HGF and TSG-6 Released by Mesenchymal Stem Cells Attenuate Colon Radiation-Induced Fibrosis
Abstract
:1. Introduction
2. Results
2.1. In Vivo Model of Colorectal Irradiation-Induced Fibrosis
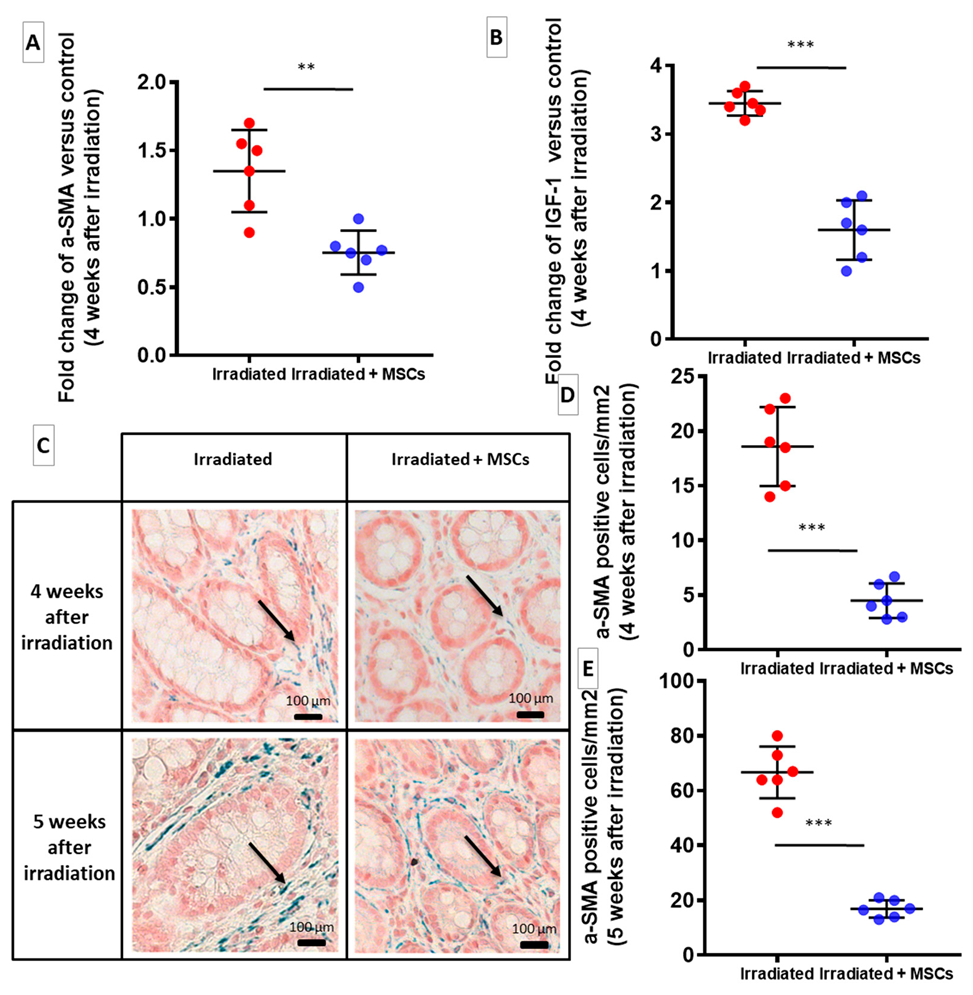
2.2. MSCs Inhibit Fibrogenesis Following Colorectal Irradiation
2.3. MSCs Act Directly on Fibroblasts and SMCs to Inhibit Their Pro-Fibrotic Phenotype
2.4. MSCs Limit Proliferation of ECM-Producing Cells
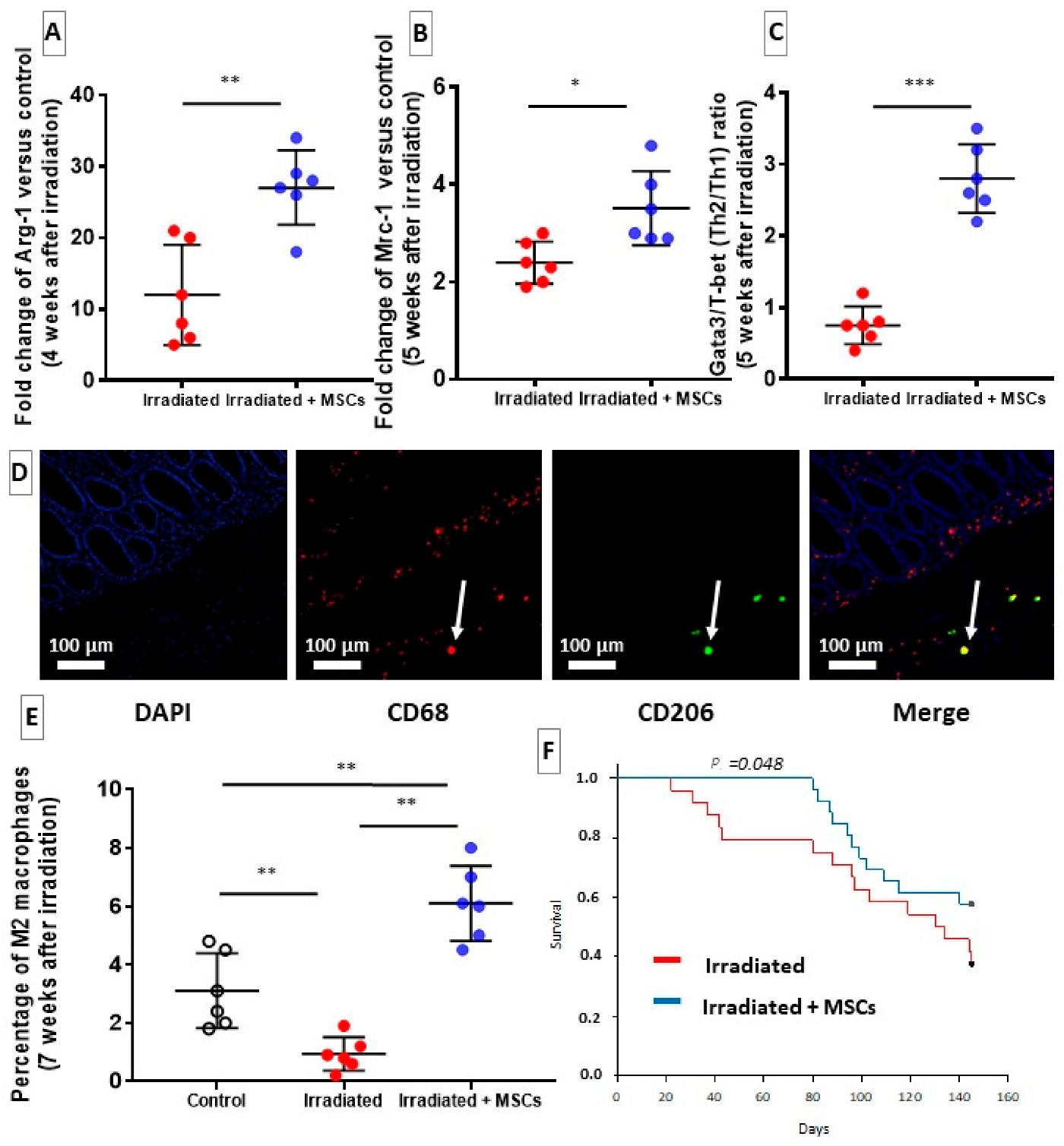
2.5. MSCs Delay Fibrosis by Favoring Th2/M2 Response
2.6. HGF Silencing in Transplanted-MSCs May Promotes Fibrosis through a TGF-β-Dependent Mechanism
2.7. TSG-6 May Inhibit Fibrosis by Deregulating Macrophage Polarization
3. Discussion
4. Materials and Methods
4.1. In Vitro Experiments
4.2. RNA Interference against HGF and TSG-6
4.3. In Vivo Experiments
4.4. Isolation, Characterization and Culture of MSCs
4.5. Configuration of Irradiation, MSC Treatment and Tissue Sampling
4.6. RNA Isolation, Reverse Transcription, Quantitative Real-Time PCR, and Array Analysis
4.7. Protein Extraction, Quantification and ELISAs
4.8. Histology and Immunohistochemistry (IHC)
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| Arg-2 | Arginine 2 |
| CD | Crohn’s Disease |
| CFU-F | Colony-Forming Unit-Fibroblasts |
| CTGF | Connective Tissue Growth Factor |
| DAPI | 4′,6-diamidino-2-phenylindole |
| DOAJ | Directory of Open Access Journals |
| ECM | Extra Cellular Matrix |
