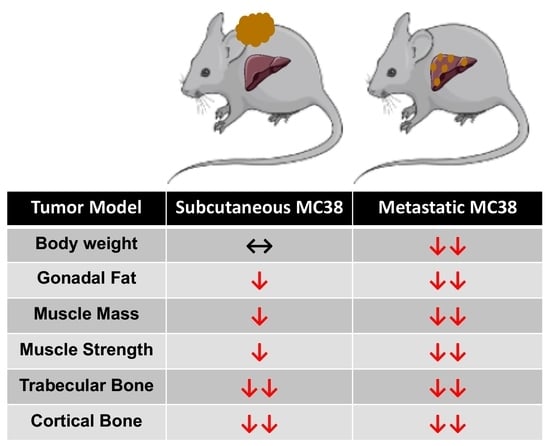
MC38 Tumors Induce Musculoskeletal Defects in Colorectal Cancer
Abstract
:1. Introduction
2. Results
2.1. Growth of Subcutaneous MC38 Tumors Causes Cachexia Consistent with Fat Wasting, Muscle Loss and Weakness
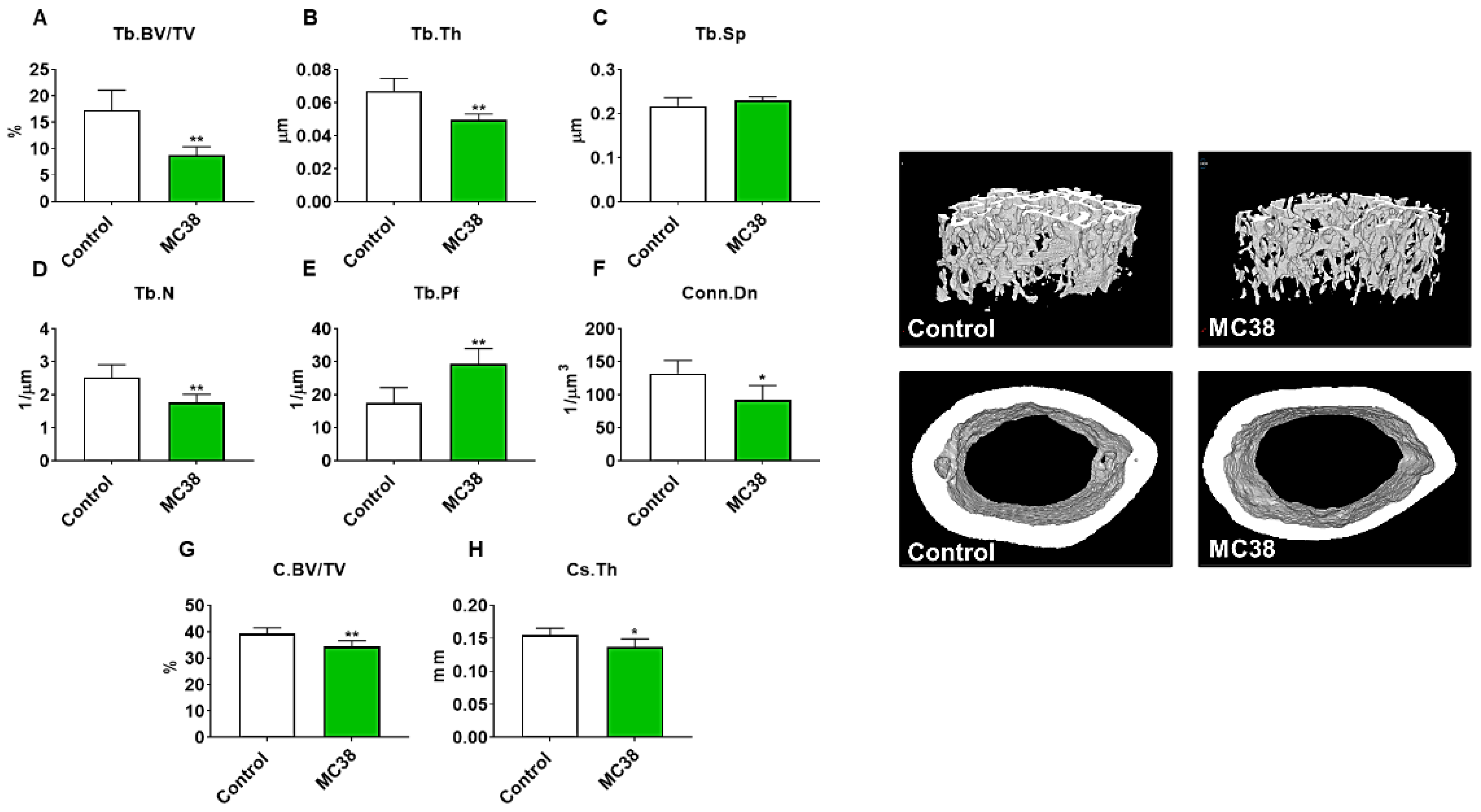
2.2. Growth of Subcutaneous MC38 Tumors Causes Loss of Cancellous and Cortical Bone
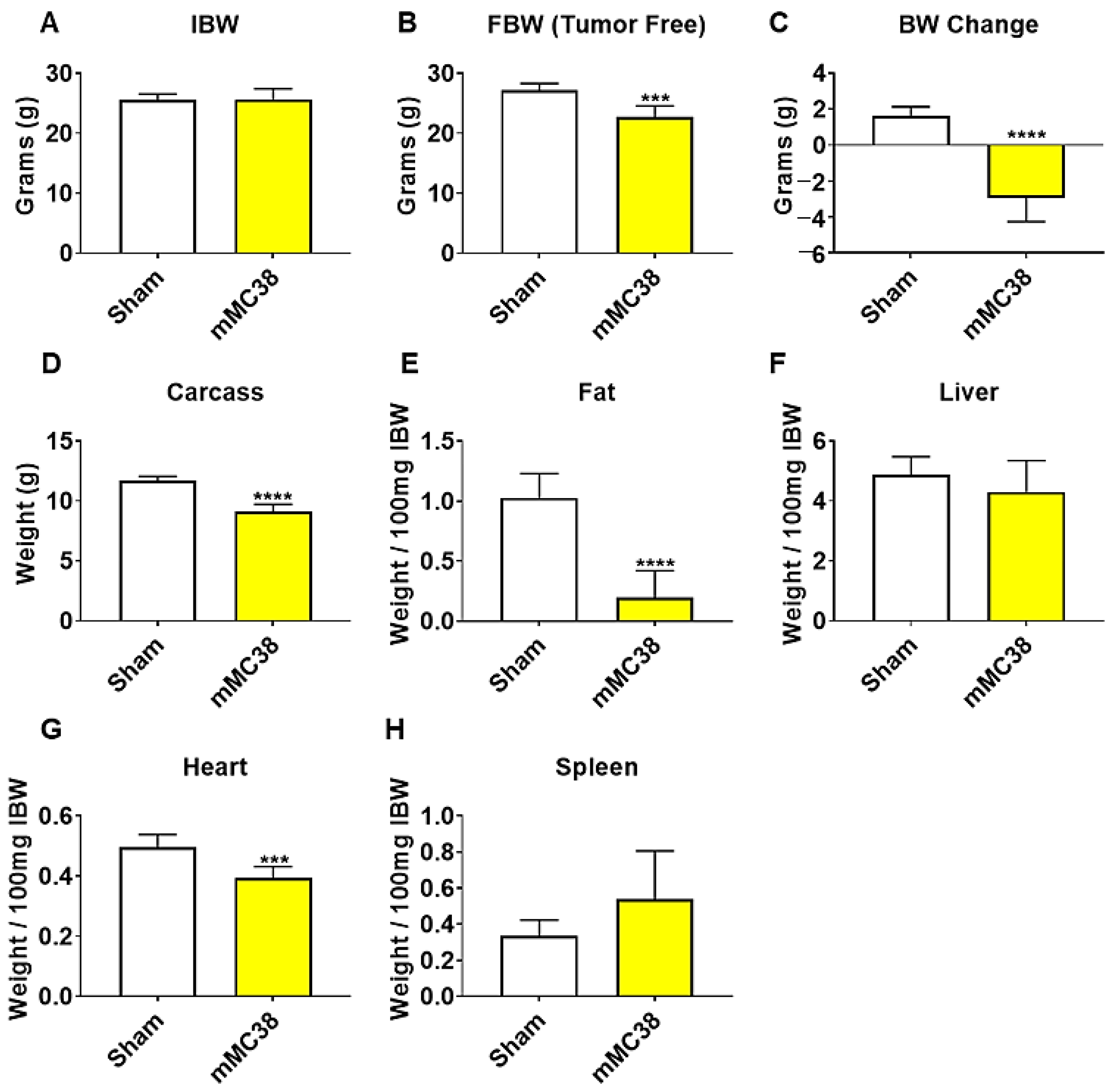
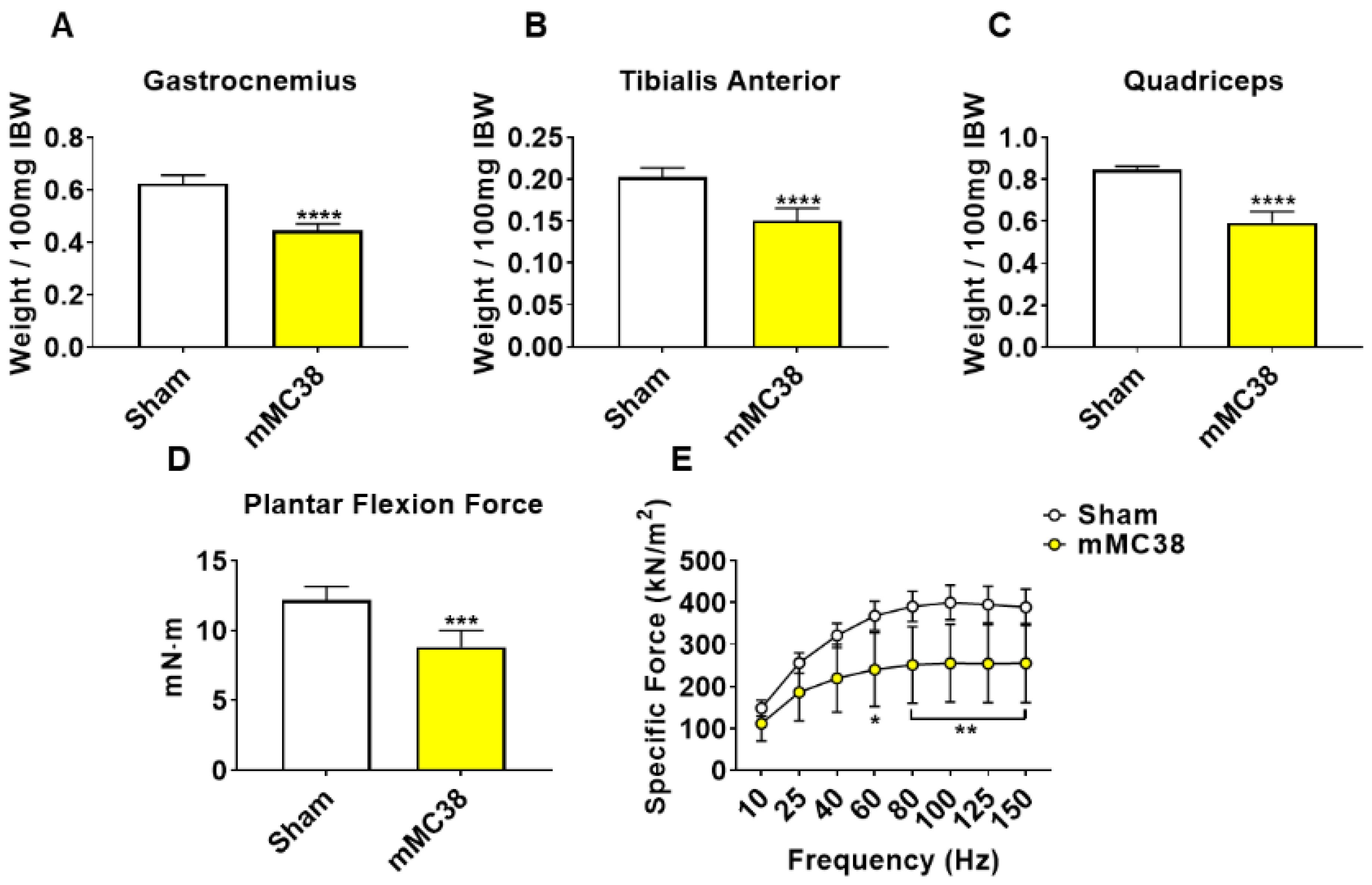
2.3. Formation of MC38 Liver Metastases Causes Severe Cachexia Consistent with Heightened Skeletal Muscle Wasting and Weakness
2.4. Formation of MC38 Liver Metastases Causes Loss of Cancellous and Cortical Bone
2.5. STAT3 Plays a Pivotal Role in MC38-Induced Skeletal Muscle Atrophy
3. Discussion
4. Materials and Methods
4.1. Cell Cultures
4.2. Animals
4.3. In Vivo Muscle Contractility
4.4. Ex Vivo Muscle Contractility
4.5. Microcomputed Tomography Analysis of Femur Bones
4.6. Myotube Diameter
4.7. Western Blotting
4.8. Statistics
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2020. CA Cancer J. Clin. 2020, 70, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.D.; Fidler-Benaoudia, M.; Keegan, T.H.; Hipp, H.S.; Jemal, A.; Siegel, R.L. Cancer statistics for adolescents and young adults, 2020. CA Cancer J. Clin. 2020, 70, 443–459. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Quan, X.Q.; Yu, S. An epidemiological survey of cachexia in advanced cancer patients and analysis on its diagnostic and treatment status. Nutr. Cancer 2015, 67, 1056–1062. [Google Scholar] [CrossRef] [PubMed]
- von Haehling, S.; Anker, S.D. Prevalence, incidence and clinical impact of cachexia: Facts and numbers-update 2014. J. Cachexia Sarcopenia Muscle 2014, 5, 261–263. [Google Scholar] [CrossRef] [PubMed]
