterial activity of plant extracts. J. Microbiol. Methods 2010, 81, 121–126. [Google Scholar] [CrossRef]
- Okamura, H.; Nishikiori, M.; Xiang, H.; Ishikawa, M.; Katoh, E. Interconversion of two GDP-bound conformations and their selection in an Arf-family small G protein. Structure 2011, 19, 988–998. [Google Scholar] [CrossRef] [Green Version]
- Xiang, H.; Takeuchi, H.; Tsunoda, Y.; Nakajima, M.; Murata, K.; Ueguchi-Tanaka, M.; Kidokoro, S.I.; Kezuka, Y.; Nonaka, T.; Matsuoka, M.; et al. Thermodynamic characterization of OsGID1-gibberellin binding using calorimetry and docking simulations. J. Mol. Recognit. 2011, 24, 275–282. [Google Scholar] [CrossRef]
- Lu, P.; Moriwaki, Y.; Zhang, M.; Katayama, Y.; Lu, Y.; Okamoto, K.; Terada, T.; Shimizu, K.; Wang, M.; Kamiya, T.; et al. Functional characterisation of two ferric-ion coordination modes of TtFbpA, the periplasmic subunit of an ABC-Type iron transporter from: Thermus thermophilus HB8. Metallomics 2019, 11, 2078–2088. [Google Scholar] [CrossRef] [PubMed]
- Adomako-Bonsu, A.G.; Chan, S.L.; Pratten, M.; Fry, J.R. Antioxidant activity of rosmarinic acid and its principal metabolites in chemical and cellular systems: Importance of physico-chemical characteristics. Toxicol. Vitr. 2017, 40, 248–255. [Google Scholar] [CrossRef]
- Erkan, N.; Ayranci, G.; Ayranci, E. Antioxidant activities of rosemary (Rosmarinus Officinalis L.) extract, blackseed (Nigella sativa L.) essential oil, carnosic acid, rosmarinic acid and sesamol. Food Chem. 2008, 110, 76–82. [Google Scholar] [CrossRef] [PubMed]
- Lamien-Meda, A.; Nell, M.; Lohwasser, U.; Börner, A.; Franz, C.; Novak, J. Investigation of antioxidant and rosmarinic acid variation in the sage collection of the genebank in gatersleben. J. Agric. Food Chem. 2010, 58, 3813–3819. [Google Scholar] [CrossRef]
- Bullen, J.J.; Leigh, L.C.; Rogers, H.J. The effect of iron compounds on the virulence of Escherichia coli for guinea-pigs. Immunology 1968, 15, 581–588. [Google Scholar] [PubMed]
- Grosse, C.; Scherer, J.; Koch, D.; Otto, M.; Taudte, N.; Grass, G. A new ferrous iron-uptake transporter, EfeU (YcdN), from Escherichia coli. Mol. Microbiol. 2006, 62, 120–131. [Google Scholar] [CrossRef] [PubMed]
- Braun, V.; Hantke, K. The Tricky Ways Bacteria Cope with Iron Limitation; Springer: Dordrecht, The Netherlands, 2013; pp. 31–66. [Google Scholar]
- Braun, V. Iron uptake by Escherichia coli. Front. Biosci. 2003, 8, 1232. [Google Scholar] [CrossRef] [PubMed]
- Cartron, M.L.; Maddocks, S.; Gillingham, P.; Craven, C.J.; Andrews, S.C. Feo—Transport of ferrous iron into bacteria. BioMetals 2006, 19, 143–157. [Google Scholar] [CrossRef] [PubMed]
- Patzer, S.I.; Hantke, K. Dual repression by Fe2+-Fur and Mn2+-MntR of the mntH gene, encoding an NRAMP-like Mn2+ transporter in Escherichia coli. J. Bacteriol. 2001, 183, 4806–4813. [Google Scholar] [CrossRef] [Green Version]
- Banerjee, S.; Paul, S.; Nguyen, L.T.; Chu, B.C.H.; Vogel, H.J. FecB, a periplasmic ferric-citrate transporter from E. coli, can bind different forms of ferric-citrate as well as a wide variety of metal-free and metal-loaded tricarboxylic acids. Metallomics 2016, 8, 125–133. [Google Scholar] [CrossRef]
