Effect of Hofmeister Ions on Transport Properties of Aqueous Solutions of Sodium Hyaluronate
Abstract
:1. Introduction
2. Results and Discussion
2.1. Apparent Tracer Diffusion Coefficients of Sodium Hyaluronate
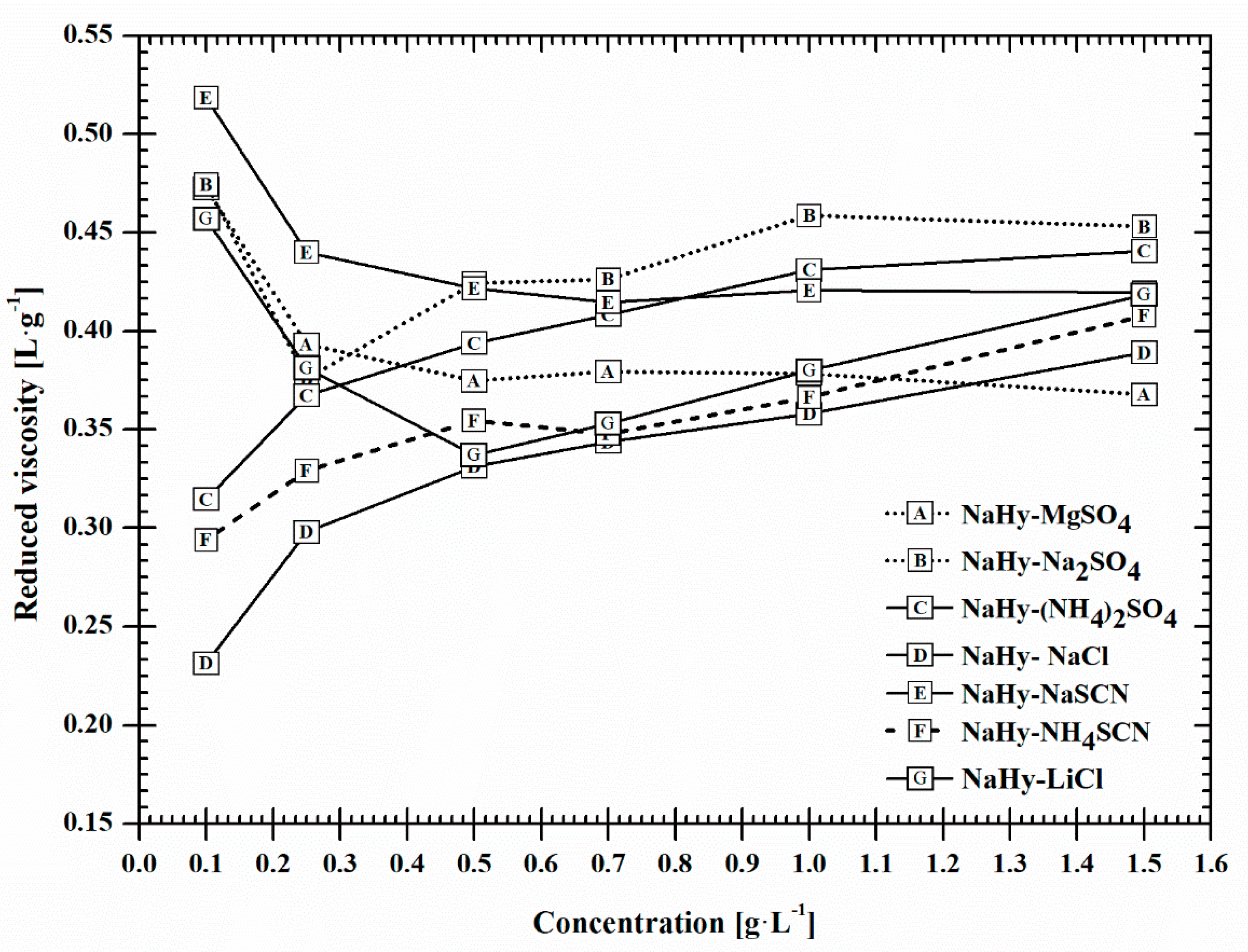
2.2. Viscosity
3. Materials and Methods
3.1. Materials
3.2. Sample Preparation for Viscosity Measuring
3.3. Measurements of Diffusion Coefficients Using Taylor Technique
3.3.1. Brief Description about Some Concepts on Diffusion
3.3.2. The Taylor Technique: Binary Diffusion
3.3.3. The Taylor Technique: Ternary Diffusion
3.3.4. Tracer and Apparent Tracer Diffusion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Meyer, K.; Palmer, J. The polysaccharide of the vitreous humor. J. Biol. Chem. 1934, 107, 629–640. [Google Scholar] [CrossRef]
- Chemistry and Biology of Hyaluronan; Garg, H.; Hales, C. (Eds.) Elsevier Ltd.: Amsterdam, The Netherlands, 2004; ISBN 9780080443829. [Google Scholar]
- McDonald, J.N.; Levick, J.R. Effect of intra-articular hyaluronan on pressure-flow relation across synovium in anaesthetized rabbits. J. Physiol. 1995, 485, 179–193. [Google Scholar] [CrossRef] [Green Version]
- Coleman, P.J.; Scott, D.; Mason, R.M.; Levick, J.R. Characterization of the effect of high molecular weight hyaluronan on trans-synovial flow in rabbit knees. J. Physiol. 1999, 514, 265–282. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Kim, J.S.; Kim, W.K.; Lee, W.; Kim, N.; Song, C.U.; Jung, J.J.; Song, J.E.; Khang, G. Evaluation of Hyaluronic Acid/Agarose Hydrogel for Cartilage Tissue Engineering Biomaterial. Macromol. Res. 2020, 28, 979–985. [Google Scholar] [CrossRef]
- Huynh, A.; Priefer, R. Hyaluronic acid applications in ophthalmology, rheumatology, and dermatology. Carbohydr. Res. 2020, 489, 107950. [Google Scholar] [CrossRef]
- Azevedo, E.F.G.; Azevedo, M.L.G.; Ribeiro, A.C.F.; Mráček, A.; Gřundĕlová, L.; Minařík, A. Hyaluronic acid transport properties and its medical applications in voice disorders. In Innovations in Physical Chemistry: Monograph Serie, Engineering Technology and Industrial Chemistry with Applications; Hagui, R., Torrens, F., Eds.; Apple Academic Press: Palm Bay, FL, USA, 2018; ISBN 9781771886376. [Google Scholar]