- Dewys, W.D.; Begg, C.; Lavin, P.T.; Band, P.R.; Bennett, J.M.; Bertino, J.R.; Cohen, M.H.; Douglass, H.O., Jr.; Engstrom, P.F.; Ezdinli, E.Z.; et al. Prognostic effect of weight loss prior to chemotherapy in cancer patients. Eastern cooperative oncology group. Am. J. Med. 1980, 69, 491–497. [Google Scholar] [CrossRef]
- Hayes, S.; Battistutta, D.; Newman, B. Objective and subjective upper body function six months following diagnosis of breast cancer. Breast Cancer Res. Treat 2005, 94, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Luctkar-Flude, M.; Groll, D.; Woodend, K.; Tranmer, J. Fatigue and physical activity in older patients with cancer: A six-month follow-up study. Oncol. Nurs. Forum 2009, 36, 194–202. [Google Scholar] [CrossRef]
- Mustian, K.M.; Peppone, L.J.; Palesh, O.G.; Janelsins, M.C.; Mohile, S.G.; Purnell, J.Q.; Darling, T.V. Exercise and cancer-related fatigue. US Oncol. 2009, 5, 20–23. [Google Scholar] [CrossRef] [Green Version]
- Huot, J.R.; Novinger, L.J.; Pin, F.; Bonetto, A. HCT116 colorectal liver metastases exacerbate muscle wasting in a mouse model for the study of colorectal cancer cachexia. Dis. Model Mech. 2020. [Google Scholar] [CrossRef] [Green Version]
- Murphy, K.T.; Struk, A.; Malcontenti-Wilson, C.; Christophi, C.; Lynch, G.S. Physiological characterization of a mouse model of cachexia in colorectal liver metastases. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2013, 304, R854–R864. [Google Scholar] [CrossRef] [Green Version]
- Wang, G.; Biswas, A.K.; Ma, W.; Kandpal, M.; Coker, C.; Grandgenett, P.M.; Hollingsworth, M.A.; Jain, R.; Tanji, K.; Lomicronpez-Pintado, S.; et al. Metastatic cancers promote cachexia through ZIP14 upregulation in skeletal muscle. Nat. Med. 2018, 24, 770–781. [Google Scholar] [CrossRef] [PubMed]
