The Responses of Bioactive Betanin Pigment and Its Derivatives from a Red Beetroot (Beta vulgaris L.) Betalain-Rich Extract to Hypochlorous Acid
Abstract
:1. Introduction
2. Results and Discussion
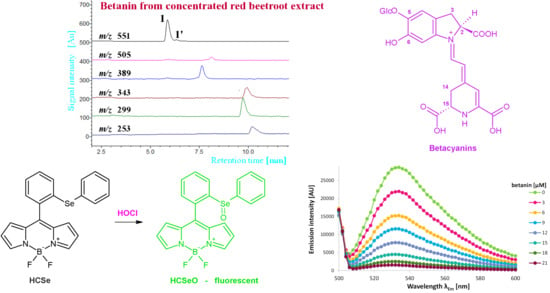
2.1. Characterization of Reactive Betalainic Pigments of B. vulgaris Concentrated Extract
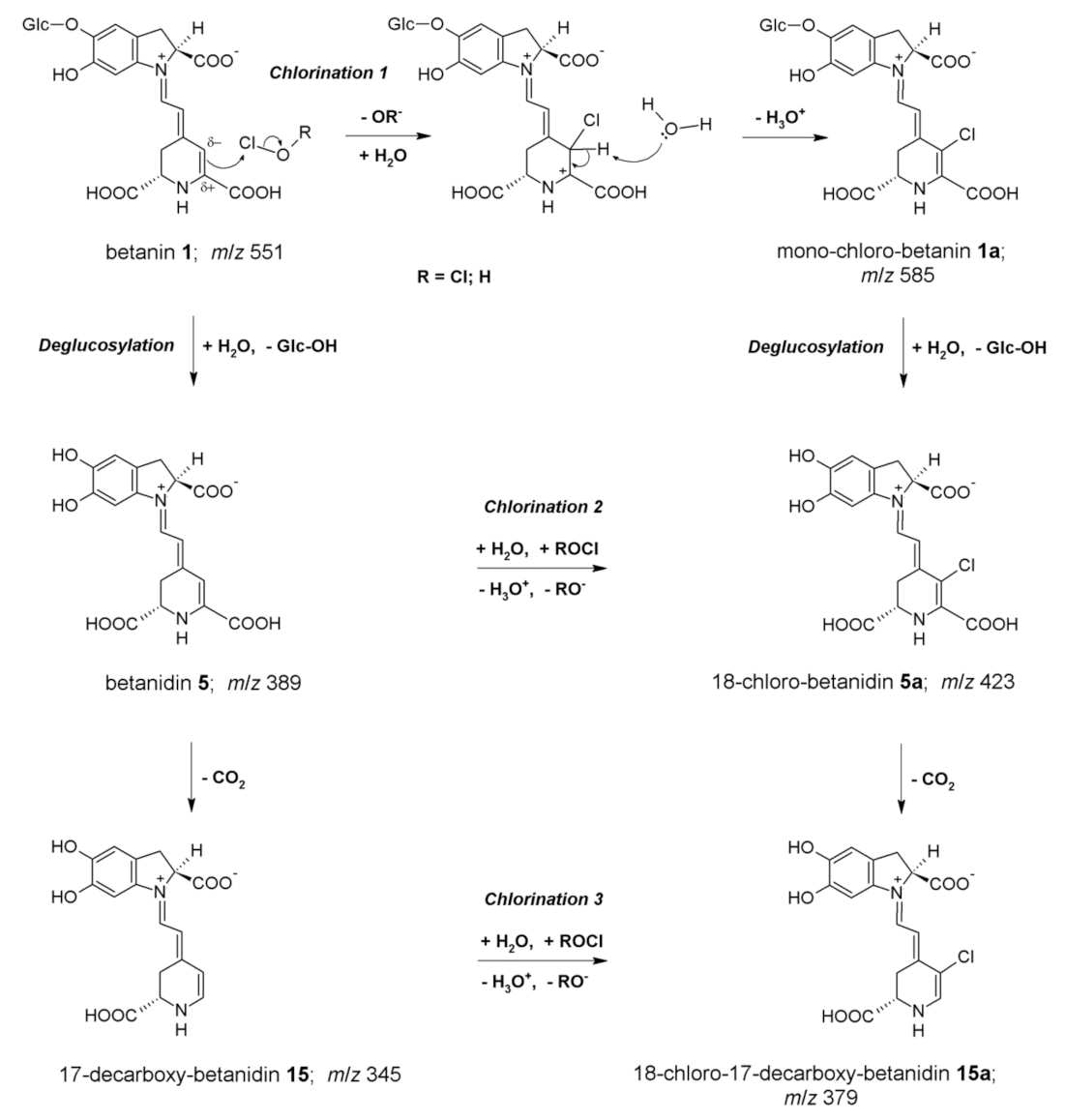
2.2. Chlorination of Betanin by Sodium Hypochlorite
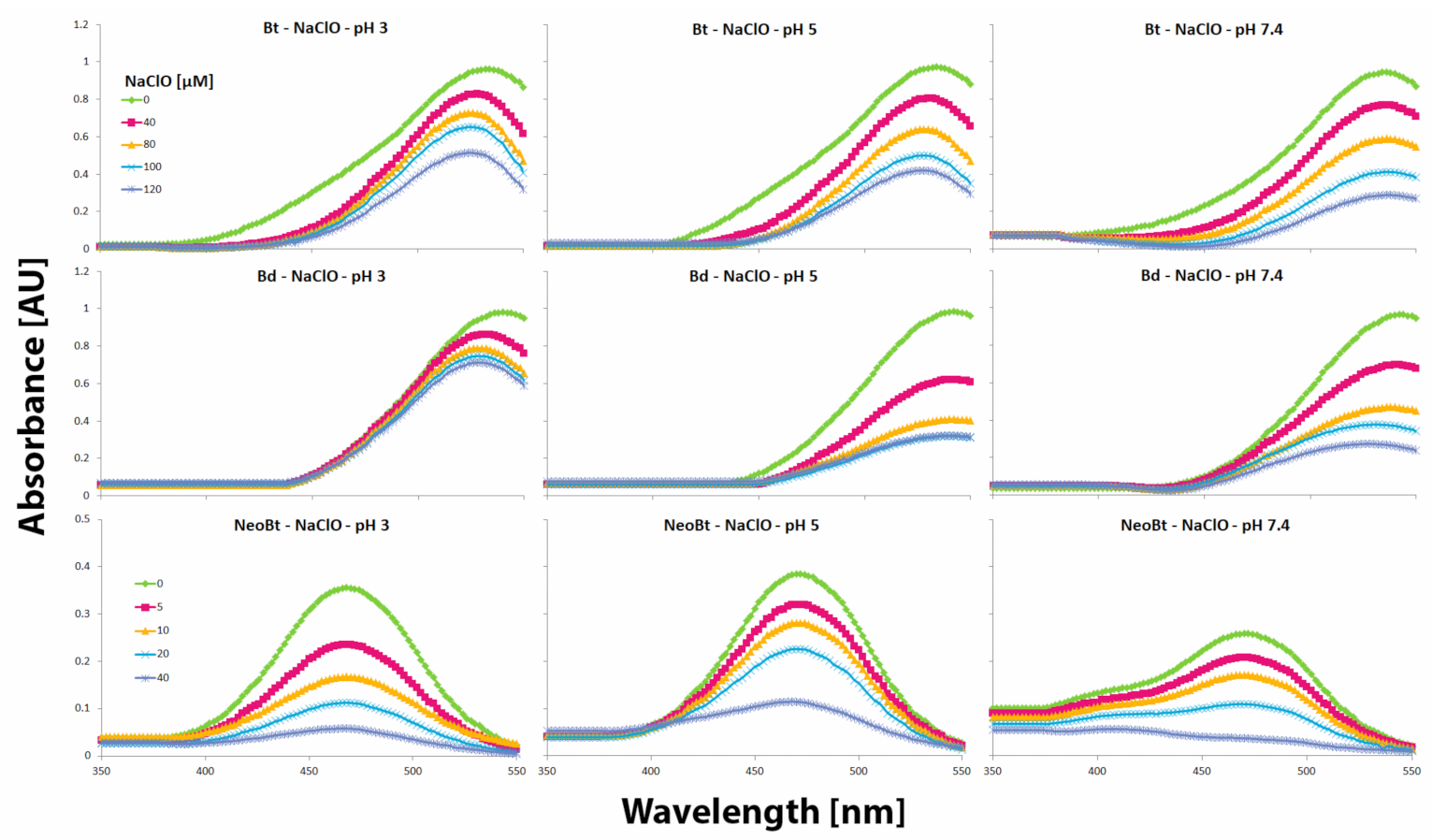
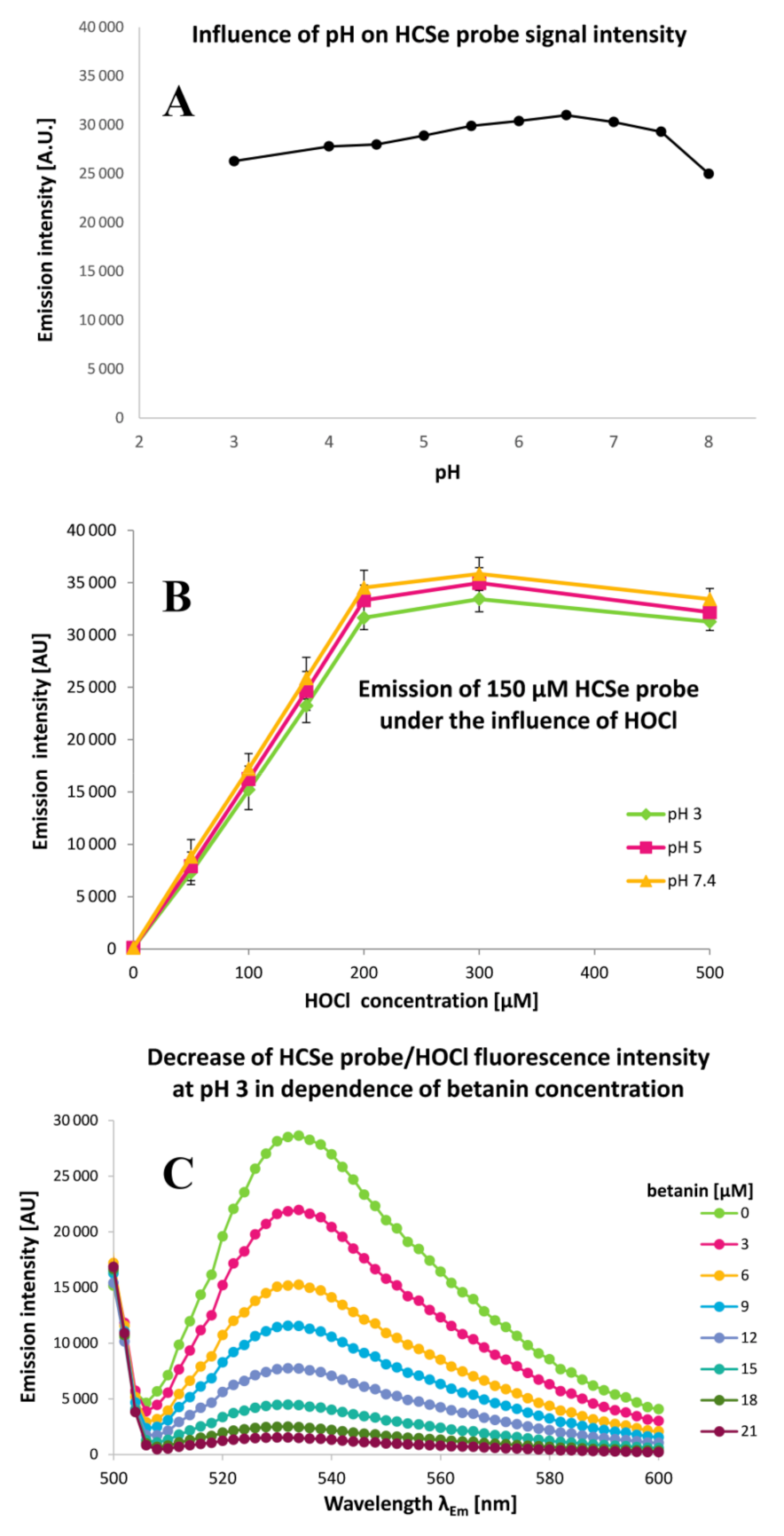
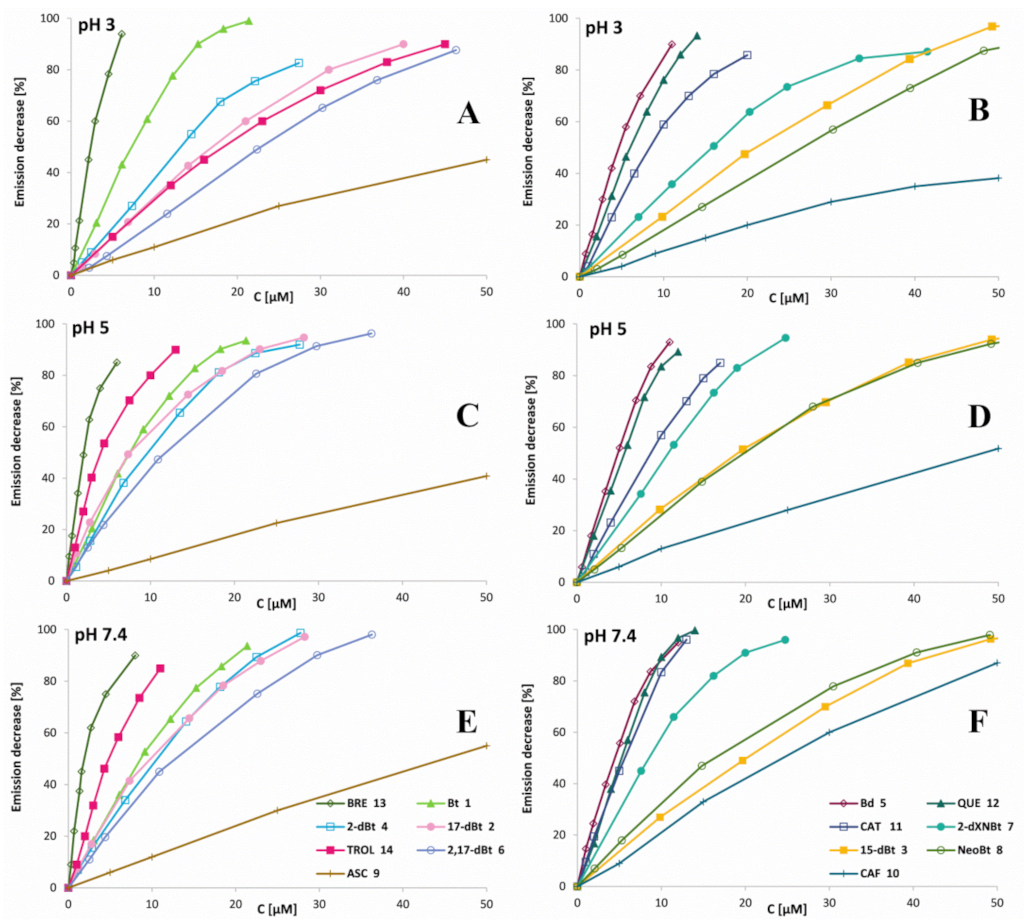
2.3. Determination of Betalainic Reactivity against Hypochlorites by Measurements of Anti-Hypochlorite Activity with the Use of HCSe and HCS Fluorescent Probes
3. Materials and Methods
3.1. Reagents
3.2. Isolation and Preparation of Betalains from B. vulgaris Extracts
3.3. Preparative Oxidation of Betanin and Neobetanin by ABTS Cation Radicals
3.4. Semi-Preparative HPLC Purification of Betalain Fractions
3.5. Chromatographic Analysis by LC-DAD-ESI-MS/MS System
3.6. HCSe and HCS Assays for Determination of Anti-Hypochlorite Activity
3.7. Measurement of Antioxidant Activity with ABTS Cation Radicals
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.I.; Giridhar, P. Plant betalains: Chemistry and biochemistry. Phytochemistry 2015, 117, 267. [Google Scholar] [CrossRef] [PubMed]
- Azeredo, H.M.C. Betalains: Properties, sources, applications, and stability—A review. Int. J. Food Sci. Tech. 2009, 44, 2365. [Google Scholar] [CrossRef] [Green Version]
- Gandía-Herrero, F.; Escribano, J.; García-Carmona, F. Biological activities of plant pigments betalains. Crit. Rev. Food Sci. 2016, 56, 937. [Google Scholar] [CrossRef]
- Khan, M.I. Plant betalains: Safety, antioxidant activity, clinical efficacy, and bioavailability. Compr. Rev. Food Sci. Food Saf. 2015, 15, 316. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wybraniec, S.; Starzak, K.; Pietrzkowski, Z. Chlorination of betacyanins in several hypochlorous acid systems. J. Agric. Food Chem. 2016, 64, 2865. [Google Scholar] [CrossRef]
