Artemether-Loaded Zein Nanoparticles: An Innovative Intravenous Dosage Form for the Management of Severe Malaria
Abstract
:1. Introduction
2. Results
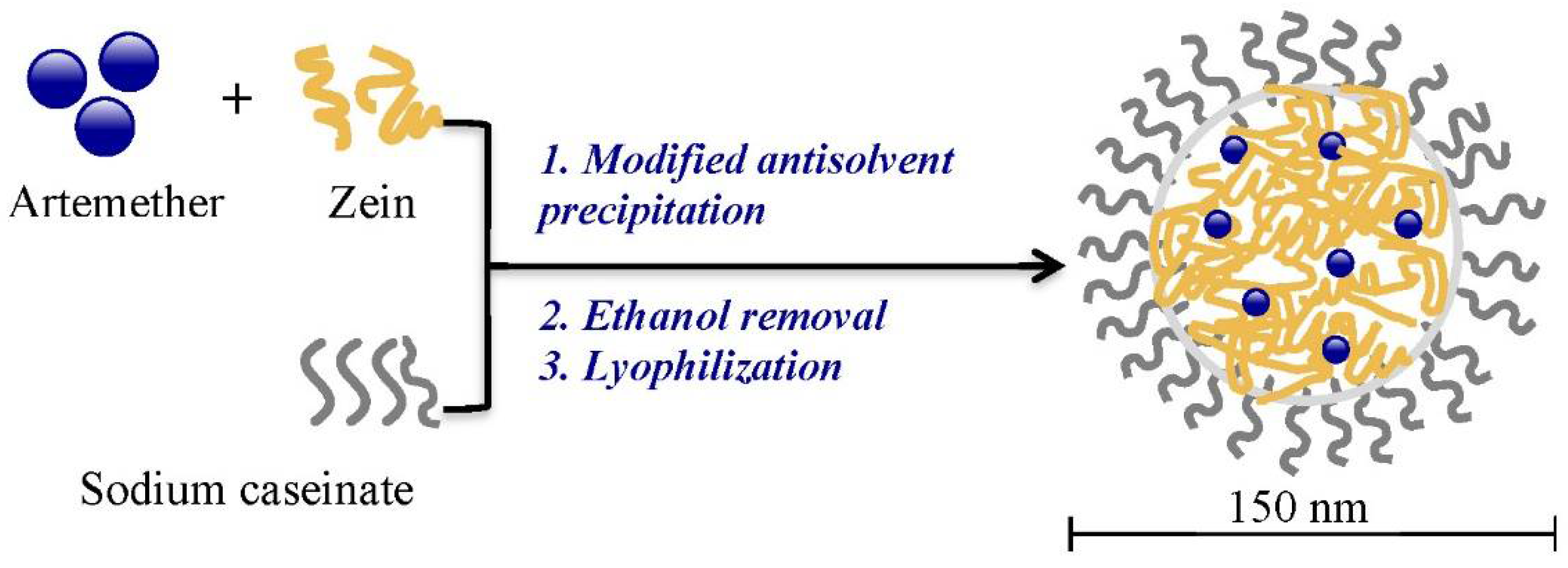
2.1. Preparation of Artemether-Load Zein Nanoparticles
2.2. Optimization of Formulation and Process Parameters
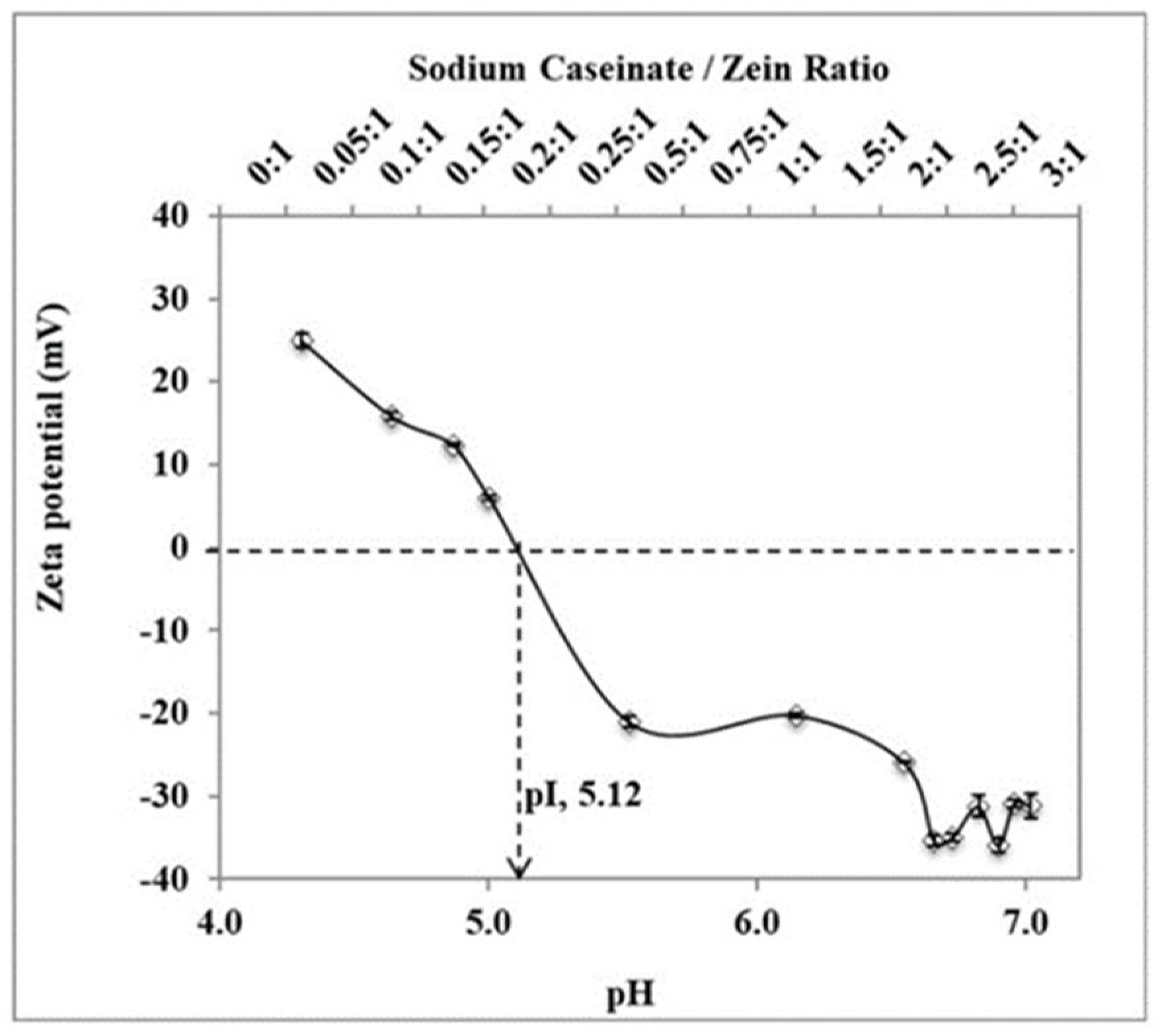
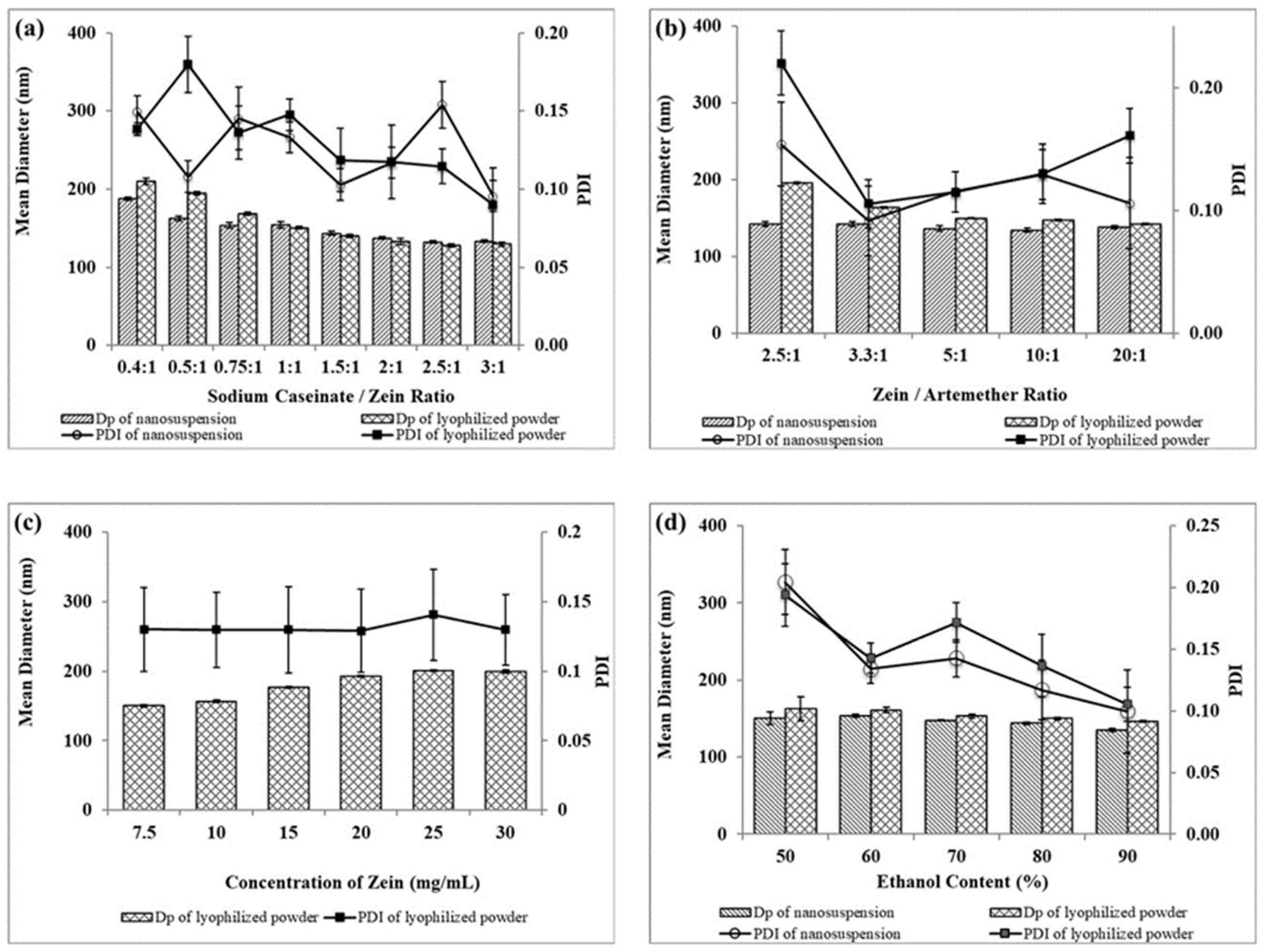
2.2.1. Sodium Caseinate/Zein Mass Ratio
2.2.2. Zein/Artemether Mass Ratio
2.2.3. Zein Concentration
2.2.4. Ethanol Concentration
2.3. Drug Content and Encapsulation Efficiency
2.4. Physical Characterization
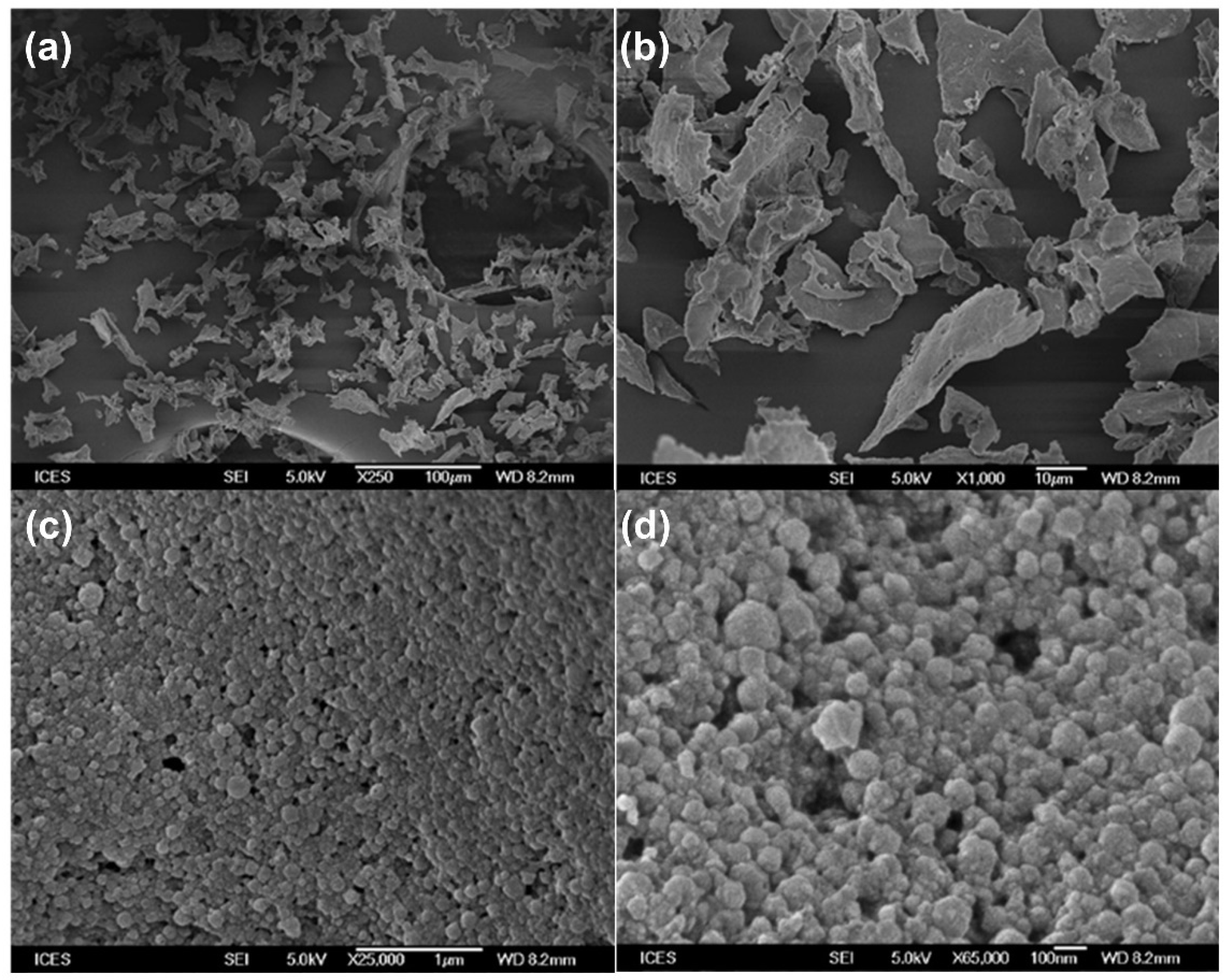
2.4.1. Particle Morphology
2.4.2. Thermal Analysis
2.4.3. Crystallinity
2.4.4. Fourier Transform Infrared (FTIR) Spectra
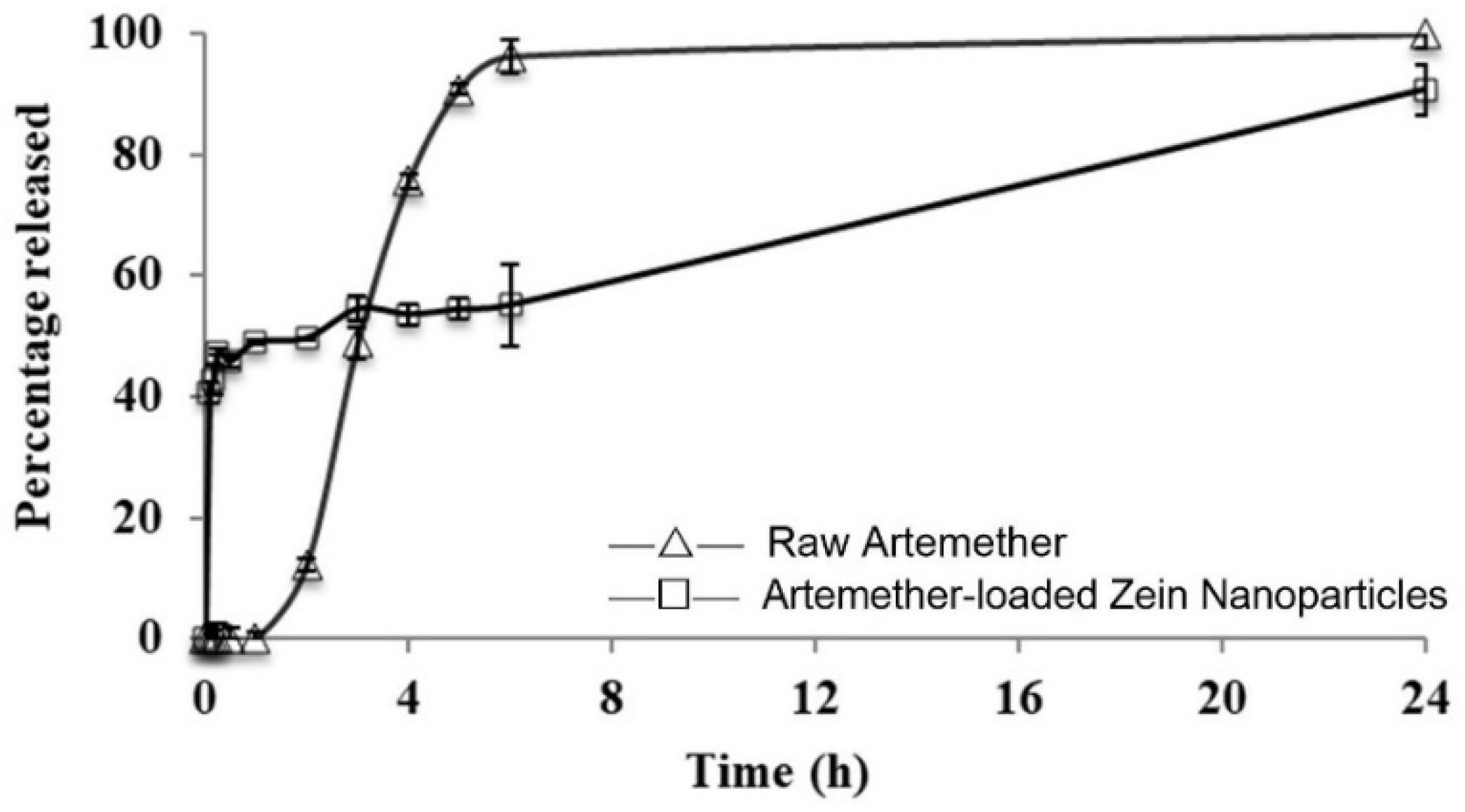
2.5. Release Profile
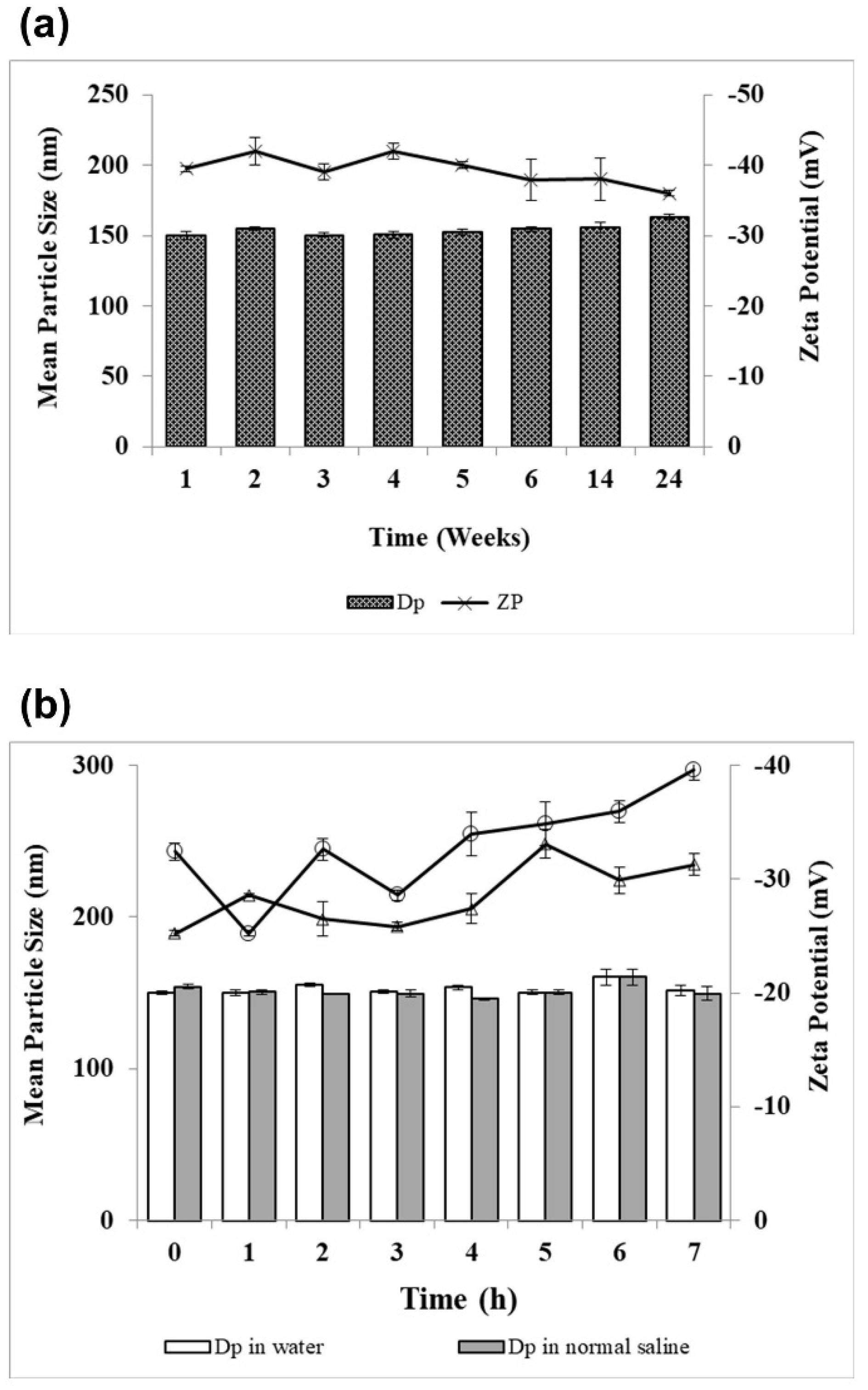
2.6. Physical Stability
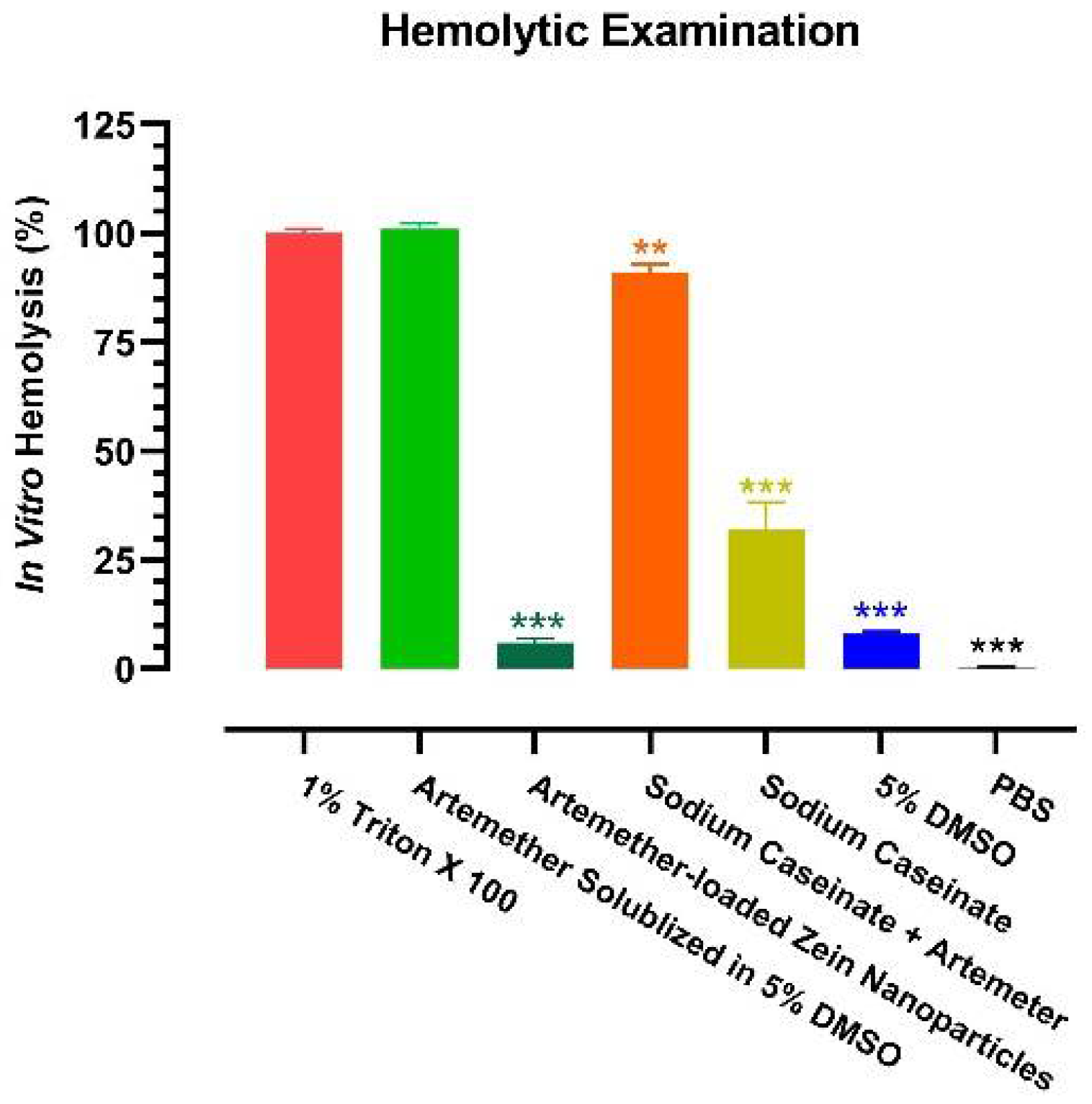
2.7. In Vitro Hemolysis Test
2.8. Pharmacokinetic Study
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Preparation of Artemether-Loaded Zein Nanoparticles
4.3. Particle Size and Zeta Potential Analyses
4.4. Drug Content and Encapsulation Efficiency
4.5. Particle Morphology
4.6. Thermal Analysis
4.7. Crystallinity
4.8. Drug–Protein Interaction
4.9. In vitro Release Study
4.10. In Vitro Hemolytic Test
4.11. Physical Stability
4.12. Pharmacokinetic Study
4.13. LC–MS/MS Analysis
4.14. Pharmacokinetic Calculation
4.15. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| CL | Clearance |
| DMSO | Dimethyl sulfoxide |
| DSC | Differential scanning calorimetry |
| FTIR | Fourier transform infrared |
| HPLC | High performance liquid chromatography |
| LC–MS/MS | Liquid chromatography-tandem mass spectrometry |
| MRT | Mean residence time |
| nm | nanometer |
| PBS | Phosphate buffer solution |
| PDI | Polydispersity index |
| pI | Isoelectric point |
| RBCs | Red blood cells |
| SD | Standard deviation |
| t1/2 λZ | Terminal elimination half-life |
| Vc | Apparent volume of distribution of the central compartment |
| WHO | World Health Organization |
References