- Lavrador, P.; Esteves, M.R.; Gaspar, V.M.; Mano, J.F. Stimuli-Responsive Nanocomposite Hydrogels for Biomedical Applications. Adv. Funct. Mater. 2020, 2020, 2005941. [Google Scholar] [CrossRef]
- Sionkowska, A.; Gadomska, M.; Musiał, K.; Piątek, J. Hyaluronic Acid as a Component of Natural Polymer Blends for Biomedical Applications: A Review. Molecules 2020, 25, 4035. [Google Scholar] [CrossRef]
- Zhong, W.; Pang, L.; Feng, H.; Dong, H.; Wang, S.; Cong, H.; Shen, Y.; Bing, Y. Recent advantage of hyaluronic acid for anti-cancer application: A review of “3S” transition approach. Carbohydr. Polym. 2020, 238, 116204. [Google Scholar] [CrossRef] [PubMed]
- Bayer, I.S. Hyaluronic Acid and Controlled Release: A Review. Molecules 2020, 25, 2649. [Google Scholar] [CrossRef]
- Vasvani, S.; Kulkarni, P.; Rawtani, D. Hyaluronic acid: A review on its biology, aspects of drug delivery, route of administrations and a special emphasis on its approved marketed products and recent clinical studies. Int. J. Biol. Macromol. 2020, 151, 1012–1029. [Google Scholar] [CrossRef]
- Später, T.; Mariyanats, A.O.; Syachina, M.A.; Mironov, A.V.; Savelyev, A.G.; Sochilina, A.V.; Menger, M.D.; Vishnyakova, P.A.; Kananykhina, E.Y.; Fatkhudinov, T.K.; et al. In Vitro and in Vivo Analysis of Adhesive, Anti-Inflammatory, and Proangiogenic Properties of Novel 3D Printed Hyaluronic Acid Glycidyl Methacrylate Hydrogel Scaffolds for Tissue Engineering. ACS Biomater. Sci. Eng. 2020, 6, 5744–5757. [Google Scholar] [CrossRef] [PubMed]
- Maiz-Fernández, S.; Pérez-Álvarez, L.; Ruiz-Rubio, L.; Vilas-Vilela, J.L.; Lanceros-Mendez, S. Polysaccharide-Based In Situ Self-Healing Hydrogels for Tissue Engineering Applications. Polymers 2020, 12, 2261. [Google Scholar] [CrossRef] [PubMed]
- Sionkowska, A.; Adamiak, K.; Musiał, K.; Gadomska, M. Collagen Based Materials in Cosmetic Applications: A Review. Materials 2020, 13, 4217. [Google Scholar] [CrossRef]
- Bhattacharya, S.; Shunmugam, R. Polymer based gels and their applications in remediation of dyes from textile effluents. J. Macromol. Sci. Part. A 2020, 57, 906–926. [Google Scholar] [CrossRef]