- Balado, M.; Puentes, B.; Couceiro, L.; Fuentes-Monteverde, J.C.; Rodríguez, J.; Osorio, C.R.; Jiménez, C.; Lemos, M.L. Secreted citrate serves as iron carrier for the marine pathogen Photobacterium damselae subsp damselae. Front. Cell. Infect. Microbiol. 2017, 7, 361. [Google Scholar] [CrossRef]
- McHugh, J.P.; Rodríguez-Quiñones, F.; Abdul-Tehrani, H.; Svistunenko, D.A.; Poole, R.K.; Cooper, C.E.; Andrews, S.C. Global iron-dependent gene regulation in Escherichia coli: A new mechanism for iron homeostasis. J. Biol. Chem. 2003, 278, 29478–29486. [Google Scholar] [CrossRef] [Green Version]
- Carrano, C.J.; Raymond, K.N. Ferric Ion Sequestering Agents. 2. Kinetics and mechanism of iron removal from transferrin by enterobactin and synthetic tricatechols. J. Am. Chem. Soc. 1979, 101, 5401–5404. [Google Scholar] [CrossRef]
- Walsh, C.T.; Liu, J.; Rusnak, F.; Sakaitani, M. Molecular studies on enzymes in chorismate metabolism and the enterobactin biosynthetic pathway. Chem. Rev. 1990, 90, 1105–1129. [Google Scholar] [CrossRef]
- Tetsumoto, S. Bactericidal activity of various seasonings against Salmonella enterica subsp. enterica serovar Typhi and Vibrio cholerae. J. Agric. Chem. Soc. Jpn. 1934, 10, 123–127. [Google Scholar] [CrossRef]
- Corral-Lugo, A.; Daddaoua, A.; Ortega, A.; Espinosa-Urgel, M.; Krell, T. Rosmarinic acid is a homoserine lactone mimic produced by plants that activates a bacterial quorum-sensing regulator. Sci. Signal. 2016, 9, ra1. [Google Scholar] [CrossRef] [PubMed]
- Trott, O.; Olson, A.J. Software news and update AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| E. coli | V. metschnikovii | |
|---|---|---|
| Fe3+ | FbpBC/A [25] | FbpBC/A a |
| Fe3+-siderophore | FhuBC/D (Fe3+-hydroxamate) [26] FepABC (Fe3+-enterobactin) [26] IutA (Fe3+-aerobactin) [26] FyuA (Fe3+-yersiniabactin) [26] | CeuBC/A (Fe3+-enterochelin) a |
| Fe2+ | FeoABC [27] EfeOUB [24] MntH [28] | FeoABC a |
| Fe-citrate | Fec(CD)E/B [29] | N.A. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, P.; Sui, M.; Zhang, M.; Wang, M.; Kamiya, T.; Okamoto, K.; Itoh, H.; Okuda, S.; Suzuki, M.; Asakura, T.; et al. Rosmarinic Acid and Sodium Citrate Have a Synergistic Bacteriostatic Effect against Vibrio Species by Inhibiting Iron Uptake. Int. J. Mol. Sci. 2021, 22, 13010. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222313010
Lu P, Sui M, Zhang M, Wang M, Kamiya T, Okamoto K, Itoh H, Okuda S, Suzuki M, Asakura T, et al. Rosmarinic Acid and Sodium Citrate Have a Synergistic Bacteriostatic Effect against Vibrio Species by Inhibiting Iron Uptake. International Journal of Molecular Sciences. 2021; 22(23):13010. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222313010
Chicago/Turabian StyleLu, Peng, Miaomiao Sui, Mimin Zhang, Mengyao Wang, Takehiro Kamiya, Ken Okamoto, Hideaki Itoh, Suguru Okuda, Michio Suzuki, Tomiko Asakura, and et al. 2021. "Rosmarinic Acid and Sodium Citrate Have a Synergistic Bacteriostatic Effect against Vibrio Species by Inhibiting Iron Uptake" International Journal of Molecular Sciences 22, no. 23: 13010. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms222313010