Potential Effects of Nonadherent on Adherent Human Umbilical Venous Endothelial Cells in Cell Culture
Abstract
:1. Introduction
2. Results
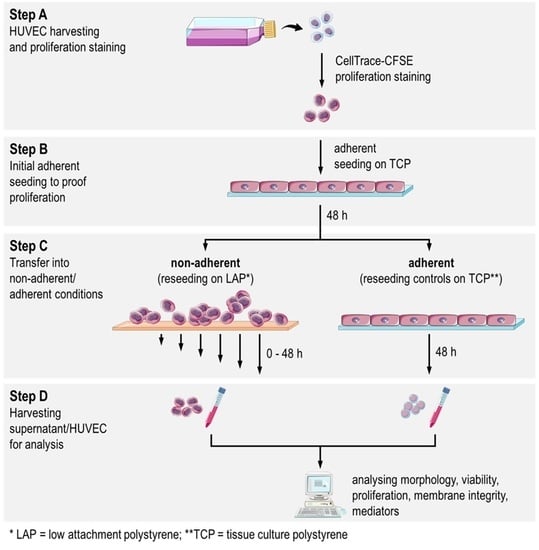
2.1. Study Design
2.2. HUVEC Adhere and Proliferate on TCP
2.3. Decrease of Proliferation Rate and High Fraction of Dead HUVEC at Nonadherent Conditions
2.4. Decreasing Cell Membrane Integrity and Accumulative Mediator Secretion of Nonadherent HUVEC
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Preliminary Study: HUVEC Adherence on TCP
4.3. Studies with Nonadherent HUVEC
4.4. Proliferation and Apoptosis/Cell Death Analyzed by Flow Cytometry and Fluorescence Microscopy
4.5. Membrane Integrity and Secretion Profile of HUVEC
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| HUVEC | Human umbilical vein endothelial cells |
| TCP | Tissue culture plates |
| LAP | Low attachment plates |
| RFU | Relative fluorescence units |
| FBS | Fetale bovine serum |
| vWF | von Willebrand factor |
| rhTNF-α | recombinant human TNF-α |
| DAPI | 6-diamidino-2-phenylindole |
| LDH | Lactate dehydrogenase |
| PGI2 | Prostacyclin |
| TXA2 | Thromboxane A2 |
| IL-1ra | Interleukin 1 receptor antagonist |
| IL-6 | Interleukin 6 |
| IL-8 | Interleukin 8 |
| IL-12 | Interleukin 12 |
| G-CSF | Granulocyte-colony stimulating factor |
| GM-CSF | Granulocyte-monocyte-colony stimulating factor |
| IFN-γ | Interferone-γ |
| MCP-1 (CCL-2) | Monocyte chemotactic protein-1 (CC-chemokine-ligand-2) |
| PDGF-BB | Platelet-derived growth factor BB |
| VEGF | Vascular endothelial growth factor |
References
- Garg, S.; Bourantas, C.; Serruys, P.W. New concepts in the design of drug-eluting coronary stents. Nat. Rev. Cardiol. 2013, 10, 248. [Google Scholar] [CrossRef]
- Spaulding, C.; Daemen, J.; Boersma, E.; Cutlip, D.E.; Serruys, P.W. A pooled analysis of data comparing sirolimus-eluting stents with bare-metal stents. N. Engl. J. Med. 2007, 356, 989–997. [Google Scholar] [CrossRef] [Green Version]
- Locker, C.; Schaff, H.V.; Dearani, J.A.; Daly, R.C. Improved late survival with arterial revascularization. Ann. Cardiothorac. Surg. 2013, 2, 467–474. [Google Scholar] [PubMed]
- Zilla, P.; Bezuidenhout, D.; Human, P. Prosthetic vascular grafts: Wrong models, wrong questions and no healing. Biomaterials 2007, 28, 5009–5027. [Google Scholar] [CrossRef] [PubMed]
- Jung, F.; Wischke, C.; Lendlein, A. Degradable, multifunctional cardiovascular implants: Challenges and hurdles. MRS Bull. 2010, 35, 607–613. [Google Scholar] [CrossRef]
- Jaffer, I.; Fredenburgh, J.; Hirsh, J.; Weitz, J. Medical device-induced thrombosis: What causes it and how can we prevent it? J. Thromb. Haemost. 2015, 13, S72–S81. [Google Scholar] [CrossRef]
- Kannan, R.Y.; Salacinski, H.J.; Butler, P.E.; Hamilton, G.; Seifalian, A.M. Current status of prosthetic bypass grafts: A review. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2005, 74, 570–581. [Google Scholar] [CrossRef]
- Liu, X.; Yuan, L.; Li, D.; Tang, Z.; Wang, Y.; Chen, G.; Chen, H.; Brash, J.L. Blood compatible materials: State of the art. J. Mater. Chem. B 2014, 2, 5718–5738. [Google Scholar] [CrossRef]
- Braune, S.; Latour, R.A.; Reinthaler, M.; Landmesser, U.; Lendlein, A.; Jung, F. In Vitro Thrombogenicity Testing of Biomaterials. Adv. Healthc. Mater. 2019, 8, 1900527. [Google Scholar] [CrossRef] [Green Version]
- Melchiorri, A.; Hibino, N.; Yi, T.; Lee, Y.; Sugiura, T.; Tara, S.; Shinoka, T.; Breuer, C.; Fisher, J. Contrasting biofunctionalization strategies for the enhanced endothelialization of biodegradable vascular grafts. Biomacromolecules 2015, 16, 437–446. [Google Scholar] [CrossRef]