- Veríssimo, L.M.P.; Valada, T.I.C.; Sobral, A.J.F.N.; Azevedo, E.E.F.G.; Azevedo, M.L.G.; Ribeiro, A.C.F. Mutual diffusion of sodium hyaluranate in aqueous solutions. J. Chem. Thermodyn. 2014, 71, 14–18. [Google Scholar] [CrossRef]
- Mráček, A.; Gřundělová, L.; Minařík, A.; Veríssimo, L.; Barros, M.; Ribeiro, A. Characterization at 25 °C of Sodium Hyaluronate in Aqueous Solutions Obtained by Transport Techniques. Molecules 2015, 20, 5812–5824. [Google Scholar] [CrossRef] [Green Version]
- Wik, K.-O.; Comper, W.D. Hyaluronate diffusion in semidilute solutions. Biopolymers 1982, 21, 583–599. [Google Scholar] [CrossRef]
- Musilová, L.; Kašpárková, V.; Mráček, A.; Minařík, A.; Minařík, M. The behaviour of hyaluronan solutions in the presence of Hofmeister ions: A light scattering, viscometry and surface tension study. Carbohydr. Polym. 2019, 212, 395–402. [Google Scholar] [CrossRef] [PubMed]
- Vlachy, N.; Jagoda-Cwiklik, B.; Vácha, R.; Touraud, D.; Jungwirth, P.; Kunz, W. Hofmeister series and specific interactions of charged headgroups with aqueous ions. Adv. Colloid Interface Sci. 2009, 146, 42–47. [Google Scholar] [CrossRef]
- Zhang, Y.; Cremer, P. Interactions between macromolecules and ions: The Hofmeister series. Curr. Opin. Chem. Biol. 2006, 10, 658–663. [Google Scholar] [CrossRef]
- Leontidis, E. Investigations of the Hofmeister series and other specific ion effects using lipid model systems. Adv. Colloid Interface Sci. 2017, 243, 8–22. [Google Scholar] [CrossRef]
- Budroni, M.A.; Rossi, F.; Wodlei, F.; Marchettini, N.; Lo Nostro, P.; Rustici, M. Hofmeister effect in self-organised chemical systems. J. Phys. Chem. C 2020. [Google Scholar] [CrossRef]
- Sarri, F.; Tatini, D.; Tanini, D.; Simonelli, M.; Ambrosi, M.; Ninham, B.W.; Capperucci, A.; Dei, L.; Lo Nostro, P. Specific ion effects in non-aqueous solvents: The case of glycerol carbonate. J. Mol. Liq. 2018, 266, 711–717. [Google Scholar] [CrossRef]
- Mazzini, V.; Liu, G.; Craig, V.S.J. Probing the Hofmeister series beyond water: Specific-ion effects in non-aqueous solvents. J. Chem. Phys. 2018, 148, 222805. [Google Scholar] [CrossRef]