- Ashley, E.A.; Pyae Phyo, A.; Woodrow, C.J. Malaria. Lancet 2018, 391, 1608–1621. [Google Scholar] [CrossRef]
- White, N.J.; Pukrittayakamee, S.; Hien, T.T.; Faiz, M.A.; Mokuolu, O.A.; Dondorp, A.M. Malaria. Lancet 2014, 383, 723–735. [Google Scholar] [CrossRef]
- WHO. World Malaria Report 2020: 20 Years of Global Progress and Challenges; World Health Organization: Geneva, Switzerlands, 2020. [Google Scholar]
- Gallup, J.L.; Sachs, J.D. The economic burden of malaria. Am. J. Trop. Med. Hyg. 2001, 64, 85–96. [Google Scholar] [CrossRef] [Green Version]
- Sachs, J.; Malaney, P. The economic and social burden of malaria. Nature 2002, 415, 680–685. [Google Scholar] [CrossRef]
- Septembre-Malaterre, A.; Lalarizo Rakoto, M.; Marodon, C.; Bedoui, Y.; Nakab, J.; Simon, E.; Hoarau, L.; Savriama, S.; Strasberg, D.; Guiraud, P.; et al. Artemisia annua, a Traditional Plant Brought to Light. Int. J. Mol. Sci. 2020, 21, 4986. [Google Scholar] [CrossRef]
- WHO. Guidelines for the Treatment of Malaria-Third Edition; World Health Organization: Geneva, Switzerland, 2015. [Google Scholar]
- Esu, E.B.; Effa, E.E.; Opie, O.N.; Meremikwu, M.M. Artemether for severe malaria. Cochrane Database Syst. Rev. 2019, 6, CD010678. [Google Scholar] [CrossRef]
- Hawkins, M.J.; Soon-Shiong, P.; Desai, N. Protein nanoparticles as drug carriers in clinical medicine. Adv. Drug Deliv. Rev. 2008, 60, 876–885. [Google Scholar] [CrossRef]
- Boateng-Marfo, Y.; Dong, Y.C.; Loh, Z.H.; Lin, H.S.; Ng, W.K. Intravenous human serum albumin (HSA)-bound artemether nanoparticles for treatment of severe malaria. Colloids Surf. A Physicochem. Eng. Asp. 2018, 536, 20–29. [Google Scholar] [CrossRef]
- Rehman, K.; Lotsch, F.; Kremsner, P.G.; Ramharter, M. Haemolysis associated with the treatment of malaria with artemisinin derivatives: A systematic review of current evidence. Int. J. Infect. Dis. 2014, 29, 268–273. [Google Scholar] [CrossRef] [Green Version]
- Paliwal, R.; Palakurthi, S. Zein in controlled drug delivery and tissue engineering. J. Control. Release 2014, 189, 108–122. [Google Scholar] [CrossRef]
- Gough, C.R.; Bessette, K.; Xue, Y.; Mou, X.; Hu, X. Air-Jet Spun Corn Zein Nanofibers and Thin Films with Topical Drug for Medical Applications. Int. J. Mol. Sci. 2020, 21, 5780. [Google Scholar] [CrossRef]