- Wybraniec, S.; Starzak, K.; Szneler, E.; Pietrzkowski, Z. Separation of chlorinated diastereomers of decarboxy-betacyanins in myeloperoxidase catalyzed chlorinated Beta vulgaris L. extract. J. Chromatogr. B 2016, 1036, 20. [Google Scholar] [CrossRef]
- Pietrzkowski, Z.; Nemzer, B.; Michałowski, T.; Wybraniec, S. Influence of betalain-rich extract on reduction of discomfort associated with osteoarthritis. New Med. 2010, 1, 12. [Google Scholar]
- Pietrzkowski, Z.; Argumedo, R.; Shu, C.; Nemzer, B.; Wybraniec, S.; Reyes-Izquierdo, T. Betalain-rich red beet concentrate (BRC) improves knee discomfort and function: A double blind, placebo-controlled clinical study. Nutr. Diet. Suppl. 2014, 6, 9. [Google Scholar]
- Kettle, A.J.; Albrett, A.M.; Chapman, A.L.; Dickerhof, N.; Forbes, L.V.; Khalilova, I.; Turner, R. Measuring chlorine bleach in biology and medicine. Biochim. Biophys. Acta Gen. Subj. 2014, 1840, 781. [Google Scholar] [CrossRef]
- Klebanoff, S.J. Myeloperoxidase: Friend and foe. J. Leukoc. Biol. 2005, 7, 598. [Google Scholar] [CrossRef]
- Zhang, R.; Song, B.; Yuan, J. Bioanalytical Methods for Hypochlorous Acid Detection: Recent Advances and Challenges. Trends Anal. Chem. 2018, 99, 1. [Google Scholar] [CrossRef]
- Albrich, J.M.; McCarthy, C.A.; Hurst, J.K. Biological reactivity of hypochlorous acid: Implications for microbicidal mechanisms of leukocyte myeloperoxidase. Proc. Natl. Acad. Sci. USA 1981, 78, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dennis, W.H.; Olivieri, V.O., Jr.; Krusé, C.W. The reaction of nucleotides with aqueous hypochlorous acid. Water Res. 1979, 13, 357. [Google Scholar] [CrossRef]
- Visser, M.C.M.; Winterbourn, C.C. Oxidative damage to fibronectin: I. The effects of the neutrophil myeloperoxidase system and HOCl. Arch. Biochem. Biophys. 1991, 285, 53. [Google Scholar] [CrossRef]