- Mráček, A.; Varhaníková, J.; Lehocký, M.; Gřundělová, L.; Pokopcová, A.; Velebný, V. The Influence of Hofmeister Series Ions on Hyaluronan Swelling and Viscosity. Molecules 2008, 13, 1025–1034. [Google Scholar] [CrossRef] [Green Version]
- Mráček, A. The Measurement of Polymer Swelling Processes by an Interferometric Method and Evaluation of Diffusion Coefficients. Int. J. Mol. Sci. 2010, 11, 532–543. [Google Scholar] [CrossRef] [Green Version]
- Mráček, A.; Benešová, K.; Minařík, A.; Urban, P.; Lapčík, L. The diffusion process of sodium hyaluronate (Na-Ha) and Na-Ha-n-alkyl derivatives films swelling. J. Biomed. Mater. Res. Part. A 2007, 83A, 184–190. [Google Scholar] [CrossRef]
- Kunz, W. Specific ion effects in colloidal and biological systems. Curr. Opin. Colloid Interface Sci. 2010, 15, 34–39. [Google Scholar] [CrossRef]
- Bastos-González, D.; Pérez-Fuentes, L.; Drummond, C.; Faraudo, J. Ions at interfaces: The central role of hydration and hydrophobicity. Curr. Opin. Colloid Interface Sci. 2016, 23, 19–28. [Google Scholar] [CrossRef]
- Walstra, P. Physical Chemistry of Foods; CRC Press: Boca Raton, FL, USA, 2002; ISBN 9780203910436. [Google Scholar]
- Cowman, M.K.; Matsuoka, S. Experimental approaches to hyaluronan structure. Carbohydr. Res. 2005, 340, 791–809. [Google Scholar] [CrossRef] [PubMed]
- Applebey, M.P. CCXI—The viscosity of salt solutions. J. Chem. Soc. Trans. 1910, 97, 2000–2025. [Google Scholar] [CrossRef]
- Tyrrell, H.J.V.; Harris, K.R. Diffusion in Liquids: A Theoretical and Experimental Study; Butterworth: London, UK, 1984; ISBN 9780408175913. [Google Scholar]
- Harned, H.; Owen, B. The Physical Chemistry of Electrolytic Solutions, 3rd ed.; Reinholds: New York, NY, USA, 1964; ISBN 978-0278917293. [Google Scholar]
- Barthel, J.; Gores, H.J.; Lohr, C.M.; Seidl, J.J. Taylor dispersion measurements at low electrolyte concentrations. I. Tetraalkylammonium perchlorate aqueous solutions. J. Solut. Chem. 1996, 25, 921–935. [Google Scholar] [CrossRef]
