Outcomes of Lower Extremity Endovascular Revascularization: Potential Predictors and Prevention Strategies
Abstract
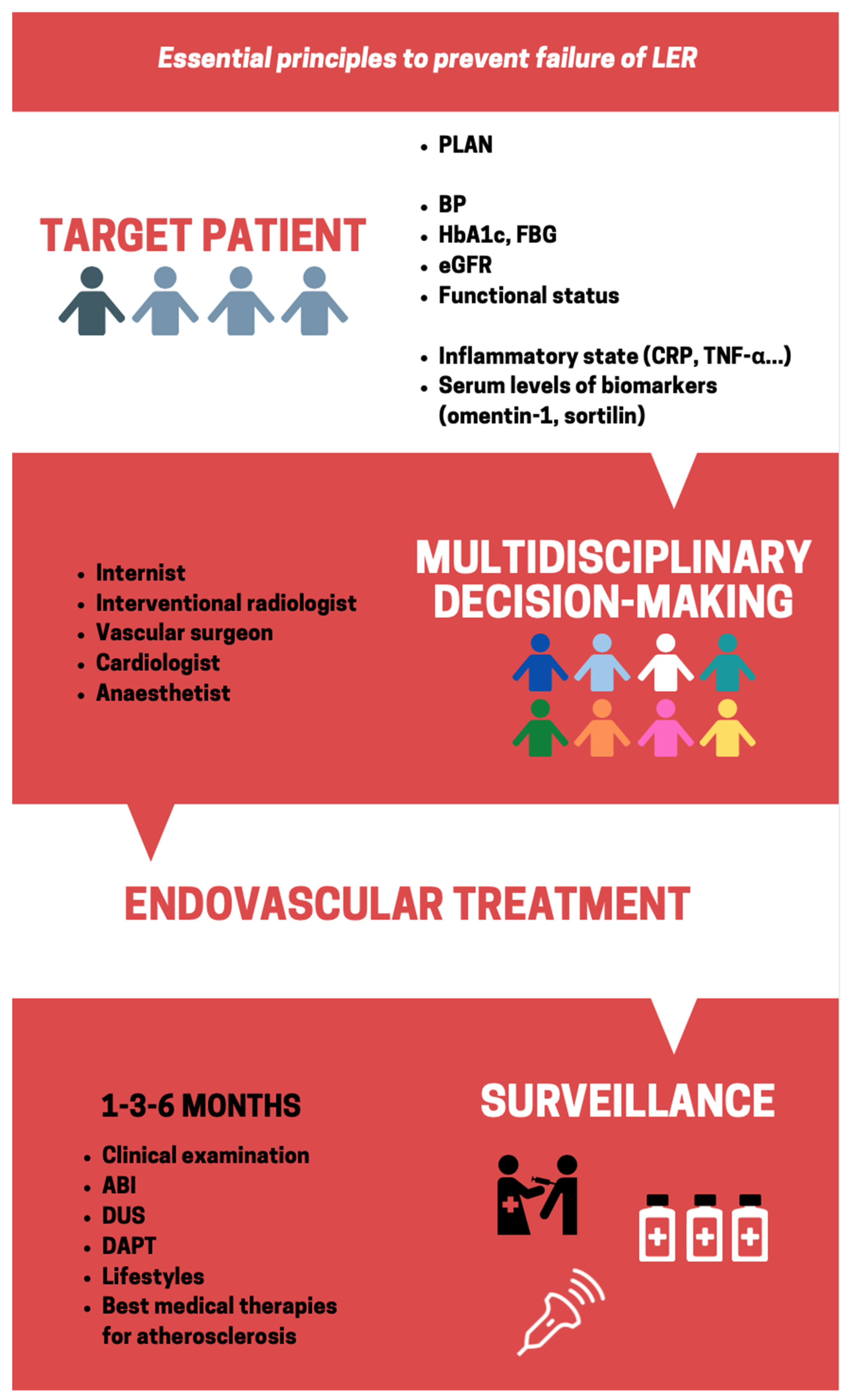
:1. Introduction
2. Definitions of Revascularization Failure
3. Revascularization: When Is It Appropriate?
3.1. Patient Risk Estimation
3.2. Limb Severity
3.3. Anatomic Pattern of Disease
4. The Unsolved Conundrum of Controlling Risk Factors
4.1. Management of Hypertension
4.2. Glycemic Control
4.3. Chronic Kidney Disease
4.4. Functional Status
5. The Inflammatory State in CLTI
6. Novel Biomarkers in CLTI
7. The Rationale of Surveillance and Post-Procedural Care
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Fowkes, F.G.R.; Rudan, D.; Rudan, I.; Aboyans, V.O.; Denenberg, J.; McDermott, M.M.E.; Norman, P.A.; Sampson, U.K.; Williams, L.J.A.; Mensah, G.; et al. Comparison of Global Estimates of Prevalence and Risk Factors for Peripheral Artery Disease in 2000 and 2010: A Systematic Review and Analysis. Lancet 2013, 382, 1329–1340. [Google Scholar] [CrossRef]
- Ouriel, K. Peripheral Arterial Disease. Lancet 2001, 358, 1257–1264. [Google Scholar] [CrossRef]
- Conte, M.S.; Bradbury, A.W.; Kolh, P.; White, J.V.; Dick, F.; Fitridge, R.; Mills, J.L.; Ricco, J.B.; Suresh, K.R.; Murad, M.H.; et al. Global Vascular Guidelines on the Management of Chronic Limb-Threatening Ischemia. Eur. J. Vasc. Endovasc. Surg. 2019, 58, S1–S109.e133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, P.; Rudan, D.; Zhu, Y.I.; Fowkes, F.J.; Rahimi, K.; Fowkes, F.G.R.; Rudan, I. Global, Regional, and National Prevalence and Risk Factors for Peripheral Artery Disease in 2015: An Updated Systematic Review and Analysis. Lancet Glob. Health 2019, 7, e1020–e1030. [Google Scholar] [CrossRef] [Green Version]
- Shu, J.; Santulli, G. Update on Peripheral Artery Disease: Epidemiology and Evidence-Based Facts. Atherosclerosis 2018, 275, 379–381. [Google Scholar] [CrossRef] [PubMed]
- Malyar, N.; Fürstenberg, T.; Wellmann, J.; Meyborg, M.; Lüders, F.; Gebauer, K.; Bunzemeier, H.; Roeder, N.; Reinecke, H. Recent Trends in Morbidity and in-Hospital Outcomes of in-Patients with Peripheral Arterial Disease: A Nationwide Population-Based Analysis. Eur. Heart J. 2013, 34, 2706–2714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Owens, C.D.; Ho, K.J.; Conte, M.S. Risk Factors for Failure of Lower-Extremity Revascularization Procedures: Are They Different for Bypass and Percutaneous Procedures? Semin. Vasc. Surg. 2008, 21, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Adam, D.J.; Beard, J.D.; Cleveland, T.; Bell, J.; Bradbury, A.W.; Forbes, J.F.; Fowkes, F.G.R.; Gillepsie, I.; Ruckley, C.V.; Raab, G.; et al. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL): Multicentre, Randomised Controlled Trial. Lancet 2005, 366, 1925–1934. [Google Scholar] [CrossRef] [PubMed]
- Brodmann, M.; Keirse, K.; Scheinert, D.; Spak, L.; Jaff, M.R.; Schmahl, R.; Li, P.; Zeller, T. Drug-Coated Balloon Treatment for Femoropopliteal Artery Disease. JACC Cardiovasc. Interv. 2017, 10, 2113–2123. [Google Scholar] [CrossRef]
- Stavroulakis, K.; Borowski, M.; Torsello, G.; Bisdas, T.; Adili, F.; Balzer, K.; Billing, A.; Böckler, D.; Brixner, D.; Debus, E.S.; et al. One-Year Results of First-Line Treatment Strategies in Patients with Critical Limb Ischemia (CRITISCH Registry). J. Endovasc. Ther. 2018, 25, 320–329. [Google Scholar] [CrossRef]
- Torsello, G.; Stavroulakis, K.; Brodmann, M.; Micari, A.; Tepe, G.; Veroux, P.; Benko, A.; Choi, D.; Vermassen, F.E.G.; Jaff, M.R.; et al. Three-Year Sustained Clinical Efficacy of Drug-Coated Balloon Angioplasty in a Real-World Femoropopliteal Cohort. J. Endovasc. Ther. 2020, 27, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Biscetti, F.; Ferraro, P.M.; Hiatt, W.R.; Angelini, F.; Nardella, E.; Cecchini, A.L.; Santoliquido, A.; Pitocco, D.; Landolfi, R.; Flex, A. Inflammatory Cytokines Associated with Failure of Lower-Extremity Endovascular Revascularization (LER): A Prospective Study of a Population with Diabetes. Diabetes Care 2019, 42, 1939–1945. [Google Scholar] [CrossRef] [PubMed]
- Sacks, D.; Marinelli, D.L.; Martin, L.G.; Spies, J.B.; Committee SoIRTA. Reporting Standards for Clinical Evaluation of New Peripheral Arterial Revascularization Devices. J. Vasc. Interv. Radiol. 2003, 14, S395–S404. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutherford, R.B.; Baker, J.; Ernst, C.; Johnston, K.; Porter, J.M.; Ahn, S.; Jones, D.N. Recommended Standards for Reports Dealing with Lower Extremity Ischemia: Revised Version. J. Vasc. Surg. 1997, 26, 517–538. [Google Scholar] [CrossRef] [Green Version]