- Carr, A.C.; Vissers, M.C.M.; Domigan, N.M.; Winterbourn, C.C. Modification of red cell membrane lipids by hypochlorous acid and haemolysis by preformed lipid chlorohydrins. Redox Rep. 1997, 3, 236. [Google Scholar] [CrossRef]
- Allegra, M.; Furtmuller, P.G.; Jantschko, W.; Zederbauer, M.; Tesoriere, L.; Livrea, M.A.; Obinger, C. Mechanism of interaction of betanin and indicaxanthin with human myeloperoxidase and hypochlorous acid. Biochem. Biophys. Res. Commun. 2005, 332, 837. [Google Scholar] [CrossRef]
- Nemzer, B.; Pietrzkowski, Z.; Spórna, A.; Stalica, P.; Thresher, W.; Michałowski, T.; Wybraniec, S. Betalainic and nutritional profiles of pigment-enriched red beet root (Beta vulgaris L.) dried extracts. Food Chem. 2011, 127, 42. [Google Scholar] [CrossRef]
- Kumorkiewicz, A.; Starzak, K.; Sutor, K.; Nemzer, B.; Pietrzkowski, Z.; Popenda, Ł.; Wybraniec, S. Structural Study on Hypochlorous Acid-Mediated Chlorination of Betanin and Its Decarboxylated Derivatives from an Anti-Inflammatory Beta vulgaris L. Extract. Molecules 2020, 25, 378. [Google Scholar] [CrossRef] [Green Version]
- Liu, S.R.; Wu, S.P. Hypochlorous acid turn-on fluorescent probe based on oxidation of diphenyl diselenide. Org. Lett. 2013, 15, 878. [Google Scholar] [CrossRef]
- Liu, S.R.; Vedamalai, M.; Wu, S.P. Hypochlorous acid turn-on boron dipyrromethene probe based on oxidation of methyl phenyl sulphide. Anal. Chim. Acta 2013, 800, 71. [Google Scholar] [CrossRef]
- Wybraniec, S. Formation of decarboxylated betacyanins in heated purified betacyanin fractions from red beet root (Beta vulgaris L.) monitored by LC-MS/MS. J. Agric. Food Chem. 2005, 59, 3483. [Google Scholar] [CrossRef]
- Nilsson, T. Studies into the pigments in beetroot (Beta vulgaris L. ssp. vulgaris var. rubra L.). Lantrukshogsk. Ann. 1970, 36, 179. [Google Scholar]
- Walker, R.B.; Everette, J.D. Comparative reaction rates of various antioxidants with ABTS radical cation. J. Agric. Food Chem. 2009, 57, 1156. [Google Scholar] [CrossRef]