- Liu, R.H.; Ong, C.S.; Fukunishi, T.; Ong, K.; Hibino, N. Review of vascular graft studies in large animal models. Tissue Eng. Part B: Rev. 2018, 24, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Krüger-Genge, A.; Blocki, A.; Franke, R.-P.; Jung, F. Vascular Endothelial Cell Biology: An Update. Int. J. Mol. Sci. 2019, 20, 4411. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gori, T. Endothelial function: A short guide for the interventional cardiologist. Int. J. Mol. Sci. 2018, 19, 3838. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- ISO 10993-1:2018 Technical Committee ISO/TC 194. Biological Evaluation of Medical Devices—Part 1: Evaluation and Testing within a Risk Management Process; ISO: Geneva, Switzerland, 2018. [Google Scholar]
- Schulz, C.; von Rüsten-Lange, M.; Krueger, A.; Lendlein, A.; Jung, F. Viability and function of primary human endothelial cells on smooth poly(ether imide) films. Clin. Hemorheol. Microcirc. 2012, 52, 267–282. [Google Scholar] [CrossRef] [Green Version]
- Salacinski, H.; Tiwari, A.; Hamilton, G.; Seifalian, A. Cellular engineering of vascular bypass grafts: Role of chemical coatings for enhancing endothelial cell attachment. Med. Biol. Eng. Comput. 2001, 39, 609–618. [Google Scholar] [CrossRef]
- Hauser, S.; Jung, F.; Pietzsch, J. Human endothelial cell models in biomaterial research. Trends Biotechnol. 2017, 35, 265–277. [Google Scholar] [CrossRef]
- Ingber, D. In Extracellular matrix as a solid-state regulator in angiogenesis: Identification of new targets for anti-cancer therapy. Semin. Cancer Biol. 1992, 3, 57–63. [Google Scholar]
- Ingber, D.E. Fibronectin controls capillary endothelial cell growth by modulating cell shape. Proc. Natl. Acad. Sci. USA 1990, 87, 3579–3583. [Google Scholar] [CrossRef] [Green Version]
- Meredith, J., Jr.; Fazeli, B.; Schwartz, M. The extracellular matrix as a cell survival factor. Mol. Biol. Cell 1993, 4, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Michel, J.-B. Anoïkis in the Cardiovascular System. Arterioscler. Thromb. Vasc. Biol. 2003, 23, 2146–2154. [Google Scholar] [CrossRef] [Green Version]
- Laplante, P.; Sirois, I.; Raymond, M.A.; Kokta, V.; Béliveau, A.; Prat, A.; Pshezhetsky, A.V.; Hébert, M.J. Caspase-3-mediated secretion of connective tissue growth factor by apoptotic endothelial cells promotes fibrosis. Cell Death Differ. 2010, 17, 291–303. [Google Scholar] [CrossRef] [PubMed]
- Chavakis, E.; Dimmeler, S. Regulation of endothelial cell survival and apoptosis during angiogenesis. Arterioscler. Thromb. Vasc. Biol. 2002, 22, 887–893. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Solovyan, V.T.; Keski-Oja, J. Apoptosis of human endothelial cells is accompanied by proteolytic processing of latent TGF-beta binding proteins and activation of TGF-beta. Cell Death Differ. 2005, 12, 815–826. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.-P.; Audemard, É.; Migneault, F.; Feghaly, A.; Brochu, S.; Gendron, P.; Boilard, É.; Major, F.; Dieudé, M.; Hébert, M.-J.; et al. Apoptotic endothelial cells release small extracellular vesicles loaded with immunostimulatory viral-like RNAs. Sci. Rep. 2019, 9, 7203. [Google Scholar] [CrossRef] [Green Version]
- Zernecke, A.; Bidzhekov, K.; Noels, H.; Shagdarsuren, E.; Gan, L.; Denecke, B.; Hristov, M.; Köppel, T.; Jahantigh, M.N.; Lutgens, E.; et al. Delivery of microRNA-126 by apoptotic bodies induces CXCL12-dependent vascular protection. Sci. Signal. 2009, 2, ra81. [Google Scholar] [CrossRef]