- Zierler, R.E.; Jordan, W.D.; Lal, B.K.; Mussa, F.; Leers, S.; Fulton, J.; Pevec, W.; Hill, A.; Murad, M.H. The Society for Vascular Surgery Practice Guidelines on Follow-up after Vascular Surgery Arterial Procedures. J. Vasc. Surg. 2018, 68, 256–284. [Google Scholar] [CrossRef] [PubMed]
- Welt, F.G.; Rogers, C. Inflammation and Restenosis in the Stent Era. Arter. Thromb. Vasc. Biol. 2002, 22, 1769–1776. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakatani, M.; Takeyama, Y.; Shibata, M.; Yorozuya, M.; Suzuki, H.; Koba, S.; Katagiri, T. Mechanisms of Restenosis after Coronary Intervention: Difference between Plain Old Balloon Angioplasty and Stenting. Cardiovasc. Pathol. 2003, 12, 40–48. [Google Scholar] [CrossRef]
- Pasterkamp, G.; De Kleijn, D.P.; Borst, C. Arterial Remodeling in Atherosclerosis, Restenosis and after Alteration of Blood Flow: Potential Mechanisms and Clinical Implications. Cardiovasc. Res. 2000, 45, 843–852. [Google Scholar] [CrossRef] [Green Version]
- Herity, N.A.; Ward, M.R.; Lo, S.; Yeung, A.C. Review: Clinical Aspects of Vascular Remodeling. J. Cardiovasc. Electrophysiol. 1999, 10, 1016–1024. [Google Scholar] [CrossRef]
- Post, M.J.; De Smet, B.J.G.L.; Van Der Helm, Y.; Borst, C.; Kuntz, R.E. Arterial Remodeling after Balloon Angioplasty or Stenting in an Atherosclerotic Experimental Model. Circulation 1997, 96, 996–1003. [Google Scholar] [CrossRef]
- Mintz, G.S.; Popma, J.J.; Pichard, A.D.; Kent, K.M.; Satler, L.F.; Wong, C.; Hong, M.K.A.; Kovach, J.; Leon, M.B. Arterial Remodeling after Coronary Angioplasty: A Serial Intravascular Ultrasound Study. Circulation 1996, 94, 35–43. [Google Scholar] [CrossRef]
- Post, M.J.; Borst, C.; Kuntz, R.E. The Relative Importance of Arterial Remodeling Compared with Intimal Hyperplasia in Lumen Renarrowing after Balloon Angioplasty. A Study in the Normal Rabbit and the Hypercholesterolemic Yucatan Micropig. Circulation 1994, 89, 2816–2821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saraidaridis, J.T.; Patel, V.I.; Lancaster, R.T.; Cambria, R.P.; Conrad, M.F. Applicability of the Society for Vascular Surgery’s Objective Performance Goals for Critical Limb Ischemia to Current Practice of Lower-Extremity Bypass. Ann. Vasc. Surg. 2016, 30, 59–65. [Google Scholar] [CrossRef] [PubMed]
- Fashandi, A.Z.; Mehaffey, J.H.; Hawkins, R.B.; Kron, I.L.; Upchurch, G.R.; Robinson, W.P. Major Adverse Limb Events and Major Adverse Cardiac Events after Contemporary Lower Extremity Bypass and Infrainguinal Endovascular Intervention in Patients with Claudication. J. Vasc. Surg. 2018, 68, 1817–1823. [Google Scholar] [CrossRef] [PubMed]
- Fleisher, L.A.; Fleischmann, K.E.; Auerbach, A.D.; Barnason, S.A.; Beckman, J.A.; Bozkurt, B. 2014 Acc/Aha Guideline on Perioperative Cardiovascular Evaluation and Management of Patients Undergoing Noncardiac Surgery: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines. J. Am. Coll Cardiol. 2014, 64, 77–137. [Google Scholar] [CrossRef] [Green Version]
- Kristensen, S.D.; Knuuti, J.; Saraste, A.; Anker, S.; Bøtker, H.E.; Hert, S.D. 2014 Esc/Esa Guidelines on Non-cardiac Surgery: Cardiovascular Assessment and Management: The Joint Task Force on Non-Cardiac Surgery: Cardiovascular Assessment and Management of the European Society of Cardiology (Esc) and the European Society of Anaesthesiology (Esa). Eur. Heart J. 2014, 35, 2383–2431. [Google Scholar] [PubMed] [Green Version]
- Kraiss, L.W.; Beckstrom, J.L.; Brooke, B.S. Frailty Assessment in Vascular Surgery and Its Utility in Preoperative Decision Making. Semin. Vasc. Surg. 2015, 28, 141–147. [Google Scholar] [CrossRef] [PubMed]
- Biancari, F.; Salenius, J.-P.; Heikkinen, M.; Luther, M.; Ylönen, K.; Lepäntalo, M. Risk-scoring Method for Prediction of 30-Day Postoperative Outcome after Infrainguinal Surgical Revascularization for Critical Lower-Limb Ischemia: A Finnvasc Registry Study. World J. Surg. 2007, 31, 217–225. [Google Scholar] [CrossRef]
- Schanzer, A.; Mega, J.; Meadows, J.; Samson, R.H.; Bandyk, D.F.; Conte, M.S. Risk Stratification in Critical Limb Ischemia: Derivation and Validation of a Model to Predict Amputation-Free Survival Using Multicenter Surgical Outcomes Data. J. Vasc. Surg. 2008, 48, 1464–1471. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bradbury, A.W.; Adam, D.J.; Bell, J.; Forbes, J.F.; Fowkes, F.G.R.; Gillespie, I.; Ruckley, C.V.; Raab, G.M. Bypass versus Angioplasty in Severe Ischaemia of the Leg (BASIL) Trial: A Survival Prediction Model to Facilitate Clinical Deci-Sion Making. J. Vasc. Surg. 2010, 51, 52S–68S. [Google Scholar] [CrossRef] [Green Version]