- Wybraniec, S.; Michałowski, T. New pathways of betanidin and betanin enzymatic oxidation studied by LC-DAD-ESI-MS/MS. J. Agric. Food Chem. 2011, 59, 9612. [Google Scholar] [CrossRef] [PubMed]
- Wybraniec, S.; Starzak, K.; Skopinska, A.; Nemzer, B.; Pietrzkowski, Z.; Michalowski, T. Studies on nonenzymatic oxidation mechanisms in neobetanin, betanin, and decarboxylated betanins. J. Agric. Food Chem. 2013, 61, 6465. [Google Scholar] [CrossRef] [PubMed]
- Kumorkiewicz, A.; Szmyr, N.; Popenda, Ł.; Pietrzkowski, Z.; Wybraniec, S. Alternative mechanisms of betacyanin oxidation by complexation and radical generation. J. Agric. Food Chem. 2019, 67, 7455. [Google Scholar] [CrossRef]
- Kumorkiewicz, A.; Szneler, E.; Wybraniec, S. Conjugation of Oxidized Betanidin and Gomphrenin Pigments from Basella alba L. Fruits with Glutathione. J. Agric. Food Chem. 2018, 66, 12815. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.R.; Liu, Y.; Feng, X.; Zhao, B.X. Recent progress in the development of fluorescent probes for the detection of hypochlorous acid. Sens. Actuat. B Chem. 2017, 240, 18. [Google Scholar] [CrossRef]
- Jin, X.; Hao, L.; Hu, Y.; She, M.; Shi, Y.; Obst, M.; Li, J.; Shi, Z. Two novel fluorescein-based fluorescent probes for hypochlorite and its real applications in tap water and biological imaging. Sens. Actuat. B Chem. 2013, 186, 56. [Google Scholar] [CrossRef]
- Huo, F.J.; Zhang, J.J.; Yang, Y.T.; Chao, J.B.; Yin, C.X.; Zhang, X.B.; Chen, T.G. A fluorescein-based highly specific colorimetric and fluorescent probe for hypochlorites in aqueous solution and its application in tap water. Sens. Actuat. B Chem. 2012, 166, 44. [Google Scholar] [CrossRef]