- Sarkar, S.; Sales, K.M.; Hamilton, G.; Seifalian, A.M. Addressing thrombogenicity in vascular graft construction. J. Biomed. Mater. Res. Part B: Appl. Biomater. 2007, 82, 100–108. [Google Scholar] [CrossRef]
- Flentje, A.; Kalsi, R.; Monahan, T.S. Small GTPases and their role in vascular disease. Int. J. Mol. Sci. 2019, 20, 917. [Google Scholar] [CrossRef] [Green Version]
- Zwaginga, J.J.; de Boer, H.C.; IJsseldijk, M.; Kerkhof, A.; Muller-Berghaus, G.; Gruhlichhenn, J.; Sixma, J.J.; de Groot, P.G. Thrombogenicity of vascular cells. Comparison between endothelial cells isolated from different sources and smooth muscle cells and fibroblasts. Arteriosclerosis 1990, 10, 437–448. [Google Scholar] [CrossRef] [Green Version]
- Hoepken, S.; Fuhrmann, R.; Jung, F.; Franke, R. Shear resistance of human umbilical endothelial cells on different materials covered with or without extracellular matrix: Controlled in-vitro study. Clin. Hemorheol. Microcirc. 2009, 43, 157–166. [Google Scholar] [CrossRef]
- Re, F.; Zanetti, A.; Sironi, M.; Polentarutti, N.; Lanfrancone, L.; Dejana, E.; Colotta, F. Inhibition of anchorage-dependent cell spreading triggers apoptosis in cultured human endothelial cells. J. Cell Biol. 1994, 127, 537–546. [Google Scholar] [CrossRef] [Green Version]
- Webb, S.J.; Harrison, D.J.; Wyllie, A.H. Apoptosis: An overview of the process and its relevance in disease. In Advances in Pharmacology; Elsevier: Amsterdam, The Netherlands, 1997; Volume 41, pp. 1–34. [Google Scholar]
- Elmore, S. Apoptosis: A review of programmed cell death. Toxicol. Pathol. 2007, 35, 495–516. [Google Scholar] [CrossRef] [PubMed]
- Markiewicz, M.; Richard, E.; Marks, N.; Ludwicka-Bradley, A. Impact of endothelial microparticles on coagulation, inflammation, and angiogenesis in age-related vascular diseases. J. Aging Res. 2013, 2013, 1–11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Colman, R.W. Biologic activities of the contact factors in vivo. Thromb. Haemost. 1999, 82, 1568–1577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jimenez, J.; Mauro, L.; Horstman, L.; Cheng, P.; Ahn, E.; Bidot, C.; Ahn, Y. Endothelial microparticles induce formation of platelet aggregates via a von Willebrand factor/ristocetin dependent pathway, rendering them resistant to dissociation. J. Thromb. Haemost. 2005, 3, 1301–1308. [Google Scholar]
- Jimenez, J.J.; Jy, W.; Mauro, L.M.; Soderland, C.; Horstman, L.L.; Ahn, Y.S. Endothelial cells release phenotypically and quantitatively distinct microparticles in activation and apoptosis. Thromb. Res. 2003, 109, 175–180. [Google Scholar] [CrossRef]
- Pallet, N.; Sirois, I.; Bell, C.; Hanafi, L.A.; Hamelin, K.; Dieudé, M.; Rondeau, C.; Thibault, P.; Desjardins, M.; Hebert, M.J. A comprehensive characterization of membrane vesicles released by autophagic human endothelial cells. Proteomics 2013, 13, 1108–1120. [Google Scholar] [CrossRef] [PubMed]
- De Caterina, R.; Dorso, C.R.; Tack-Goldman, K.; Weksler, B. Nitrates and endothelial prostacyclin production: Studies in vitro. Circulation 1985, 71, 176–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Franke, R.; Fuhrmann, R.; Schnittler, H.; Petrow, W.; Simons, G. Humane Endothelzellen in vitro unter hydrodynamischer Scherbelastung: Pharmakologische Einflüsse auf Haftfähigkeit und Nonthrombogenität der Gefäßinnenwandzellen. VASA 1988, 24, 11–16. [Google Scholar]