- Li, K.-K.; Yin, S.-W.; Yin, Y.-C.; Tang, C.-H.; Yang, X.-Q.; Wen, S.-H. Preparation of water-soluble antimicrobial zein nanoparticles by a modified antisolvent approach and their characterization. J. Food. Eng. 2013, 119, 343–352. [Google Scholar] [CrossRef]
- Luo, Y.; Teng, Z.; Wang, T.T.; Wang, Q. Cellular uptake and transport of zein nanoparticles: Effects of sodium caseinate. J. Agric. Food Chem. 2013, 61, 7621–7629. [Google Scholar] [CrossRef]
- Patel, A.R.; Bouwens, E.C.; Velikov, K.P. Sodium caseinate stabilized zein colloidal particles. J. Agric. Food Chem. 2010, 58, 12497–12503. [Google Scholar] [CrossRef]
- Dickinson, E. Properties of emulsions stabilized with milk proteins: Overview of some recent developments. J. Dairy Sci. 1997, 80, 2607–2619. [Google Scholar] [CrossRef]
- Shukla, R.; Cheryan, M. Zein: The industrial protein from corn. Ind. Crop Prod. 2001, 13, 171–192. [Google Scholar] [CrossRef]
- Strange, E.; Holsinger, V.; Kleyn, D. Chemical properties of thiolated and succinylated caseins. J. Agric. Food Chem. 1993, 41, 30–36. [Google Scholar] [CrossRef]
- Podaralla, S.; Perumal, O. Influence of formulation factors on the preparation of zein nanoparticles. AAPS PharmSciTech 2012, 13, 919–927. [Google Scholar] [CrossRef] [Green Version]
- Zhong, Q.; Jin, M. Zein nanoparticles produced by liquid-liquid dispersion. Food Hydrocoll. 2018, 23, 2380–2387. [Google Scholar] [CrossRef]
- Dong, F.; Padua, G.W.; Wang, Y. Controlled formation of hydrophobic surfaces by self-assembly of an amphiphilic natural protein from aqueous solutions. Soft Matter 2013, 9, 5933–5941. [Google Scholar] [CrossRef]
- Ansari, M.T.; Hussain, A.; Nadeem, S.; Majeed, H.; Saeed-Ul-Hassan, S.; Tariq, I.; Mahmood, Q.; Khan, A.K.; Murtaza, G. Preparation and characterization of solid dispersions of artemether by freeze-dried method. BioMed Res. Int. 2015, 2015, 109563. [Google Scholar] [CrossRef]
- Lai, L.; Guo, H. Preparation of new 5-fluorouracil-loaded zein nanoparticles for liver targeting. Int. J. Pharm. 2011, 404, 317–323. [Google Scholar] [CrossRef]