- Setsukinai, K.I.; Urano, Y.; Kakinuma, K.; Majima, H.J.; Nagano, T. Development of novel fluorescence probes that can reliably detect reactive oxygen species and distinguish specific species. J. Biol. Chem. 2003, 278, 3170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Zheng, Y.; Hang, W.; Yan, X.; Zhao, Y. Sensitive and selective off-on rhodamine hydrazide fluorescent chemosensor for hypochlorous acid detection and bioimaging. Talanta 2011, 85, 779. [Google Scholar] [CrossRef] [PubMed]
- Koide, Y.; Urano, Y.; Hanaoka, K.; Terai, T.; Nagano, T. Development of an Si-rhodamine-based far-red to near-infrared fluorescence probe selective for hypochlorous acid and its applications for biological imaging. Am. Chem. Soc. 2011, 133, 5680. [Google Scholar] [CrossRef] [PubMed]
- Kenmoku, S.; Urano, Y.; Kojima, H.; Nagano, T. Development of a highly specific rhodamine-based fluorescence probe for hypochlorous acid and its application to real-time imaging of phagocytosis. J. Am. Chem. Soc. 2007, 129, 7313. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, H.; Choi, Y.; Kim, Y. A ratiometric fluorescent probe based on a BODIPY–DCDHF conjugate for the detection of hypochlorous acid in living cells. Analyst 2013, 138, 3368. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.; Huo, F.; Yue, Y.; Wen, Y.; Chao, J.; Zhang, Y.; Yin, C. A solvent depend on ratiometric fluorescent probe for hypochlorous acid and its application in living cells. Dye. Pigment. 2017, 136, 852. [Google Scholar] [CrossRef]
- Xu, X.X.; Qian, Y. A novel pyridyl triphenylamine–BODIPY aldoxime: Naked-eye visible and fluorometric chemodosimeter for hypochlorite. Spectrochim. Acta Mol. Biomol. Spectrosc. 2017, 183, 356. [Google Scholar] [CrossRef]
- Wang, L.; Li, W.; Zhi, W.; Ye, D.; Zhang, W.; Ni, L. Rapid detection of hypochlorite by a coumarin-based hydrazide in aqueous solution and its application in live-cell imaging. Sens. Actuat. B Chem. 2018, 255, 1112. [Google Scholar] [CrossRef]
- Song, X.; Dong, B.; Kong, X.; Wang, C.; Zhang, N.; Lin, W. Construction of a ratiometric fluorescent probe with an extremely large emission shift for imaging hypochlorite in living cells. Spectrochim. Acta Mol. Biomol. Spectrosc. 2018, 188, 394. [Google Scholar] [CrossRef]