| FBS | Foetal Bovine Serum |
| FGM | Fibroblasts Growth Medium |
| GAPDH | Glyceraldehyde-3-phosphate dehydrogenase |
| GvHD | Graft-versus-Host Disease |
| hCoSMC | human Colonic Smooth Muscle Cells |
| HES | Hematoxylin-Eosin-Safran |
| HGF | Hepatocyt Growth Factor |
| hIF | human Intestinal Fibroblasts |
| IBD | Intestinal Bowel Diseases |
| IGF | Insulin-like Growth Factor |
| LD | Linear Dichroism |
| MDPI | Multidisciplinary Digital Publishing Institute |
| MMP | Matrix MetalloProteinases |
| MRC | Mannose Receptor |
| MSC | Mesenchymal stem cell |
| NOS-2 | Nitric Oxide Synthase 2 |
| PAI-1 | Plasminogen Activator Inhibitor 1 |
| PBS | Phosphate Buffered Saline |
| PD-L1 | Programmed Cell Death Ligand-1 |
| NTRK | Neurotrophin Tyrosine Kinase Receptor |
| PRD | Pelvic Radiotherapy Disease |
| SERPINE -1 | Serpin Family E Member 1 |
| SERPINH 1 | Serpin Family H Member 1 |
| SMAD | Small Mother Against Decapentaplegic |
| SMC | Smooth Muscle Cells |
| SPARC | Secreted Pprotein Aacidic and Rrich in Ccysteine |
| TGF-β | Transforming Growth Factor β |
| Th | T-helper |
| TIMP | Tissue Inhibitors of Metalloproteinases |
| TLA | Three Letter Acronym |
| TSG-6 | Tumor Necrosis Factor-stimulated gene 6 |
| UC | Ulcerative Colitis |
| α-SMA | α-Smooth Muscle Actin |
References
- Usunier, B.; Benderitter, M.; Tamarat, R.; Chapel, A. Management of Fibrosis: The Mesenchymal Stromal Cells Breakthrough. Stem Cells Int. 2014, 2014, 340257. [Google Scholar] [CrossRef] [Green Version]