- Colloidal Dynamics Inc. The Zeta Potential. In Electroacoustic Tutorials; Colloidal Dynamics Inc.: Ponte Vedra Beach, FL, USA, 1999; pp. 1–4. [Google Scholar]
- Ibrahim, N.; Ibrahim, H.; Dormoi, J.; Briolant, S.; Pradines, B.; Moreno, A.; Mazier, D.; Legrand, P.; Nepveu, F. Albumin-bound nanoparticles of practically water-insoluble antimalarial lead greatly enhance its efficacy. Int. J. Pharm. 2014, 464, 214–224. [Google Scholar] [CrossRef]
- Joshi, M.; Pathak, S.; Sharma, S.; Patravale, V. Design and in vivo pharmacodynamic evaluation of nanostructured lipid carriers for parenteral delivery of artemether: Nanoject. Int. J. Pharm. 2008, 364, 119–126. [Google Scholar] [CrossRef]
- Li, Q.G.; Peggins, J.O.; Fleckenstein, L.L.; Masonic, K.; Heiffer, M.H.; Brewer, T.G. The pharmacokinetics and bioavailability of dihydroartemisinin, arteether, artemether, artesunic acid and artelinic acid in rats. J. Pharm. Pharmacol. 1998, 50, 173–182. [Google Scholar] [CrossRef]
- Lin, H.S.; Ho, P.C. Preclinical pharmacokinetic evaluation of resveratrol trimethyl ether in Sprague-Dawley rats: The impacts of aqueous solubility, dose escalation, food and repeated dosing on oral bioavailability. J. Pharm. Sci. 2011, 100, 4491–4500. [Google Scholar] [CrossRef]
- Yeo, S.C.; Ho, P.C.; Lin, H.S. Pharmacokinetics of pterostilbene in Sprague-Dawley rats: The impacts of aqueous solubility, fasting, dose escalation, and dosing route on bioavailability. Mol. Nutr. Food Res. 2013, 57, 1015–1025. [Google Scholar] [CrossRef]
- German, P.I.; Aweeka, F.T. Clinical pharmacology of artemisinin-based combination therapies. Clin. Pharmacokinet. 2008, 47, 91–102. [Google Scholar]
- Medhi, B.; Patyar, S.; Rao, R.S.; Byrav, D.S.P.; Prakash, A. Pharmacokinetic and toxicological profile of artemisinin compounds: An update. Pharmacology 2009, 84, 323–332. [Google Scholar] [CrossRef]
- Morris, C.A.; Duparc, S.; Borghini-Fuhrer, I.; Jung, D.; Shin, C.S.; Fleckenstein, L. Review of the clinical pharmacokinetics of artesunate and its active metabolite dihydroartemisinin following intravenous, intramuscular, oral or rectal administration. Malar. J. 2011, 10, 263. [Google Scholar] [CrossRef] [Green Version]