- Zhang, P.; Wang, H.; Zhang, D.; Zeng, X.; Zeng, R.; Xiao, L.; Tao, H.; Long, Y.; Yi, P.; Chen, J. Two-photon fluorescent probe for lysosome-targetable hypochlorous acid detection within living cells. Sens. Actuat. B Chem. 2018, 255, 2223. [Google Scholar] [CrossRef]
- Deng, B.; Ren, M.; Kong, X.; Zhou, K.; Lin, W. Development of an enhanced turn-on fluorescent HOCl probe with a large Stokes shift and its use for imaging HOCl in cells and zebrafish. Sens. Actuat. B Chem. 2018, 255, 963. [Google Scholar] [CrossRef]
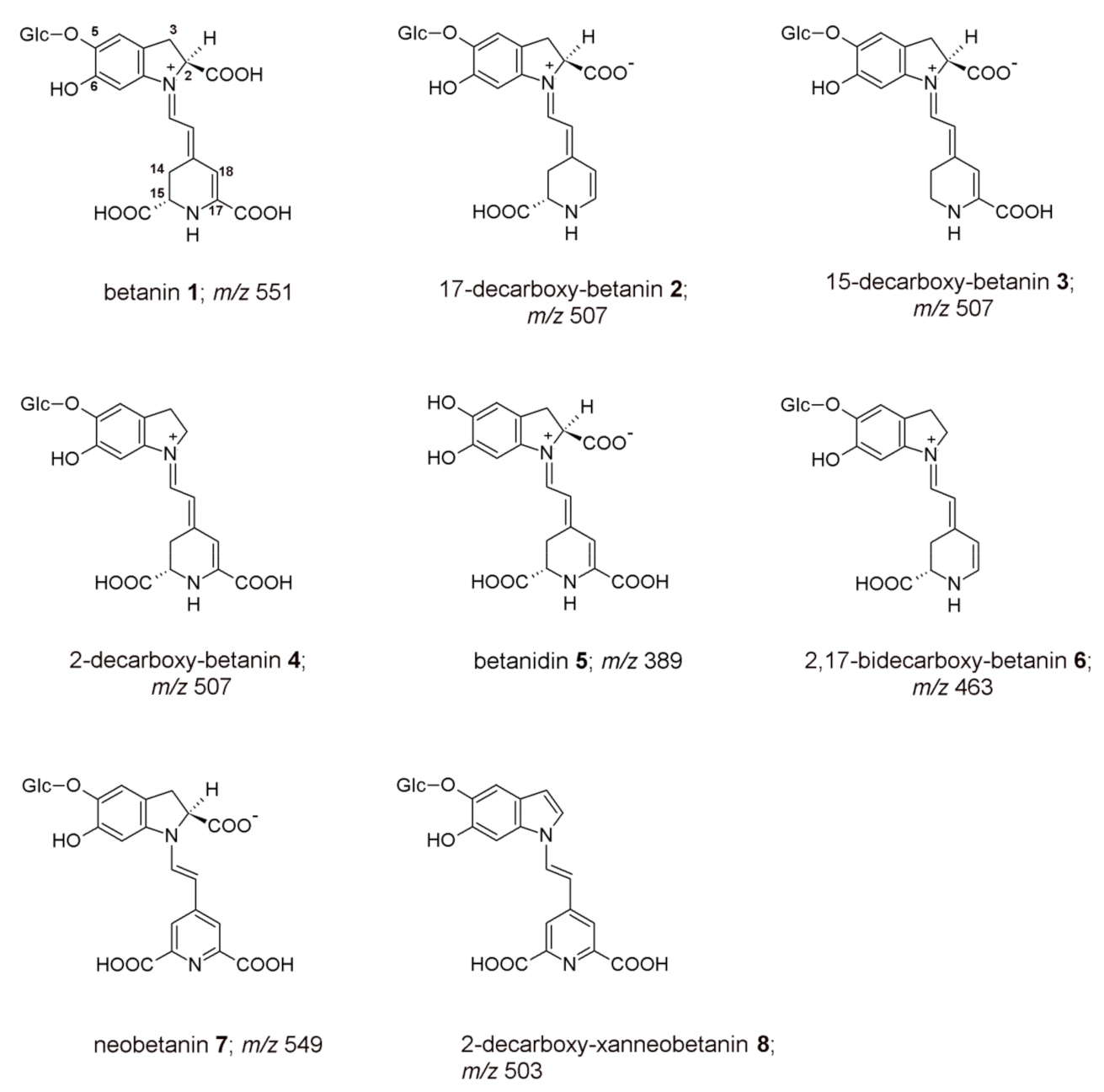
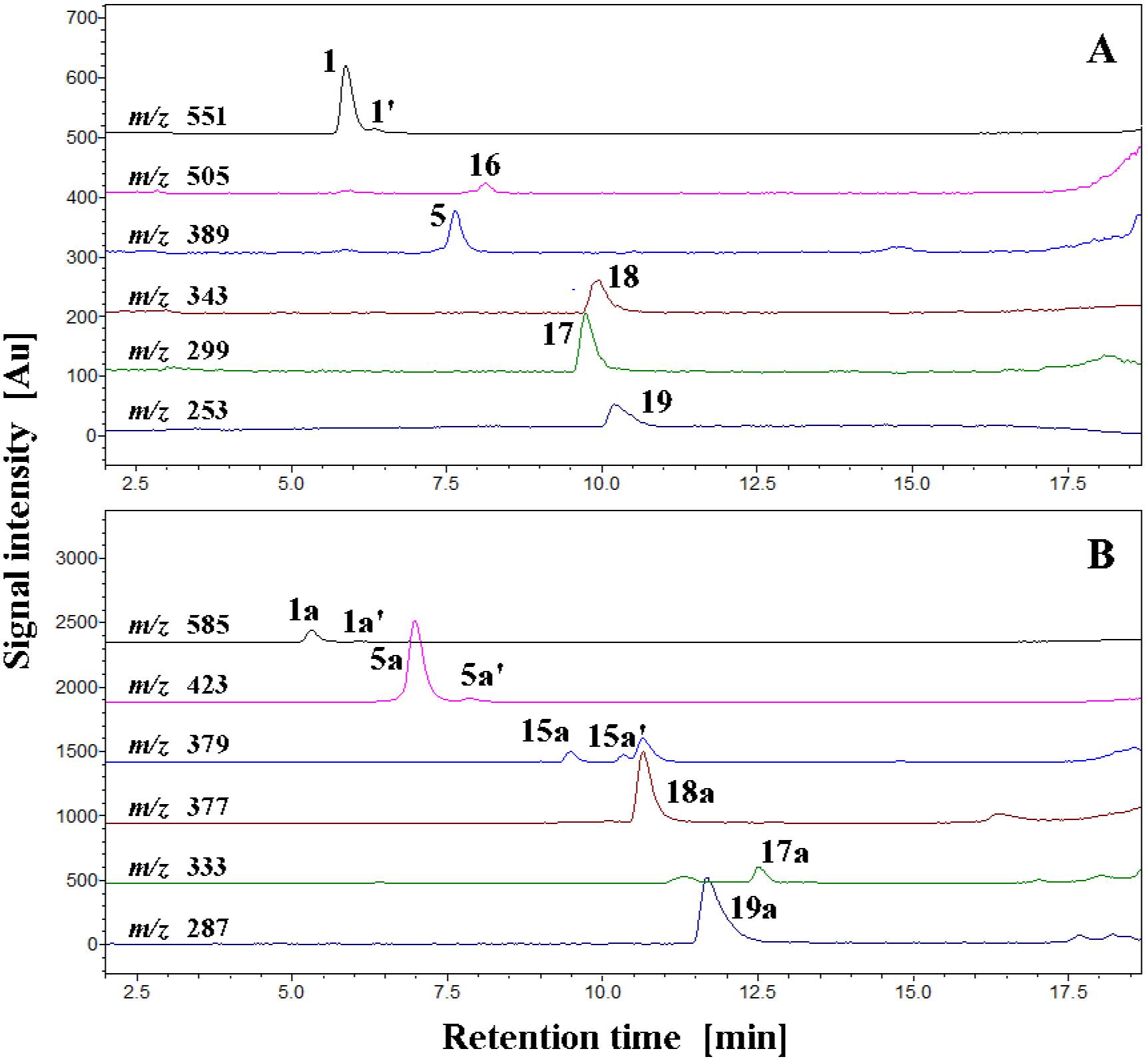


| No. | Compound | λmax [nm] | m/z [M+H]+ | m/z from MS/MS of [M+H]+ |
|---|---|---|---|---|
| 1 | betanin | 538 | 551 | 389 |
| 2 | 17-decarboxy-betanin | 505 | 507 | 345 |
| 3 | 15-decarboxy-betanin | 528 | 507 | 345 |
| 4 | 2-decarboxy-betanin | 533 | 507 | 345 |
| 5 | betanidin | 540 | 389 | 345 |
| 6/6′ | 2,17-bidecarboxy-betanin/-isobetanin | 507 | 463 | 301 |
| 7 | neobetanin | 466 | 549 | 387; 343; 299 |
| 8 | 2-decarboxy-xanneobetanin | 422 | 503 | 341; 297; 253 |
| No. | Compound | Rt [min] | λmax [nm] | m/z [M+H]+ | m/z from MS/MS of [M+H]+ |
|---|---|---|---|---|---|
| Betanin oxidation, deglucosylation, and decarboxylation products formed at pH 7.4 | |||||
| 1 | betanin | 5.9 | 538 | 551 | 389 |
| 1′ | isobetanin | 6.3 | 538 | 551 | 389 |
| 5 | betanidin | 7.7 | 540 | 389 | 345 |
| 15 | 17-decarboxy-betanidin | 7.8 | 511 | 345 | 299; 255 |
| 15′ | 17-decarboxy-isobetanidin | 8.4 | 511 | 345 | 299; 255 |
| 16 | 2-decarboxy-xanbetanin | 8.2 | 445 | 505 | 343; 297 |
| 17 | 2,17-bidecarboxy-xanbetanidin | 9.7 | 467 | 299 | 255 |
| 18 | 2-decarboxy-xanbetanidin | 9.9 | 490 | 343 | 299; 255 |
| 19 | 2,15,17-tridecarboxy-xanneobetanidin | 10.2 | 401 | 253 | 253 |
| Betanin chlorination products formed at pH 7.4 | |||||