- Huot, J.R.; Novinger, L.J.; Pin, F.; Narasimhan, A.; Zimmers, T.A.; O’Connell, T.M.; Bonetto, A. Formation of colorectal liver metastases induces musculoskeletal and metabolic abnormalities consistent with exacerbated cachexia. JCI Insight 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huot, J.R.; Pin, F.; Narasimhan, A.; Novinger, L.J.; Keith, A.S.; Zimmers, T.A.; Willis, M.S.; Bonetto, A. ACVR2B antagonism as a countermeasure to multi-organ perturbations in metastatic colorectal cancer cachexia. J. Cachexia Sarcopenia Muscle 2020, 11, 1779–1798. [Google Scholar] [CrossRef] [PubMed]
- Bae, T.; Jang, J.; Lee, H.; Song, J.; Chae, S.; Park, M.; Son, C.G.; Yoon, S.; Yoon, Y. Paeonia lactiflora root extract suppresses cancer cachexia by down-regulating muscular NF-kappaB signalling and muscle-specific E3 ubiquitin ligases in cancer-bearing mice. J. Ethnopharm. 2020, 246, 112222. [Google Scholar] [CrossRef] [PubMed]
- Schafer, M.; Oeing, C.U.; Rohm, M.; Baysal-Temel, E.; Lehmann, L.H.; Bauer, R.; Volz, H.C.; Boutros, M.; Sohn, D.; Sticht, C.; et al. Ataxin-10 is part of a cachexokine cocktail triggering cardiac metabolic dysfunction in cancer cachexia. Mol. Metab. 2016, 5, 67–78. [Google Scholar] [CrossRef] [PubMed]
- Bonetto, A.; Kays, J.K.; Parker, V.A.; Matthews, R.R.; Barreto, R.; Puppa, M.J.; Kang, K.S.; Carson, J.A.; Guise, T.A.; Mohammad, K.S.; et al. Differential bone loss in mouse models of colon cancer cachexia. Front Physiol. 2016, 7, 679. [Google Scholar] [CrossRef] [Green Version]
- Bonetto, A.; Aydogdu, T.; Jin, X.; Zhang, Z.; Zhan, R.; Puzis, L.; Koniaris, L.G.; Zimmers, T.A. JAK/STAT3 pathway inhibition blocks skeletal muscle wasting downstream of IL-6 and in experimental cancer cachexia. Am. J. Physiol. Endocrinol. Metab. 2012, 303, E410–E421. [Google Scholar] [CrossRef] [Green Version]