- Garg, A.; Tomar, D.S.; Bhalala, K.; Wahajuddin, M. Development and investigation of Artemether loaded binary solid lipid nanoparticles: Physicochemical characterization and In-Situ single-pass intestinal permeability. J. Drug Deliv. Sci. Technol. 2020, 60, 102072. [Google Scholar] [CrossRef]
- Khatri, H.; Chokshi, N.; Rawal, S.; Patel, B.M.; Badanthadka, M.; Patel, M.M. Fabrication and In Vivo evaluation of ligand appended paclitaxel and artemether loaded lipid nanoparticulate systems for the treatment of NSCLC: A nanoparticle assisted combination oncotherapy. Int. J. Pharm. 2020, 583, 119386. [Google Scholar] [CrossRef]
- RinTalib, S.; Ahmed, N.; Khan, D.; Khan, G.M.; Ur Rehman, A. Chitosan-chondroitin based artemether loaded nanoparticles for transdermal drug delivery system. J. Drug Deliv. Sci. Technol. 2021, 61, 102281. [Google Scholar]
- Pawar, S.; Shende, P. 2(2) factorial design-based biocompatible microneedle arrays containing artemether co-loaded with lumefantrine nanoparticles for transepidermal delivery. Biomed. Microdevices 2020, 22, 19. [Google Scholar] [CrossRef]
- Souza, A.C.M.; Mosqueira, V.C.F.; Silveira, A.P.A.; Antunes, L.R.; Richard, S.; Guimaraes, H.N.; Grabe-Guimaraes, A. Reduced cardiotoxicity and increased oral efficacy of artemether polymeric nanocapsules in Plasmodium berghei-infected mice. Parasitology 2018, 145, 1075–1083. [Google Scholar] [CrossRef]
- Parashar, D.; Aditya, N.P.; Murthy, R.S. Development of artemether and lumefantrine co-loaded nanostructured lipid carriers: Physicochemical characterization and In Vivo antimalarial activity. Drug Deliv. 2016, 23, 123–129. [Google Scholar] [CrossRef]
- Sidhaye, A.A.; Bhuran, K.C.; Zambare, S.; Abubaker, M.; Nirmalan, N.; Singh, K.K. Bio-inspired artemether-loaded human serum albumin nanoparticles for effective control of malaria-infected erythrocytes. Nanomedicine 2016, 11, 2809–2828. [Google Scholar] [CrossRef] [Green Version]
- Jain, K.; Sood, S.; Gowthamarajan, K. Optimization of artemether-loaded NLC for intranasal delivery using central composite design. Drug Deliv. 2015, 22, 940–954. [Google Scholar] [CrossRef] [Green Version]