| 1a | 18-chloro-betanin | 5.3 | 522 | 585 | 423; 387 |
| 1a’ | 18-chloro-isobetanin | 6.1 | 522 | 585 | 423; 387 |
| 5a | 18-chloro-betanidin | 7.0 | 527 | 423 | 387; 341 |
| 5a’ | 18-chloro-isobetanidin | 7.8 | 527 | 423 | 387; 341 |
| 15a | 18-chloro-17-decarboxy-betanidin | 9.5 | 525 | 379 | 343; 333 |
| 15a’ | 18-chloro-17-decarboxy-isobetanidin | 10.3 | 525 | 379 | 343; 333 |
| 17a | 18-chloro-2,17-bidecarboxy-xanbetanidin | 12.5 | 499 | 333 | 287; 253 |
| 18a | 18-chloro-2-decarboxy-xanbetanidin | 10.7 | 494 | 377 | 331; 287; 253 |
| 19a | 18-chloro-2,15,17-tridecarboxy-xanneobetanidin | 11.7 | 401 | 287 | 251 |
| Compound | C50 [μM] | “a” Factor of Initial Slope | Anti-HA | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| (No.) | pH 3 | pH 5 | pH 7.4 | pH 3 | pH 5 | pH 7.4 | pH 3 | pH 5 | pH 7.4 | |||||||||
| Bt (1) | 7.37 | 0.4 | 7.20 | 0.4 | 8.14 | 0.9 | 6.72 | 0.4 | 6.49 | 0.4 | 5.75 | 0.4 | 2.78 | 0.1 | 0.54 | 0.3 | 0.50 | 0.1 |
| 17-dBt (2) | 15.5 | 1.1 | 5.82 | 0.6 | 8.66 | 1.1 | 2.91 | 1.1 | 8.15 | 0.5 | 5.61 | 0.1 | 1.20 | 0.1 | 0.32 | 0.3 | 0.40 | 0.3 |
| 15-dBt (3) | 20.6 | 1.4 | 19.0 | 1.9 | 22.6 | 2.4 | 2.41 | 0.2 | 2.62 | 0.2 | 2.20 | 0.2 | 1.00 | 0.1 | 0.22 | 0.1 | 0.19 | 0.0 |
| 2-dBt (4) | 10.9 | 0.3 | 8.11 | 0.9 | 11.2 | 1.3 | 3.78 | 0.3 | 5.67 | 0.4 | 4.40 | 0.3 | 1.56 | 0.1 | 0.47 | 0.2 | 0.38 | 0.2 |
| Bd (5) | 5.37 | 0.3 | 5.26 | 0.7 | 4.95 | 0.5 | 8.38 | 0.7 | 8.85 | 1.1 | 9.59 | 1.0 | 3.47 | 0.3 | 0.73 | 0.5 | 0.83 | 0.1 |
| 2,17-dBt (6) | 19.9 | 1.4 | 9.77 | 1.0 | 12.1 | 1.4 | 2.21 | 0.5 | 4.95 | 0.2 | 4.12 | 0.4 | 0.91 | 0.2 | 0.41 | 0.2 | 0.36 | 0.2 |
| NeoBt (7) | 25.8 | 3.0 | 20.1 | 2.0 | 15.8 | 1.6 | 1.85 | 0.1 | 2.45 | 0.1 | 3.16 | 0.2 | 0.76 | 0.1 | 0.20 | 0.0 | 0.27 | 0.2 |
| 2-dXNBt (8) | 13.9 | 1.1 | 11.0 | 1.1 | 9.26 | 0.9 | 3.14 | 0.2 | 4.42 | 0.4 | 5.30 | 0.5 | 1.30 | 0.1 | 0.37 | 0.3 | 0.46 | 0.3 |