- Callendar, R.; Leaist, D.G. Diffusion Coefficients for Binary, Ternary, and Polydisperse Solutions from Peak-Width Analysis of Taylor Dispersion Profiles. J. Solut. Chem. 2006, 35, 353–379. [Google Scholar] [CrossRef]
- Price, W.E. Theory of the taylor dispersion technique for three-component-system diffusion measurements. J. Chem. Soc. Faraday Trans. Phys. Chem. Condens. Phases 1988, 84, 2431. [Google Scholar] [CrossRef]
- Deng, Z.; Leaist, D.G. Ternary mutual diffusion coefficients of MgCl2 + MgSO4 + H2O and Na2SO4 + MgSO4 + H2O from Taylor dispersion profiles. Can. J. Chem. 1991, 69, 1548–1553. [Google Scholar] [CrossRef] [Green Version]
- Galindres, D.M.; Ribeiro, A.C.F.; Esteso, M.A.; Vargas, E.F.; Leaist, D.G.; Rodrigo, M.M. The effects of sodium chloride on the diffusion of sulfonated resorcinarenes in aqueous solutions. Fluid Phase Equilib. 2020, 518, 112629. [Google Scholar] [CrossRef]
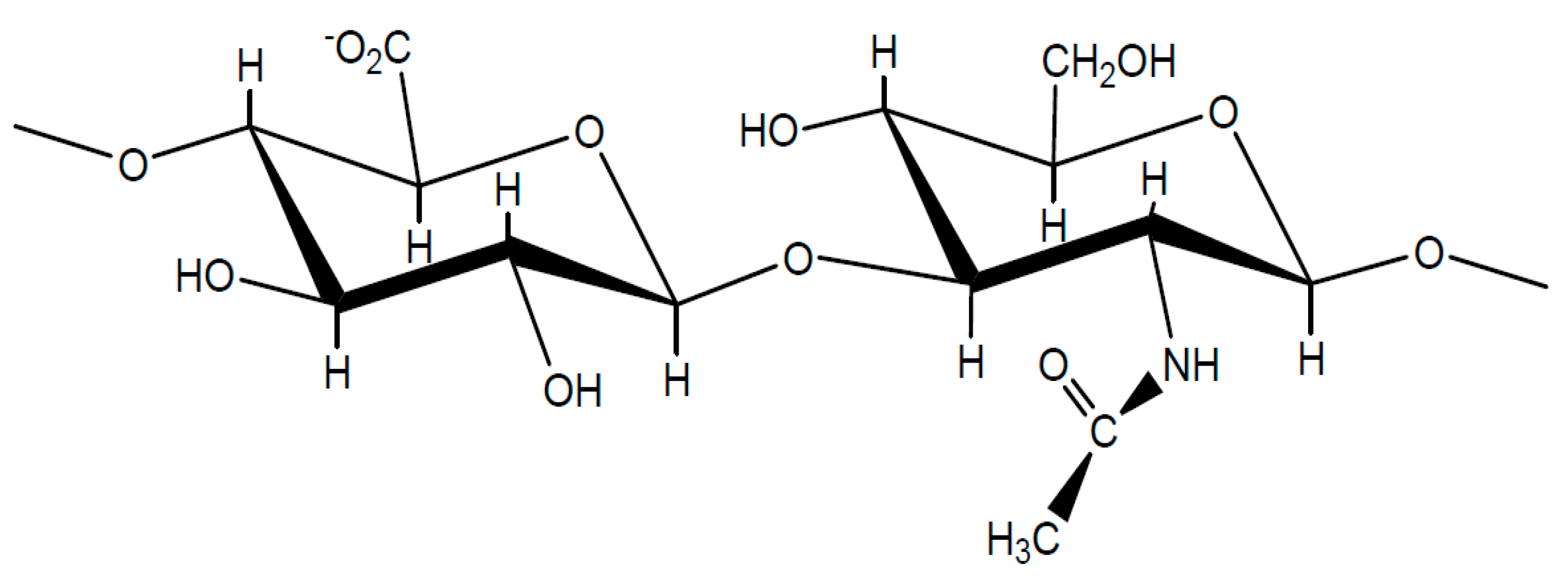


| Cation (KC or CHC) 2 | Anion (KA or CHA) 2 | appD01T ± SD/(10−9m2 s−1) (0.01 mol dm−3) | (ΔappD01T/D0) % 3 | appD01T ± SD/(10−9m2 s−1) (0.1 mol dm−3) | (ΔappD01T/D0) % 3 | |
|---|---|---|---|---|---|---|
| MgSO4 | (Mg2+, Kc) | (SO42−, KA) | 0.609 ± 0.004 | −54.3 | 0.715 ± 0.005 | −46.4 |
| NaSCN | (Na+, KC) | (SCN−, CHA) | 0.858 ± 0.004 | −35.6 | 1.421 ± 0.004 | 6.6 |
| Na2SO4 | (Na+, KC) | (SO42−, KA) | 0.860 ± 0.019 | −35.5 | 1.016 ± 0.003 | −23.8 |
| NH4SCN | (NH4+, CHC) | (SCN−, CHA) | 1.080 ± 0.020 | −18.9 | 1.595 ± 0.007 | 19.7 |
| NaCl | (Na+, KC) | (Cl−, CHA) | 1.090 ± 0.020 | −25.7 | 1.435 ± 0.008 | 7.6 |
| LiCl | (Li+, KC) | (Cl−, CHA) | 1.360 ± 0.003 | 2.0 | 1.351 ± 0.005 | 1.3 |
| (NH4)2SO4 | (NH4+, CHC) | (SO42−, KA) | 1.109 ± 0.008 | −16.8 | 1.313 ± 0.004 | −1.5 |
| Cation (KC or CHC) 2 | Anion (KA or CHA) 2 | appD01T ± SD/(10−9m2 s−1) (0.01 mol dm−3) | (ΔappD01T/D0) % 3 | appD01T ± SD/(10−9m2 s−1) (0.1 mol dm−3) | (ΔappD01T/D0) % 3 | |
|---|---|---|---|---|---|---|
| MgSO4 | (Mg2+, Kc) | (SO42−, KA) | 0.709 ± 0.002 | −31.6 | 0.754 ± 0.005 | −27.2 |
| NaSCN | (Na+, KC) | (SCN−, CHA) | 1.136 ± 0.002 | 9.6 | 1.325 ± 0.010 | 27.8 |
| Na2SO4 | (Na+, KC) | (SO42−, KA) | 0.868 ± 0.010 | −16.2 | 1.075 ± 0.020 | 3.8 |