- Buchanan, F.G.; Chang, W.; Sheng, H.; Shao, J.; Morrow, J.D.; DuBois, R.N. Up-regulation of the enzymes involved in prostacyclin synthesis via Ras induces vascular endothelial growth factor. Gastroenterology 2004, 127, 1391–1400. [Google Scholar] [CrossRef]
- Cai, J.; Jiang, W.G.; Ahmed, A.; Boulton, M. Vascular endothelial growth factor-induced endothelial cell proliferation is regulated by interaction between VEGFR-2, SH-PTP1 and eNOS. Microvasc. Res. 2006, 71, 20–31. [Google Scholar] [CrossRef]
- Battegay, E.J.; Rupp, J.; Iruela-Arispe, L.; Sage, E.H.; Pech, M. PDGF-BB modulates endothelial proliferation and angiogenesis in vitro via PDGF beta-receptors. J. Cell Biol. 1994, 125, 917–928. [Google Scholar] [CrossRef] [PubMed]
- Au, P.; Tam, J.; Duda, D.G.; Lin, P.-C.; Munn, L.L.; Fukumura, D.; Jain, R.K. Paradoxical effects of PDGF-BB overexpression in endothelial cells on engineered blood vessels in vivo. Am. J. Pathol. 2009, 175, 294–302. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Papadopoulos, N.; Lennartsson, J. The PDGF/PDGFR pathway as a drug target. Mol. Asp. Med. 2018, 62, 75–88. [Google Scholar] [CrossRef]
- Chu, L.-y.; Liou, J.-Y.; Wu, K.K. Prostacyclin protects vascular integrity via PPAR/14-3-3 pathway. Prostaglandins Other Lipid Mediat. 2015, 118, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Waugh, D.J.; Wilson, C. The interleukin-8 pathway in cancer. Clin. Cancer Res. 2008, 14, 6735–6741. [Google Scholar] [CrossRef] [Green Version]
- Salcedo, R.; Ponce, M.L.; Young, H.A.; Wasserman, K.; Ward, J.M.; Kleinman, H.K.; Oppenheim, J.J.; Murphy, W.J. Human endothelial cells express CCR2 and respond to MCP-1: Direct role of MCP-1 in angiogenesis and tumor progression. Blood 2000, 96, 34–40. [Google Scholar] [CrossRef]
- Krüger, A.; Fuhrmann, R.; Jung, F.; Franke, R. Influence of the coating with extracellular matrix and the number of cell passages on the endothelialization of a polystyrene surface. Clin. Hemorheol. Microcirc. 2015, 60, 153–161. [Google Scholar] [CrossRef]
- Krueger-Genge, A.; Schulz, C.; Kratz, K.; Lendlein, A.; Jung, F. Comparison of two substrate materials used as negative control in endothelialization studies: Glass versus polymeric tissue culture plate. Clin. Hemorheol. Microcirc. 2018, 69, 437–445. [Google Scholar] [CrossRef]
- Milo, R.; Jorgensen, P.; Moran, U.; Weber, G.; Springer, M. BioNumbers—the database of key numbers in molecular and cell biology. Nucleic Acids Res. 2009, 38 (Suppl. S1), D750–D753. [Google Scholar] [CrossRef] [Green Version]








Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schulz, C.; Krüger-Genge, A.; Lendlein, A.; Küpper, J.-H.; Jung, F. Potential Effects of Nonadherent on Adherent Human Umbilical Venous Endothelial Cells in Cell Culture. Int. J. Mol. Sci. 2021, 22, 1493. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031493
Schulz C, Krüger-Genge A, Lendlein A, Küpper J-H, Jung F. Potential Effects of Nonadherent on Adherent Human Umbilical Venous Endothelial Cells in Cell Culture. International Journal of Molecular Sciences. 2021; 22(3):1493. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031493
Chicago/Turabian StyleSchulz, Christian, Anne Krüger-Genge, Andreas Lendlein, Jan-Heiner Küpper, and Friedrich Jung. 2021. "Potential Effects of Nonadherent on Adherent Human Umbilical Venous Endothelial Cells in Cell Culture" International Journal of Molecular Sciences 22, no. 3: 1493. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22031493