- Meltzer, A.J.; Graham, A.; Connolly, P.H.; Meltzer, E.C.; Karwowski, J.K.; Bush, H.L.; Schneider, D.B. The Comprehensive Risk Assessment for Bypass (CRAB) Facilitates Efficient Perioperative Risk Assessment for Patients with Critical Limb Ischemia. J. Vasc. Surg. 2013, 57, 1186–1195. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simons, J.P.; Goodney, P.P.; Flahive, J.; Hoel, A.W.; Hallett, J.W.; Kraiss, L.W.; Schanzer, A.; Initiative, S.F.V.S.V.Q. A Comparative Evaluation of Risk-Adjustment Models for Benchmarking Amputation-Free Survival after Lower Extremity Bypass. J. Vasc. Surg. 2016, 63, 990–997. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soga, Y.; Iida, O.; Takahaera, M.; Hirano, K.; Suzuki, K.; Kawasaki, D.; Miyashita, Y.; Tsuchiya, T. Two-Year Life Expectancy in Patients with Critical Limb Ischemia. JACC Cardiovasc. Interv. 2014, 7, 1444–1449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, S.M.; Kalbaugh, C.A.; Blackhurst, D.W.; Cass, A.L.; Trent, E.A.; Langan, E.M.; Youkey, J.R. Determinants of Functional Outcome after Revascularization for Critical Limb Ischemia: An Analysis of 1000 Consecutive Vascular Interventions. J. Vasc. Surg. 2006, 44, 747–756. [Google Scholar] [CrossRef] [Green Version]
- Zhan, L.X.; Branco, B.C.; Armstrong, D.G.; Mills, J.L. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System Based on Wound, Ischemia, and Foot Infection (WIfI) Correlates with Risk of Major Amputation and Time to Wound Healing. J. Vasc. Surg. 2015, 61, 939–944. [Google Scholar] [CrossRef] [Green Version]
- Robinson, W.P.; Loretz, L.; Hanesian, C.; Flahive, J.; Bostrom, J.; Lunig, N.; Schanzer, A.; Messina, L. Society for Vascular Surgery Wound, Ischemia, foot Infection (WIfI) Score Correlates with the Intensity of Multimodal Limb Treatment and Patient-Centered Outcomes in Patients with Threatened Limbs Managed in a Limb Preservation Center. J. Vasc. Surg. 2017, 66, 488–498.e2. [Google Scholar] [CrossRef]
- Mills, J.L.; Conte, M.S.; Armstrong, D.G.; Pomposelli, F.B.; Schanzer, A.; Sidawy, A.N.; Andros, G. The Society for Vascular Surgery Lower Extremity Threatened Limb Classification System: Risk Stratification based on Wound, Ischemia, and foot Infection (WIfI). J. Vasc. Surg. 2014, 59, 220–234.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mills, J.L. Update and Validation of the Society for Vascular Surgery Wound, Ischemia, and Foot Infection Threatened Limb Classification System. Semin. Vasc. Surg. 2014, 27, 16–22. [Google Scholar] [CrossRef]
- Cull, D.L.; Manos, G.; Hartley, M.C.; Taylor, S.M.; Langan, E.M.; Eidt, J.F.; Johnson, B.L. An early validation of the Society for Vascular Surgery Lower Extremity Threatened Limb Classification System. J. Vasc. Surg. 2014, 60, 1535–1542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Darling, J.D.; McCallum, J.C.; Soden, P.A.; Guzman, R.; Raul, J.; Wyers, M.; Mark, C.; Hamdan, A.; Allen, D.; Verhagen, H.J.; et al. Predictive ability of the Society for Vascular Surgery Wound, Ischemia, and foot Infection (WIfI) Classification System after First-Time Lower Extremity Revascularizations. J. Vasc. Surg. 2017, 65, 695–704. [Google Scholar] [CrossRef] [Green Version]
- Mayor, J.; Chung, J.; Zhang, Q.; Montero-Baker, M.; Schanzer, A.; Conte, M.S.; Mills, J.L. Using the Society for Vascular Surgery Wound, Ischemia, and Foot Infection Classification to Identify Patients Most Likely to Benefit from Revascularization. J. Vasc. Surg. 2019, 70, 776–785.e1. [Google Scholar] [CrossRef]
- Jaff, M.R.; White, C.J.; Hiatt, W.R.; Fowkes, G.R.; Dormandy, J.; Razavi, M.; Reekers, J.; Norgren, L. An Update on Methods for Revascularization and Expansion of the Tasc Lesion Classification to Include below-the-Knee Arteries: A Supplement to the Inter-Society Consensus for the Management of Peripheral Arterial Disease (Tasc ii). J. Endovasc. Ther. 2015, 22, 663–677. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bollinger, A.; Breddin, K.; Hess, H.; Heystraten, F.; Kollath, J.; Konttila, A.; Pouliadis, G.; Marshall, M.; Mey, T.; Mietaschk, A.; et al. Semiquantitative Assessment of Lower Limb Atherosclerosis from Routine Angiographic Images. Atherosclerosis 1981, 38, 339–346. [Google Scholar] [CrossRef]
- Kodama, A.; Meecham, L.; Popplewell, M.; Bate, G.; Conte, M.S.; Bradbury, A.W. Relationship between Global Anatomic Staging System (Glass) and Clinical Outcomes Following Revascularisation for Chronic Limb Threatening Ischaemia in the Bypass versus Angioplasty in Severe Ischaemia of the Leg (Basil)-1 Trial. Eur. J. Vasc. Endovasc. Surg. 2020, 60, 687–695. [Google Scholar] [CrossRef]