| NH4SCN | (NH4+, CHC) | (SCN−, CHA) | 1.380 ± 0.010 | 33.2 | 1.563 ± 0.001 | 50.9 |
| NaCl | (Na+, KC) | (Cl−, CHA) | 0.947 ± 0.002 | −8.6 | 1.426 ± 0.005 | 37.6 |
| LiCl | (Li+, KC) | (Cl−, CHA) | 1.282 ± 0.005 | 23.4 | 1.228 ± 0.005 | 18.5 |
| (NH4)2SO4 | (NH4+, CHC) | (SO42−, KA) | 1.200 ± 0.005 | 15.8 | 1.490 ± 0.004 | 43.8 |
| Salts Behaviour | |||||||
|---|---|---|---|---|---|---|---|
| appD0IT × 10−9 (m2·s−1) | MgSO4 | Na2SO4 | LiCl | NaSCN | NaCl | (NH4)2SO4 | NH4SCN |
| KK | KK | KCH | KCH | KCH | CHK | CHCH | |
| ηred water (L·g−1) | NH4SCN | (NH4)2SO4 | NaSCN | NaCl | Na2SO4 | LiCl | MgSO4 |
| CHCH | CHK | KCH | KCH | KK | KCH | KK | |
| ηred NaHy at 0.1 g L−1 (L·g−1) | NaCl | NH4SCN | (NH4)2SO4 | LiCl | Na2SO4 | MgSO4 | NaSCN |
| ↓ | ↓ | ↓ | ↑ | ↑ | ↑ | ↑ | |
| KCH | CHCH | CHK | KCH | KK | KK | KCH | |
| ηred NaHy at 1.5 g L−1(L·g−1) | MgSO4 | NaCl | NH4SCN | LiCl | NaSCN | (NH4)2SO4 | Na2SO4 |
| KK | KCH | CHCH | KCH | KCH | CHK | KK | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Musilová, L.; Mráček, A.; Kašpárková, V.; Minařík, A.; Valente, A.J.M.; Azevedo, E.F.G.; Veríssimo, L.M.P.; Rodrigo, M.M.; Esteso, M.A.; Ribeiro, A.C.F. Effect of Hofmeister Ions on Transport Properties of Aqueous Solutions of Sodium Hyaluronate. Int. J. Mol. Sci. 2021, 22, 1932. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22041932
Musilová L, Mráček A, Kašpárková V, Minařík A, Valente AJM, Azevedo EFG, Veríssimo LMP, Rodrigo MM, Esteso MA, Ribeiro ACF. Effect of Hofmeister Ions on Transport Properties of Aqueous Solutions of Sodium Hyaluronate. International Journal of Molecular Sciences. 2021; 22(4):1932. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22041932
Chicago/Turabian StyleMusilová, Lenka, Aleš Mráček, Věra Kašpárková, Antonín Minařík, Artur J. M. Valente, Eduarda F. G. Azevedo, Luis M. P. Veríssimo, M. Melia Rodrigo, Miguel A. Esteso, and Ana C. F. Ribeiro. 2021. "Effect of Hofmeister Ions on Transport Properties of Aqueous Solutions of Sodium Hyaluronate" International Journal of Molecular Sciences 22, no. 4: 1932. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22041932