- Eltayeb, S.E.; Su, Z.; Shi, Y.; Li, S.; Xiao, Y.; Ping, Q. Preparation and optimization of transferrin-modified-artemether lipid nanospheres based on the orthogonal design of emulsion formulation and physically electrostatic adsorption. Int. J. Pharm. 2013, 452, 321–332. [Google Scholar] [CrossRef]
- Aditya, N.P.; Patankar, S.; Madhusudhan, B.; Murthy, R.S.; Souto, E.B. Arthemeter-loaded lipid nanoparticles produced by modified thin-film hydration: Pharmacokinetics, toxicological and In Vivo anti-malarial activity. Eur. J. Pharm. Sci. 2010, 40, 448–455. [Google Scholar] [CrossRef]
- Sun, D.; Zhou, S.; Gao, W. What Went Wrong with Anticancer Nanomedicine Design and How to Make It Right. ACS Nano 2020, 14, 12281–12290. [Google Scholar] [CrossRef]
- Alhakamy, N.A.; Ahmed, O.A.A.; Aldawsari, H.M.; Alfaifi, M.Y.; Eid, B.G.; Abdel-Naim, A.B.; Fahmy, U.A. Encapsulation of Lovastatin in Zein Nanoparticles Exhibits Enhanced Apoptotic Activity in HepG2 Cells. Int. J. Mol. Sci. 2019, 20, 5788. [Google Scholar] [CrossRef] [Green Version]
- Algandaby, M.M.; Al-Sawahli, M.M.; Ahmed, O.A.A.; Fahmy, U.A.; Abdallah, H.M.; Hattori, M.; Ashour, O.M.; Abdel-Naim, A.B. Curcumin-Zein Nanospheres Improve Liver Targeting and Antifibrotic Activity of Curcumin in Carbon Tetrachloride-Induced Mice Liver Fibrosis. J. Biomed. Nanotechnol. 2016, 12, 1746–1757. [Google Scholar] [CrossRef]
- Cheng, W.; Wang, B.; Zhang, C.; Dong, Q.; Qian, J.; Zha, L.; Chen, W.; Hong, L. Preparation and preliminary pharmacokinetics study of GNA-loaded zein nanoparticles. J. Pharm. Pharmacol. 2019, 71, 1626–1634. [Google Scholar] [CrossRef]
- Dokunmu, T.M.; Ahanonu, C.L.; Abegunde, O.O.; Adeyemi, O.A. Artemisinin-induced delayed hemolysis after administration of artesunate and artesunate-amodiaquine in malaria-free Wistar rats. Biomed. Res. Ther. 2017, 4, 1246–1260. [Google Scholar] [CrossRef] [Green Version]
- D’Souza, S. A review of in vitro drug release test methods for nano-sized dosage forms. Adv. Pharmaceut. 2014, 2014, 304757. [Google Scholar]