- O’Donnell, T.F.; Deery, S.E.; Darling, J.D.; Shean, K.E.; Mittleman, M.A.; Yee, G.N.; Dernbach, M.R.; Schermerhorn, M.L. Adherence to Lipid Management Guidelines Is Associated with Lower Mortality and Major Adverse Limb Events in Patients Un-Dergoing Revascularization for Chronic Limb-Threatening Ischemia. J. Vasc. Surg. 2017, 66, 572–578. [Google Scholar] [CrossRef] [PubMed]
- Bavry, A.A.; Anderson, R.D.; Gong, Y.; Denardo, S.J.; Cooper-Dehoff, R.M.; Handberg, E.M.; Pepine, C.J. Outcomes among Hypertensive Patients with Concomitant Peripheral and Coronary Artery Disease: Findings from the Interna-Tional Verapamil-SR/Trandolapril Study. Hypertension 2010, 55, 48–53. [Google Scholar] [CrossRef] [Green Version]
- Heart Outcomes Prevention Evaluation Study Investigators; Yusuf, S.; Sleight, P.; Pogue, J.; Bosch, J.; Davies, R.; Dagenais, G. Effects of an Angiotensin-Converting–Enzyme Inhibitor, Ramipril, on Cardiovascular Events in High-Risk Patients. N. Engl. J. Med. 2000, 342, 145–153. [Google Scholar] [CrossRef]
- Sleight, P. The HOPE Study (Heart Outcomes Prevention Evaluation). J. Renin-Angiotensin-Aldosterone Syst. 2000, 1, 18–20. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, E.J.; Chen, D.C.; Singh, G.D.A.; Amsterdam, E.; Laird, J.R. Angiotensin-Converting Enzyme Inhibitor or Angiotensin Receptor Blocker Use Is Associated with Reduced Major Adverse Cardiovascular Events among Patients with Critical Limb Ischemia. Vasc. Med. 2015, 20, 237–244. [Google Scholar] [CrossRef] [Green Version]
- Kray, E.J.; Dombrovskiy, V.Y.; Vogel, T.R. Use of Angiotensin-Converting Enzyme Inhibitors and Freedom from Amputation after Lower Extremity Revascularization. Vasc. Health Risk Manag. 2017, 13, 269–274. [Google Scholar] [CrossRef]
- Høgh, A.; Lindholt, J.S.; Nielsen, H.; Jensen, L.P.; Johnsen, S.P. Use of Angiotensin-Converting Enzyme Inhibitors and Cardiovascular Outcomes Following Primary Vascular Surgery: A Nation-Wide Propensity Score Matched Follow-Up Study. Vasc Endovasc. Surg. 2012, 46, 515–523. [Google Scholar] [CrossRef]
- Khan, S.Z.; Montross, B.; Rivero, M.; Cherr, G.S.; Harris, L.M.; Dryjski, M.L.; Dosluoglu, H.H. Angiotensin Converting Enzyme Inhibitors and Angiotensin II Receptor Blockers (ACEI/ARB) are Associated with Improved Limb Salvage after Infrapopliteal Interventions for Critical Limb Ischemia. Ann. Vasc. Surg. 2020, 63, 275–286. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.H.; Tsao, Y.A.; McLoughlin, G.; Breckenridge, A. Placebo-Controlled Comparison of Captopril, Atenolol, Labetalol, and Pindolol in Hypertension Complicated by Intermittent Claudication. Lancet 1987, 2, 650–653. [Google Scholar] [CrossRef]
- Lepantalo, M. Chronic Effects of Metoprolol and Methyldopa on Calf Blood Flow in Intermittent Claudication. Br. J. Clin. Pharmacol. 1984, 18, 90–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paravastu, S.C.; Mendonca, D.A.; Da Silva, A. Beta Blockers for Peripheral Arterial Disease. Cochrane Database Syst Rev. 2013, CD005508. [Google Scholar] [CrossRef] [PubMed]
- Manapurathe, T.D.; Krishna, S.M.; Dewdney, B.; Moxon, J.V.; Biros, E.; Golledge, J. Effect of Blood Pressure Lowering Medications on Leg Ischemia in Peripheral Artery Disease Patients: A Meta-Analysis of Ran-Domised Controlled Trials. PLoS ONE 2017, 12, e0178713. [Google Scholar] [CrossRef] [Green Version]
- Vrsalovic, M. Diabetes and Peripheral Artery Disease: A Bad Combination. Am. J. Surg. 2018, 216, 182–183. [Google Scholar] [CrossRef] [PubMed]
- Britton, K.A.; Mukamal, K.J.; Ix, J.H.; Siscovick, D.S.; Newman, A.B.; De Boer, I.H.; Thacker, E.L.; Biggs, M.L.; Gaziano, J.M.; Djoussé, L. Insulin Resistance and Incident Peripheral Artery Disease in the Cardiovascular Health Study. Vasc. Med. 2012, 17, 85–93. [Google Scholar] [CrossRef] [Green Version]
- Joosten, M.M.; Pai, J.K.; Bertoia, M.L.; Rimm, E.B.; Spiegelman, D.; Mittleman, M.A.; Mukamal, K.J. Associations Between Conventional Cardiovascular Risk Factors and Risk of Peripheral Artery Disease in Men. JAMA 2012, 308, 1660–1667. [Google Scholar] [CrossRef]
- Collaboration PSCaAPCS. SEx-specific Relevance of Diabetes to Occlusive Vascular and Other Mortality: A Collaborative Meta-Analysis of Individual Data from 980 793 Adults from 68 Prospective Studies. Lancet Diabetes Endocrinol. 2018, 6, 538–546. [Google Scholar] [CrossRef]
- Domingueti, C.P.; Dusse, L.M.S.; Carvalho, M.D.G.; De Sousa, L.P.; Gomes, K.B.; Fernandes, A.P. Diabetes Mellitus: The Linkage between Oxidative Stress, Inflammation, Hypercoagulability and Vascular Complications. J. Diabetes Complicat. 2016, 30, 738–745. [Google Scholar] [CrossRef]
- Vrsalovic, M.; Vucur, K.; Presecki, A.V.; Fabijanic, D.; Milosevic, M. Impact of Diabetes on Mortality in Peripheral Artery Disease: A Meta-Analysis. Clin. Cardiol. 2017, 40, 287–291. [Google Scholar] [CrossRef]