- Haydont, V.; Vozenin-Brotons, M.C. Maintenance of radiation-induced intestinal fibrosis: Cellular and molecular features. World J. Gastroenterol. 2007, 13, 2675–2683. [Google Scholar] [CrossRef] [Green Version]
- Graham, M.F.; Bryson, G.R.; Diegelmann, R.F. Transforming growth factor beta 1 selectively augments collagen synthesis by human intestinal smooth muscle cells. Gastroenterology 1990, 99, 447–453. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-beta: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Strup-Perrot, C.; Mathe, D.; Linard, C.; Violot, M.; Milliat, F.; François, A.; Bourhis, J.; Vozenin-Brotons, M.-C. Global gene expression profiles reveal an increase in mRNA levels of collagens, MMPs, and TIMPs in late radiation enteritis. Am. J. Physiol. Liver Physiol. 2004, 287, G875–G885. [Google Scholar] [CrossRef]
- François, A.; Milliat, F.; Guipaud, O.; Benderitter, M. Inflammation and Immunity in Radiation Damage to the Gut Mucosa. Biomed. Res. Int. 2013, 2013, 123241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- The Global Cancer Observatory: International Agency for Research on Cancer: World Health Organization 2020 (Globocan). Number of New Cases in 2020 Both Sexes, all Ages and Number of Deaths in 2020, Both Sexes, All Ages. November 2020. Available online: https://gco.iarc.fr/today/data/factsheets/populations/900-world-fact-sheets.pdf (accessed on 25 January 2021).