- Chen, W.; Yeo, S.C.; Elhennawy, M.G.; Xiang, X.; Lin, H.S. Determination of naturally occurring resveratrol analog trans-4,4’-dihydroxystilbene in rat plasma by liquid chromatography-tandem mass spectrometry: Application to a pharmacokinetic study. Anal. Bioanal. Chem. 2015, 407, 5793–5801. [Google Scholar] [CrossRef]
- Yeo, S.C.M.; Fenwick, P.S.; Barnes, P.J.; Lin, H.S.; Donnelly, L.E. Isorhapontigenin, a bioavailable dietary polyphenol, suppresses airway epithelial cell inflammation through a corticosteroid-independent mechanism. Br. J. Pharmacol. 2017, 174, 2043–2059. [Google Scholar] [CrossRef]
- Dai, Y.; Yeo, S.C.M.; Barnes, P.J.; Donnelly, L.E.; Loo, L.C.; Lin, H.S. Pre-clinical Pharmacokinetic and Metabolomic Analyses of Isorhapontigenin, a Dietary Resveratrol Derivative. Front. Pharmacol. 2018, 9, 753. [Google Scholar] [CrossRef] [Green Version]
- Dai, Y.; Lim, J.X.; Yeo, S.C.M.; Xiang, X.; Tan, K.S.; Fu, J.H.; Huang, L.; Lin, H.S. Biotransformation of Piceatannol, a Dietary Resveratrol Derivative: Promises to Human Health. Mol. Nutr. Food Res. 2020, 64, e1900905. [Google Scholar] [CrossRef]
- Chen, W.; Yeo, S.C.M.; Elhennawy, M.; Lin, H.S. Oxyresveratrol: A bioavailable dietary polyphenol. J. Funct. Foods 2016, 22, 122–131. [Google Scholar] [CrossRef]
- Lindegardh, N.; Hanpithakpong, W.; Kamanikom, B.; Pattayaso, J.; Singhasivanon, P.; White, N.J.; Day, N.P. Quantification of dihydroartemisinin, artesunate and artemisinin in human blood: Overcoming the technical challenge of protecting the peroxide bridge. Bioanalysis 2011, 3, 1613–1624. [Google Scholar] [CrossRef]
- Huang, L.; Jayewardene, A.L.; Li, X.; Marzan, F.; Lizak, P.S.; Aweeka, F.T. Development and validation of a high-performance liquid chromatography/tandem mass spectrometry method for the determination of artemether and its active metabolite dihydroartemisinin in human plasma. J. Pharm. Biomed. Anal. 2009, 50, 959–965. [Google Scholar] [CrossRef] [Green Version]
- Elhennawy, M.G.; Lin, H.S. Determination of Tangeretin in Rat Plasma: Assessment of Its Clearance and Absolute Oral Bioavailability. Pharmaceutics 2018, 10, 3. [Google Scholar] [CrossRef] [Green Version]