- Haltmayer, M.; Mueller, T.; Horvath, W.; Luft, C.; Poelz, W.; Haidinger, D. Impact of Atherosclerotic Risk Factors on the Anatomical Distribution of Peripheral Arterial Disease. Int. Angiol. 2001, 20, 200–207. [Google Scholar]
- Thiruvoipati, T.; Kielhorn, C.E.; Armstrong, E.J. Peripheral Artery Disease in Patients with Diabetes: Epidemiology, Mechanisms, and Outcomes. World J. Diabetes 2015, 6, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Jude, E.B.; Oyibo, S.O.; Chalmers, N.; Boulton, A.J. Peripheral Arterial Disease in Diabetic and Nondiabetic Patients: A Comparison of Severity and Outcome. Diabetes Care 2001, 24, 1433–1437. [Google Scholar] [CrossRef] [Green Version]
- Arya, S.; Binney, Z.O.; Khakharia, A.; Long, C.A.; Brewster, L.P.; Wilson, P.W.; Rajani, R.R.; Goodney, P.P.; Jordan, W.D. High Hemoglobin A. J. Vasc. Surg. 2018, 67, 217–228.e211. [Google Scholar] [CrossRef] [Green Version]
- Fowkes, F.G.R.; Aboyans, V.; McDermott, M.M.; Sampson, U.K.A.; Criqui, M.H. Peripheral Artery Disease: Epidemiology and Global Perspectives. Nat. Rev. Cardiol. 2017, 14, 156–170. [Google Scholar] [CrossRef] [PubMed]
- Kallio, M.; Forsblom, C.; Groop, P.-H.; Groop, L.; Lepäntalo, M. Development of New Peripheral Arterial Occlusive Disease in Patients with Type 2 Diabetes during a Mean Follow-up of 11 Years. Diabetes Care 2003, 26, 1241–1245. [Google Scholar] [CrossRef] [Green Version]
- Conte, M.S.; Pomposelli, F.B.; Clair, D.G.; Geraghty, P.J.; McKinsey, J.F.; Mills, J.L.; Moneta, G.L.; Murad, M.H.; Powell, R.J.; Reed, A.B.; et al. Society for Vascular Surgery Practice Guidelines for Atherosclerotic Occlusive Disease of the Lower Extremities: Management of Asymptomatic Disease and Claudication. J. Vasc. Surg. 2015, 61, 2S–41S.e1. [Google Scholar] [CrossRef] [Green Version]
- Hingorani, A.; LaMuraglia, G.M.; Henke, P.; Meissner, M.H.; Loretz, L.; Zinszer, K.M.; Driver, V.R.; Frykberg, R.; Carman, T.L.; Marston, W.; et al. The Management of Diabetic Foot: A Clinical Practice Guideline by the Society for Vascular Surgery in Collaboration with the American Podiatric Medical Association and the Society for Vascular Medicine. J. Vasc. Surg. 2016, 63, 3S–21S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Selvin, E.; Marinopoulos, S.; Berkenblit, G.; Rami, T.; Brancati, F.L.; Powe, N.R.; Golden, S.H. Meta-Analysis: Glycosylated Hemoglobin and Cardiovascular Disease in Diabetes Mellitus. Ann. Intern. Med. 2004, 141, 421–431. [Google Scholar] [CrossRef]
- Gerstein, H.C.E.; Miller, M.; Byington, R.P.; Goff, D.C.; Bigger, J.T.; Buse, J.B.; Cushman, W.C.; Genuth, S.; Ismail-Beigi, F.; Grimm, R.H.; et al. Effects of Intensive Glucose Lowering in Type 2 Diabetes. N. Engl. J. Med. 2008, 358, 2545–2559. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patel, A.; MacMahon, S.; Chalmers, J.; Neal, B.; Billot, L.; Joshi, R.; Woodward, M.; Marre, M.; Travert, F.; Cooper, M.; et al. Intensive Blood Glucose Control and Vascular Outcomes in Patients with Type 2 Diabetes. N. Engl. J. Med. 2008, 358, 2560–2572. [Google Scholar] [CrossRef] [Green Version]
- Duckworth, W.; Abraira, C.; Moritz, T.; Reda, D.; Emanuele, N.; Reaven, P.D.; Zieve, F.J.; Marks, J.; Davis, S.N.; Hayward, R.; et al. Glucose Control and Vascular Complications in Veterans with Type 2 Diabetes. N. Engl. J. Med. 2009, 360, 129–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cavender, M.A.; Scirica, B.M.; Raz, I.; Steg, P.G.; McGuire, D.K.; Leiter, L.A.; Hirshberg, B.; Davidson, J.; Cahn, A.; Mosenzon, O.; et al. Cardiovascular Outcomes of Patients in SAVOR-TIMI 53 by Baseline Hemoglobin A1c. Am. J. Med. 2016, 129, 340.e1–340.e8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.C.C.; Blomster, J.I.; Heizer, G.; Berger, J.S.; Baumgartner, I.; Fowkes, F.G.R.; Held, P.; Katona, B.G.; Norgren, L.; Jones, W.S. Cardiovascular and Limb Outcomes in Patients with Diabetes and Peripheral Artery Disease: The Euclid Trial. J. Am. Coll. Cardiol. 2018, 72, 3274–3284. [Google Scholar] [CrossRef]
- Goldman, M.P.; Clark, C.J.; Craven, T.E.; Davis, R.P.; Williams, T.K.; Velazquez-Ramirez, G.; Hurie, J.B.; Edwards, M.S. Effect of Intensive Glycemic Control on Risk of Lower Extremity Amputation. J. Am. Coll. Surg. 2018, 227, 596–604. [Google Scholar] [CrossRef]