- Heichler, C.; Scheibe, K.; Schmied, A.; Geppert, C.I.; Schmid, B.; Wirtz, S.; Thoma, O.M.; Kramer, V.; Waldner, M.J.; Buttner, C.; et al. STAT3 activation through IL-6/IL-11 in cancer-associated fibroblasts promotes colorectal tumour development and correlates with poor prognosis. Gut 2019, 69, 1269–1282. [Google Scholar] [CrossRef]
- Bonetto, A.; Aydogdu, T.; Kunzevitzky, N.; Guttridge, D.C.; Khuri, S.; Koniaris, L.G.; Zimmers, T.A. STAT3 activation in skeletal muscle links muscle wasting and the acute phase response in cancer cachexia. PLoS ONE 2011, 6, e22538. [Google Scholar] [CrossRef] [Green Version]
- Pin, F.; Barreto, R.; Kitase, Y.; Mitra, S.; Erne, C.E.; Novinger, L.J.; Zimmers, T.A.; Couch, M.E.; Bonewald, L.F.; Bonetto, A. Growth of ovarian cancer xenografts causes loss of muscle and bone mass: A new model for the study of cancer cachexia. J. Cachexia Sarcopenia Muscle 2018, 9, 685–700. [Google Scholar] [CrossRef] [Green Version]
- Ruers, T.; Bleichrodt, R.P. Treatment of liver metastases, an update on the possibilities and results. Eur. J. Cancer 2002, 38, 1023–1033. [Google Scholar] [CrossRef]
- Meeske, K.; Smith, A.W.; Alfano, C.M.; McGregor, B.A.; McTiernan, A.; Baumgartner, K.B.; Malone, K.E.; Reeve, B.B.; Ballard-Barbash, R.; Bernstein, L. Fatigue in breast cancer survivors two to five years post diagnosis: A HEAL Study report. Qual Life Res. 2007, 16, 947–960. [Google Scholar] [CrossRef] [PubMed]
- Barton, B.E. IL-6-like cytokines and cancer cachexia: Consequences of chronic inflammation. Immunol. Res. 2001, 23, 41–58. [Google Scholar] [CrossRef]