- Breen, W.G.; Leventakos, K.; Dong, H.; Merrell, K.W. Radiation and immunotherapy: Emerging mechanisms of synergy. J. Thorac. Dis. 2020, 12, 7011–7023. [Google Scholar] [CrossRef] [PubMed]
- Hauer-Jensen, M.; Denham, J.W.; Andreyev, H.J.N. Radiation enteropathy—Pathogenesis, treatment and prevention. Nat. Rev. Gastroenterol. Hepatol. 2014, 11, 470–479. [Google Scholar] [CrossRef] [PubMed]
- Walters, J.R.; Wotherspoon, A.; Denham, J.W.; Hauer-Jensen, M. “Pelvic radiation disease”: New understanding and new solutions for a new disease in the era of cancer survivorship. Scand. J. Gastroenterol. 2010, 46, 389–397. [Google Scholar]
- Qi, Y.; Jiang, D.; Sindrilaru, A.; Stegemann, A.; Schatz, S.; Treiber, N.; Rojewski, M.; Schrezenmeier, H.; Beken, S.V.; Wlaschek, M.; et al. TSG-6 Released from Intradermally Injected Mesenchymal Stem Cells Accelerates Wound Healing and Reduces Tissue Fibrosis in Murine Full-Thickness Skin Wounds. J. Investig. Derm. 2014, 134, 526–537. [Google Scholar] [CrossRef] [Green Version]
- Wu, H.; Yiu, W.H.; Li, R.X.; Wong, D.W.L.; Leung, J.C.K.; Chan, L.Y.Y.; Zhang, Y.; Lian, Q.; Lin, M.; Tse, H.-F.; et al. Mesenchymal Stem Cells Modulate Albumin-Induced Renal Tubular Inflammation and Fibrosis. PLoS ONE 2014, 9, e90883. [Google Scholar] [CrossRef] [Green Version]
- Linard, C.; Busson, E.; Holler, V.; Strup-Perrot, C.; Lacave-Lapalun, J.-V.; Lhomme, B.; Prat, M.; Devauchelle, P.; Sabourin, J.-C.; Simon, J.-M.; et al. Repeated Autologous Bone Marrow-Derived Mesenchymal Stem Cell Injections Improve Radiation-Induced Proctitis in Pigs. Stem Cells Transl. Med. 2013, 2, 916–927. [Google Scholar] [CrossRef] [PubMed]
- Bessout, R.; Demarquay, C.; Moussa, L.; René, A.; Doix, B.; Benderitter, M.; Sémont, A.; Mathieu, N. TH17 predominant T-cell responses in radiation-induced bowel disease are modulated by treatment with adipose-derived mesenchymal stromal cells. J. Pathol. 2015, 237, 435–446. [Google Scholar] [CrossRef] [PubMed]
- Moussa, L.; Usunier, B.; Demarquay, C.; Benderitter, M.; Tamarat, R.; Sémont, A.; Mathieu, N. Bowel Radiation Injury: Complexity of the Pathophysiology and Promises of Cell and Tissue Engineering. Cell Transpl. 2016, 25, 1723–1746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Durand, C.; Pezet, S.; Eutamène, H.; Demarquay, C.; Mathieu, N.; Moussa, L.; Daudin, R.; Holler, V.; Sabourin, J.-C.; Milliat, F.; et al. Persistent visceral allodynia in rats exposed to colorectal irradiation is reversed by mesenchymal stromal cell treatment. Pain 2015, 156, 1465–1476. [Google Scholar] [CrossRef]
- émont, A.; Demarquay, C.; Bessout, R.; Durand, C.; Benderitter, M.; Mathieu, N. Mesenchymal Stem Cell Therapy Stimulates Endogenous Host Progenitor Cells to Improve Colonic Epithelial Regeneration. PLoS ONE 2013, 8, e70170. [Google Scholar]
- Bessout, R.; Semont, A.; Demarquay, C.; Charcosset, A.; Benderitter, M.; Mathieu, N. Mesenchymal stem cell therapy induces glucocorticoid synthesis in colonic mucosa and suppresses radiation-activated T cells: New insights into MSC immunomodulation. Mucosal Immunol. 2014, 7, 656–669. [Google Scholar] [CrossRef]