- Takahara, M.; Kaneto, H.; Iida, O.; Gorogawa, S.-I.; Katakami, N.; Matsuoka, T.-A.; Ikeda, M.; Shimomura, I. The Influence of Glycemic Control on the Prognosis of Japanese Patients Undergoing Percutaneous Transluminal Angioplasty for Critical Limb Ischemia. Diabetes Care 2010, 33, 2538–2542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, S.; Armstrong, E.J.; Sherif, W.; Alvandi, B.; Westin, G.G.; Singh, G.D.A.; Amsterdam, E.; Laird, J.R. Association of Elevated Fasting Glucose with Lower Patency and Increased Major Adverse Limb Events among Patients with DI-Abetes Undergoing Infrapopliteal Balloon Angioplasty. Vasc. Med. 2014, 19, 307–314. [Google Scholar] [CrossRef] [Green Version]
- Cha, J.-J.; Kim, H.; Ko, Y.-G.; Choi, D.; Lee, J.-H.; Yoon, C.-H.; Chae, I.-H.; Yu, C.W.; Lee, S.W.; Lee, S.-R.; et al. Influence of Preprocedural Glycemic Control on Clinical Outcomes of Endovascular Therapy in Diabetic Patients with Lower Ex-tremity Artery Disease: An Analysis from a Korean Multicenter Retrospective Registry Cohort. Cardiovasc. Diabetol. 2020, 19, 1–10. [Google Scholar] [CrossRef]
- Singh, N.; Zeng, C.; Lewinger, J.P.; Wolfson, A.M.; Shavelle, D.; Weaver, F.; Garg, P.K. Preoperative Hemoglobin A1c Levels and Increased Risk of Adverse Limb Events in Diabetic Patients Undergoing Infrainguinal Lower Extremity Bypass Surgery in the Vascular Quality Initiative. J. Vasc. Surg. 2019, 70, 1225–1234.e1. [Google Scholar] [CrossRef] [PubMed]
- Biscetti, F.; Pitocco, D.; Straface, G.; Zaccardi, F.; De Cristofaro, R.; Rizzo, P.; Lancellotti, S.; Arena, V.; Stigliano, E.; Musella, T.; et al. Glycaemic Variability Affects Ischaemia-Induced Angiogenesis in Diabetic Mice. Clin. Sci. 2011, 121, 555–564. [Google Scholar] [CrossRef]
- Gorst, C.; Kwok, C.S.; Aslam, S.; Buchan, I.; Kontopantelis, E.; Myint, P.K.; Heatlie, G.; Loke, Y.; Rutter, M.K.; Mammas, M.A. Long-Term Glycemic Variabilityand Risk of Adverse Outcomes: A Systematic Review and Meta-Analysis. Diabetes Care 2015, 38, 2354–2369. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-P.; Lin, C.-C.; Li, C.-I.; Liu, C.-S.; Lin, C.-H.; Hwang, K.-L.; Yang, S.-Y.; Li, T.-C. Fasting Plasma Glucose Variability and HbA1c Are Associated with Peripheral Artery Disease Risk in Type 2 Diabetes. Cardiovasc. Diabetol. 2020, 19, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Brooke, B.S.; Kraiss, L.W.; Stone, D.H.; Nolan, B.; De Martino, R.R.; Reiber, G.E.; Goodman, D.C.; Cronenwett, J.L.; Goodney, P.P. Improving Outcomes for Diabetic Patients Undergoing Revascularization for Critical Limb Ischemia: Does the Quality of Outpatient Diabetic Care Matter? Ann. Vasc. Surg. 2014, 28, 1719–1728. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.R.; Everett, B.M.; Birtcher, K.K.; Brown, J.M.; Cefalu, W.T.; Januzzi, J.L.; Kalyani, R.R.; Kosiborod, M.; Magwire, M.L.; Morris, P.B.; et al. 2018 ACC Expert Consensus Decision Pathway on Novel Therapies for Cardiovascular Risk Reduction in Patients with Type 2 Diabetes and Atherosclerotic Cardiovascular Disease: A Report of the American College of Cardiology Task Force on Expert Consensus Decision Pathways. J. Am. Coll. Cardiol. 2018, 72, 3200–3223. [Google Scholar] [PubMed]
- Neal, B.; Perkovic, V.; Mahaffey, K.W.; De Zeeuw, D.; Fulcher, G.; Erondu, N.; Shaw, W.; Law, G.; Desai, M.; Matthews, D.R. Canagliflozin and Cardiovascular and Renal Events in Type 2 Diabetes. N. Engl. J. Med. 2017, 377, 644–657. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, S.; Bandyopadhyay, D.; Ghosh, R.K.; Majumdar, U.; Aneja, A.; Lavie, C.J.; Deedwania, P. SGLT-2 Inhibitors and Peripheral Artery Disease: A Statistical Hoax or Reality? Curr. Probl. Cardiol. 2019, 44, 207–222. [Google Scholar] [CrossRef]
- Li, D.; Yang, J.Y.; Wang, T.; Shen, S.; Tang, H. Risks of Diabetic Foot Syndrome and Amputation Associated with Sodium Glucose Co-transporter 2 Inhibitors: A Meta-Analysis of Randomized Controlled Trials. Diabetes Metab. 2018, 44, 410–414. [Google Scholar] [CrossRef]
- Potier, L.; Roussel, R.; Velho, G.; Saulnier, P.-J.; Bumbu, A.; Matar, O.; Schneider, F.; Ragot, S.; Marre, M.; Mohammedi, K.; et al. Lower Limb Events in Individuals with Type 2 Diabetes: Evidence for an Increased Risk Associated with Diuretic Use. Diabetology 2019, 62, 939–947. [Google Scholar] [CrossRef] [Green Version]
- Xie, W.; Song, X.; Liu, Z. Impact of Dipeptidyl-Peptidase 4 Inhibitors on Cardiovascular Diseases. Vasc. Pharmacol. 2018, 109, 17–26. [Google Scholar] [CrossRef]
- O’Hare, A.M.; Vittinghoff, E.; Hsia, J.; Shlipak, M.G. Renal Insufficiency and the Risk of Lower Extremity Peripheral Arterial Disease: Results from the Heart and Estrogen/Progestin Replacement Study (HERS). J. Am. Soc. Nephrol. 2004, 15, 1046–1051. [Google Scholar] [CrossRef] [PubMed]