- Loberg, R.D.; Bradley, D.A.; Tomlins, S.A.; Chinnaiyan, A.M.; Pienta, K.J. The lethal phenotype of cancer: The molecular basis of death due to malignancy. CA Cancer J. Clin. 2007, 57, 225–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tisdale, M.J. Mechanisms of cancer cachexia. Physiol. Rev. 2009, 89, 381–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arora, G.K.; Gupta, A.; Narayanan, S.; Guo, T.; Iyengar, P.; Infante, R.E. Cachexia-associated adipose loss induced by tumor-secreted leukemia inhibitory factor is counterbalanced by decreased leptin. JCI Insight 2018, 3. [Google Scholar] [CrossRef] [Green Version]
- Rohm, M.; Schafer, M.; Laurent, V.; Ustunel, B.E.; Niopek, K.; Algire, C.; Hautzinger, O.; Sijmonsma, T.P.; Zota, A.; Medrikova, D.; et al. An AMP-activated protein kinase-stabilizing peptide ameliorates adipose tissue wasting in cancer cachexia in mice. Nat. Med. 2016, 22, 1120–1130. [Google Scholar] [CrossRef]
- Barreto, R.; Kitase, Y.; Matsumoto, T.; Pin, F.; Colston, K.C.; Couch, K.E.; O’Connell, T.M.; Couch, M.E.; Bonewald, L.F.; Bonetto, A. ACVR2B/Fc counteracts chemotherapy-induced loss of muscle and bone mass. Sci. Rep. 2017, 7, 14470. [Google Scholar] [CrossRef]
- Essex, A.L.; Pin, F.; Huot, J.R.; Bonewald, L.F.; Plotkin, L.I.; Bonetto, A. Bisphosphonate treatment ameliorates chemotherapy-induced bone and muscle abnormalities in young mice. Front Endocrinol. 2019, 10, 809. [Google Scholar] [CrossRef]
- Hain, B.A.; Jude, B.; Xu, H.; Smuin, D.M.; Fox, E.J.; Elfar, J.C.; Waning, D.L. Zoledronic acid improves muscle function in healthy mice treated with chemotherapy. J. Bone. Miner. Res. 2019. [Google Scholar] [CrossRef] [Green Version]