- Munneke, J.M.; Spruit, M.J.; Cornelissen, A.S.; van Hoeven, V.; Voermans, C.; Hazenberg, M.D. The Potential of Mesenchymal Stromal Cells as Treatment for Severe Steroid-Refractory Acute Graft-Versus-Host Disease: A Critical Review of the Literature. Transplantation 2016, 100, 2309–2314. [Google Scholar] [CrossRef]
- Tamarat, R.; Lataillade, J.J.; Bey, E.; Gourmelon, P.; Benderitter, M. Stem cell therapy: From bench to bedside. Radiat. Prot. Dosim. 2012, 151, 633–639. [Google Scholar] [CrossRef]
- Gazdhar, A.; Susuri, N.; Hostettler, K.K.; Gugger, M.; Knudsen, L.; Rotha, M.; Ochs, M.; Geiser, T. HGF Expressing Stem Cells in Usual Interstitial Pneumonia Originate from the Bone Marrow and Are Antifibrotic. PLoS ONE 2013, 8, e65453. [Google Scholar] [CrossRef] [Green Version]
- Seo, K.-W.; Sohn, S.-Y.; Bhang, D.-H.; Nam, M.-J.; Lee, H.-W.; Youn, H. Therapeutic effects of hepatocyte growth factor-overexpressing human umbilical cord blood-derived mesenchymal stem cells on liver fibrosis in rats. Cell Biol. Int. 2013, 38, 106–116. [Google Scholar] [CrossRef] [PubMed]
- Lai, L.; Chen, J.; Wei, X.; Huang, M.; Hu, X.; Yang, R.; Jiang, X.; Shan, H. Transplantation of MSCs Overexpressing HGF into a Rat Model of Liver Fibrosis. Mol. Imaging Biol. 2015, 18, 43–51. [Google Scholar] [CrossRef] [PubMed]
- Vozenin-Brotons, M.C.; Milliat, F.; Sabourin, J.C.; de Gouville, A.C.; Francois, A.; Lasser, P.; Morice, P.; Haie-Meder, C.; Lusinchi, A.; Antoun, S.; et al. Fibrogenic signals in patients with radiation enteritis are associated with increased connective tissue growth factor expression. Int. J. Radiat. Oncol. Biol. Phys. 2003, 56, 561–572. [Google Scholar] [CrossRef]
- Sanggaard, K.W.; Scavenius, C.; Rasmussen, A.J.; Wisniewski, H.G.; Thogersen, I.B.; Enghild, J.J. The TSG-6/HC2-mediated transfer is a dynamic process shuffling heavy chains between glycosaminoglycans. J. Biol. Chem. 2010, 285, 21988–21993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tolg, C.; Hamilton, S.R.; Zalinska, E.; McCulloch, L.; Amin, R.; Akentieva, N.; Winnik, F.; Savani, R.; Bagli, D.J.; Luyt, L.G.; et al. A RHAMM mimetic peptide blocks hyaluronan signaling and reduces inflammation and fibrogenesis in excisional skin wounds. Am. J. Pathol. 2012, 181, 1250–1270. [Google Scholar] [CrossRef] [PubMed] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Usunier, B.; Brossard, C.; L’Homme, B.; Linard, C.; Benderitter, M.; Milliat, F.; Chapel, A. HGF and TSG-6 Released by Mesenchymal Stem Cells Attenuate Colon Radiation-Induced Fibrosis. Int. J. Mol. Sci. 2021, 22, 1790. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22041790
Usunier B, Brossard C, L’Homme B, Linard C, Benderitter M, Milliat F, Chapel A. HGF and TSG-6 Released by Mesenchymal Stem Cells Attenuate Colon Radiation-Induced Fibrosis. International Journal of Molecular Sciences. 2021; 22(4):1790. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22041790
Chicago/Turabian StyleUsunier, Benoît, Clément Brossard, Bruno L’Homme, Christine Linard, Marc Benderitter, Fabien Milliat, and Alain Chapel. 2021. "HGF and TSG-6 Released by Mesenchymal Stem Cells Attenuate Colon Radiation-Induced Fibrosis" International Journal of Molecular Sciences 22, no. 4: 1790. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22041790