- Lacroix, P.; Aboyans, V.; Desormais, I.; Kowalsky, T.; Cambou, J.P.; Constans, J.; Rivière, A.B. Chronic Kidney Disease and the Short-Term Risk of Mortality and Amputation in Patients Hospitalized for Peripheral Artery Disease. J. Vasc. Surg. 2013, 58, 966–971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harfouch, B.; Prasad, A. Implications of Renal Disease in Patients Undergoing Peripheral Arterial Interventions. Interv. Cardiol. Clin. 2020, 9, 345–356. [Google Scholar] [CrossRef] [PubMed]
- O’Hare, A.M.; Glidden, D.V.; Fox, C.S.; Hsu, C.Y. High Prevalence of Peripheral Arterial Disease in Persons with Renal Insufficiency: Results from the National Health and Nutri-Tion Examination Survey 1999–2000. Circulation 2004, 109, 320–323. [Google Scholar] [CrossRef] [Green Version]
- Rajagopalan, S.; Dellegrottaglie, S.; Furniss, A.L.; Gillespie, B.W.; Satayathum, S.; Lameire, N.; Saito, A.; Akiba, T.; Jadoul, M.; Ginsberg, N.; et al. Peripheral Arterial Disease in Patients with End-Stage Renal Disease: Observations from the Dialysis Outcomes and Practice Pat-Terns Study (Dopps). Circulation 2006, 114, 1914–1922. [Google Scholar] [CrossRef] [Green Version]
- O’Hare, A.; Johansen, K. Lower-Extremity Peripheral Arterial Disease among Patients with End-Stage Renal Disease. J. Am. Soc. Nephrol. 2001, 12, 2838–2847. [Google Scholar]
- Herzog, C.A.; Asinger, R.W.; Berger, A.K.; Charytan, D.M.; Diez, J.D.M.; Hart, R.G.; Eckardt, K.-U.; Kasiske, B.L.; McCullough, P.A.; Passman, R.S.; et al. Cardiovascular Disease in Chronic Kidney Disease. a Clinical Update from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int. 2011, 80, 572–586. [Google Scholar] [CrossRef] [Green Version]
- Workgroup, K. K/DOQI Clinical Practice Guidelines for Cardiovascular Disease in Dialysis Patients. Am. J. Kidney Dis. 2005, 45, S1–S153. [Google Scholar]
- Kolte, D.; Kennedy, K.; Shishehbor, M.; Abbott, J.; Khera, S.; Soukas, P. Thirty-Day Readmissions After Endovascular or Surgical Therapy for Critical Limb Ischemia: Analysis of the 2013 to 2014 Nationwide Readmissions Databases. J. Vasc. Surg. 2017, 66, 1911. [Google Scholar] [CrossRef]
- Gerhard-Herman, M.D.; Gornik, H.L.; Barrett, C.; Barshes, N.R.; Corriere, M.A.; Drachman, D.E.; Rowkes, F.G.R.; Hamburg, N.M.; Kinlay, S.; Lookstein, R.; et al. 2016 Aha/Acc Guideline on the Management of Patients with Lower Extremity Peripheral Artery Disease: A Report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. Circulation 2017, 135, e726–e779. [Google Scholar] [PubMed]
- Smilowitz, N.R.; Bhandari, N.; Berger, J.S. Chronic Kidney Disease and Outcomes of Lower Extremity Revascularization for Peripheral Artery Disease. Atherosclerosis 2020, 297, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.E.; Gamboa, C.; Warnock, D.G.; Muntner, P. Chronic Kidney Disease and Risk of Death from Infection. Am. J. Nephrol. 2011, 34, 330–336. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ašćerić, R.R.; Dimković, N.B.; Trajković, G.; Ristić, B.S.; Janković, A.N.; Durić, P.S.; Ilijevski, N.S. Prevalence, Clinical Characteristics, and Predictors of Peripheral Arterial Disease in Hemodialysis Patients: A Cross-Sectional Study. BMC Nephrol. 2019, 20, 281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Arinze, N.V.; Gregory, A.; Francis, J.M.; Farber, A.; Chitalia, V.C. Unique Aspects of Peripheral Artery Disease in Patients with Chronic Kidney Disease. Vasc. Med. 2019, 24, 251–260. [Google Scholar] [CrossRef]
- Okamoto, S.; Iida, O.; Mano, T. Current Perspective on Hemodialysis Patients with Peripheral Artery Disease. Ann. Vasc. Dis. 2017, 10, 88–91. [Google Scholar] [CrossRef] [Green Version]
- Patel, V.I.; Mukhopadhyay, S.; Guest, J.M.; Conrad, M.F.; Watkins, M.T.; Kwolek, C.J.; LaMuraglia, G.M.; Cambria, R.P. Impact of Severe Chronic Kidney Disease on Outcomes of Infrainguinal Peripheral Arterial Intervention. J. Vasc. Surg. 2014, 59, 368–375. [Google Scholar] [CrossRef] [Green Version]
- Kim, H.O.; Kim, J.-M.; Woo, J.S.; Choi, D.; Ahn, C.-M.; Lee, S.-W.; Lee, J.-H.; Choi, S.-H.; Yu, C.W.; Min, P.K. Young-Guk other The Korean Vascular Intervention Society (K-VIS) Endovascular therapy in Lower Limb Artery diseases registry (ELLA) Registry Investigators; Effects of Chronic Kidney Disease on Clinical Outcomes in Patients with Peripheral Artery Disease Under-going Endovascular Treatment: Analysis from the K-VISELLA Registry. Int. J. Cardiol. 2018, 262, 32–37. [Google Scholar] [CrossRef]
- O’Hare, A.M.; Bertenthal, D.; Shlipak, M.G.; Sen, S.; Chren, M.-M. Impact of Renal Insufficiency on Mortality in Advanced Lower Extremity Peripheral Arterial Disease. J. Am. Soc. Nephrol. 2004, 16, 514–519. [Google Scholar] [CrossRef]