| ASC (9) | 54.6 | 5.3 | 50.6 | 4.3 | 42.7 | 3.3 | 0.71 | 0.6 | 0.83 | 0.1 | 1.00 | 0.0 | 0.30 | 0.2 | 0.07 | 0.0 | 0.09 | 0.0 |
| CAF (10) | 18.8 | 2.0 | 28.5 | 2.0 | 28.2 | 2.0 | 1.00 | 0.8 | 1.02 | 1.0 | 1.76 | 0.1 | 0.41 | 0.3 | 0.08 | 0.0 | 0.15 | 0.1 |
| CAT (11) | 7.10 | 0.6 | 7.18 | 0.6 | 6.00 | 0.4 | 6.04 | 0.5 | 5.92 | 0.5 | 8.29 | 0.6 | 2.50 | 0.2 | 0.49 | 0.2 | 0.72 | 0.2 |
| QUE (12) | 5.75 | 0.8 | 5.35 | 0.8 | 5.21 | 0.5 | 8.11 | 0.7 | 8.93 | 0.8 | 9.56 | 0.8 | 3.36 | 0.2 | 0.74 | 0.5 | 0.83 | 0.6 |
| BRE (13) | 2.94 | 0.3 | 2.19 | 0.3 | 1.99 | 0.1 | 16.0 | 1.2 | 19.4 | 1.9 | 23.3 | 1.6 | 6.62 | 0.3 | 1.60 | 0.1 | 2.02 | 0.2 |
| TROL (14) | 18.6 | 1.3 | 3.72 | 1.3 | 3.91 | 0.3 | 2.42 | 0.3 | 12.1 | 1.0 | 10.9 | 0.9 | 1.00 | 0.0 | 1.00 | 0.0 | 1.00 | 0.0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Starzak, K.; Sutor, K.; Świergosz, T.; Nemzer, B.; Pietrzkowski, Z.; Popenda, Ł.; Liu, S.-R.; Wu, S.-P.; Wybraniec, S. The Responses of Bioactive Betanin Pigment and Its Derivatives from a Red Beetroot (Beta vulgaris L.) Betalain-Rich Extract to Hypochlorous Acid. Int. J. Mol. Sci. 2021, 22, 1155. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031155
Starzak K, Sutor K, Świergosz T, Nemzer B, Pietrzkowski Z, Popenda Ł, Liu S-R, Wu S-P, Wybraniec S. The Responses of Bioactive Betanin Pigment and Its Derivatives from a Red Beetroot (Beta vulgaris L.) Betalain-Rich Extract to Hypochlorous Acid. International Journal of Molecular Sciences. 2021; 22(3):1155. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031155
Chicago/Turabian StyleStarzak, Karolina, Katarzyna Sutor, Tomasz Świergosz, Boris Nemzer, Zbigniew Pietrzkowski, Łukasz Popenda, Shi-Rong Liu, Shu-Pao Wu, and Sławomir Wybraniec. 2021. "The Responses of Bioactive Betanin Pigment and Its Derivatives from a Red Beetroot (Beta vulgaris L.) Betalain-Rich Extract to Hypochlorous Acid" International Journal of Molecular Sciences 22, no. 3: 1155. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031155