- Barreto, R.; Mandili, G.; Witzmann, F.A.; Novelli, F.; Zimmers, T.A.; Bonetto, A. Cancer and chemotherapy contribute to muscle loss by activating common signaling pathways. Front Physiol. 2016, 7, 472. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barreto, R.; Waning, D.L.; Gao, H.; Liu, Y.; Zimmers, T.A.; Bonetto, A. Chemotherapy-related cachexia is associated with mitochondrial depletion and the activation of ERK1/2 and p38 MAPKs. Oncotarget 2016, 7, 43442–43460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huot, J.R.; Essex, A.L.; Gutierrez, M.; Barreto, R.; Wang, M.; Waning, D.L.; Plotkin, L.I.; Bonetto, A. Chronic treatment with multi-kinase inhibitors causes differential toxicities on skeletal and cardiac muscles. Cancers 2019, 11, 571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouxsein, M.L.; Boyd, S.K.; Christiansen, B.A.; Guldberg, R.E.; Jepsen, K.J.; Muller, R. Guidelines for assessment of bone microstructure in rodents using micro-computed tomography. J. Bone. Miner. Res. 2010, 25, 1468–1486. [Google Scholar] [CrossRef] [PubMed]
- Schneider, C.A.; Rasband, W.S.; Eliceiri, K.W. NIH image to imagej: 25 years of image analysis. Nat. Methods 2012, 9, 671–675. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huot, J.R.; Pin, F.; Essex, A.L.; Bonetto, A. MC38 Tumors Induce Musculoskeletal Defects in Colorectal Cancer. Int. J. Mol. Sci. 2021, 22, 1486. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031486
Huot JR, Pin F, Essex AL, Bonetto A. MC38 Tumors Induce Musculoskeletal Defects in Colorectal Cancer. International Journal of Molecular Sciences. 2021; 22(3):1486. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031486
Chicago/Turabian StyleHuot, Joshua R., Fabrizio Pin, Alyson L. Essex, and Andrea Bonetto. 2021. "MC38 Tumors Induce Musculoskeletal Defects in Colorectal Cancer" International Journal of Molecular Sciences 22, no. 3: 1486. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031486