- Elhennawy, M.G.; Lin, H.S. Dose- and time-dependent pharmacokinetics of apigenin trimethyl ether. Eur. J. Pharm. Sci. 2018, 118, 96–102. [Google Scholar] [CrossRef]
- Yeo, S.C.; Sviripa, V.M.; Huang, M.; Kril, L.; Watt, D.S.; Liu, C.; Lin, H.S. Analysis of trans-2,6-difluoro-4′-(N,N-dimethylamino)stilbene (DFS) in biological samples by liquid chromatography-tandem mass spectrometry: Metabolite identification and pharmacokinetics. Anal. Bioanal. Chem. 2015, 407, 7319–7332. [Google Scholar] [CrossRef]




| Zein/Artemether Ratio | Artemether Content (% w/w) | Encapsulated Drug (% w/w) | EE (%) |
|---|---|---|---|
| 2.5:1 | 16.3 ± 0.7 | 5.07 ± 0.32 | 31.0 ± 2.4 |
| 3.3:1 | 12.8 ± 0.4 | 5.86 ± 0.31 | 45.9 ± 1.9 |
| 5:1 | 8.90 ± 0.20 | 4.64 ± 0.17 | 52.1 ± 2.0 |
| 10:1 | 4.60 ± 0.20 | 2.53 ± 0.17 | 56.6 ± 2.5 |
| 20:1 | 2.42 ± 0.09 | 1.51 ± 0.07 | 62.3 ± 3.0 |
| Formulation | Artemether Solubilized in Cosolvent | Artemether-Loaded Zein Nanoparticles |
|---|---|---|
| Dose (mg/kg) | 5 | 5 |
| Vc (L/kg) | 2.14 ± 0.98 | 1.95 ± 0.85 |
| AUC0→last (104× min·ng/mL) | 6.22 ± 1.22 | 8.14 ± 4.52 |
| CL (mL/min/kg) | 83.1 ± 17.4 | 72.4 ± 25.7 |
| t1/2 λZ (min) | 165 ± 47 | 297 ± 132 |
| MRT0→last (min) | 45.6 ± 16.4 | 82.9 ± 15.2 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boateng-Marfo, Y.; Dong, Y.; Ng, W.K.; Lin, H.-S. Artemether-Loaded Zein Nanoparticles: An Innovative Intravenous Dosage Form for the Management of Severe Malaria. Int. J. Mol. Sci. 2021, 22, 1141. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031141
Boateng-Marfo Y, Dong Y, Ng WK, Lin H-S. Artemether-Loaded Zein Nanoparticles: An Innovative Intravenous Dosage Form for the Management of Severe Malaria. International Journal of Molecular Sciences. 2021; 22(3):1141. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031141
Chicago/Turabian StyleBoateng-Marfo, Yaa, Yuancai Dong, Wai Kiong Ng, and Hai-Shu Lin. 2021. "Artemether-Loaded Zein Nanoparticles: An Innovative Intravenous Dosage Form for the Management of Severe Malaria" International Journal of Molecular Sciences 22, no. 3: 1141. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031141