- Lüders, F.; Bunzemeier, H.; Engelbertz, C.; Malyar, N.M.; Meyborg, M.; Roeder, N.; Berger, K.; Reinecke, H. CKD and Acute and Long-Term Outcome of Patients with Peripheral Artery Disease and Critical Limb Ischemia. Clin. J. Am. Soc. Nephrol. 2015, 11, 216–222. [Google Scholar] [CrossRef] [Green Version]
- Heideman, P.P.; Rajebi, M.R.; McKusick, M.A.; Bjarnason, H.; Oderich, G.S.; Friese, J.L.; Fleming, M.D.; Stockland, A.H.; Harmsen, W.S.; Mandrekar, J.; et al. Impact of Chronic Kidney Disease on Clinical Outcomes of Endovascular Treatment for Femoropopliteal Arterial Disease. J. Vasc. Interv. Radiol. 2016, 27, 1204–1214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ortmann, J.; Gahl, B.; Diehm, N.; Dick, F.; Traupe, T.; Baumgartner, I. Survival Benefits of Revascularization in Patients with Critical Limb Ischemia and Renal Insufficiency. J. Vasc. Surg. 2012, 56, 737–745.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fallon, J.M.; Goodney, P.P.; Stone, D.H.; Patel, V.I.; Nolan, B.W.; Kalish, J.A.; Zhao, Y.; Hamdan, A.D. Vascular Study Group of New England Outcomes of Lower Extremity Revascularization among the Hemodialysis-Dependent. J. Vasc. Surg. 2015, 62, 1183–1191.e1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, J.X.; Glorioso, T.J.; Dattilo, P.B.; Aggarwal, V.; Ho, P.M.; Barón, A.E.; Donaldson, D.; Armstrong, E.J.; Klein, A.; Giri, J.; et al. Effect of Chronic Kidney Disease on Mortality in Patients Who Underwent Lower Extremity Peripheral Vascular Intervention. Am. J. Cardiol. 2017, 119, 669–674. [Google Scholar] [CrossRef] [PubMed]
- Meyer, A.; Schinz, K.; Lang, W.; Schmid, A.; Regus, S.; Rother, U. Outcomes and Influence of the Pedal Arch in Below-the-Knee Angioplasty in Patients with End-Stage Renal Disease and Critical Limb Ischemia. Ann. Vasc. Surg. 2016, 35, 121–129. [Google Scholar] [CrossRef]
- Nishibe, T.; Kondo, Y.; Dardik, A.; Muto, A.; Koizumi, J.; Nishibe, M. Stent Placement in the Superficial Femoral Artery for Patients on Chronic Hemodialysis with Peripheral Artery Disease. Int. Angiol. 2009, 28, 484–489. [Google Scholar]
- Meyer, A.; Lang, W.; Borowski, M.; Torsello, G.; Bisdas, T.; Schmitz-Rixen, T.; Gkremoutis, A.; Steinbauer, M.; Betz, T.; Eckstein, H.-H.; et al. In-Hospital Outcomes in Patients with Critical Limb Ischemia and End-Stage Renal Disease after Revascularization. J. Vasc. Surg. 2016, 63, 966–973. [Google Scholar] [CrossRef] [Green Version]
- Garimella, P.S.; Balakrishnan, P.; Correa, A.; Poojary, P.; Annapureddy, N.; Chauhan, K.; Patel, A.; Patel, S.; Konstantinidis, I.; Chan, L.; et al. Nationwide Trends in Hospital Outcomes and Utilization After Lower Limb Revascularization in Patients on Hemodialysis. JACC Cardiovasc. Interv. 2017, 10, 2101–2110. [Google Scholar] [CrossRef]
- Khan, T.; Plotkin, A.; Magee, G.A.; Shin, L.; Woelfel, S.L.; Ziegler, K.R.; Shih, C.D.; Weaver, F.A.; Armstrong, D.G.; Rowe, V.L. Functional Ambulatory Status as a Potential Adjunctive Decision-Making Tool Following Wound, Level of Ischemia, and Severity of Foot Infection Assessment. J. Vasc. Surg. 2020, 72, 738–746. [Google Scholar] [CrossRef]
- Mills, J.L. Modern Treatment of Chronic Limb-Threatening Ischemia Requires a Plan, Clinical Judgment, and Shared Decision Making. J. Vasc. Surg. 2020, 72, 389–390. [Google Scholar] [CrossRef]
- Cesari, M.; Penninx, B.W.; Newman, A.B.; Kritchevsky, S.B.; Nicklas, B.J.; Sutton-Tyrrell, K. Inflammatory Markers and Onset of Cardiovascular Events: Results from the Health ABC Study. Circulation 2003, 108, 2317–2322. [Google Scholar] [CrossRef] [Green Version]
- Burger-Kentischer, A.; Goebel, H.; Seiler, R.; Fraedrich, G.; Schaefer, H.E.; Dimmeler, S.; Kleemann, R.; Bernhagen, J.; Ihling, C. Expression of Macrophage Migration Inhibitory Factor in Different Stages of Human Atherosclerosis. Circulation 2002, 105, 1561–1566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biscetti, F.; Straface, G.; Bertoletti, G.; Vincenzoni, C.; Snider, F.; Arena, V.; Landolfi, R.; Flex, A. Identification of a Potential Proinflammatory Genetic Profile Influencing Carotid Plaque Vulnerability. J. Vasc. Surg. 2015, 61, 374–381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmidt, M.I.; Duncan, B.B.; Sharrett, A.R.; Lindberg, G.; Savage, P.J.; Offenbacher, S.; Azambuja, M.I.; Tracy, R.P.; Heiss, G. Markers of Inflammation and Prediction of Diabetes Mellitus in Adults (Atherosclerosis Risk in Communities Study): A Cohort Study. Lancet 1999, 353, 1649–1652. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Katsiki, N. Oxidative Stress and Inflammation: Their Role in the Pathogenesis of Peripheral Artery Disease with or without Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2018, 16, 547–554. [Google Scholar] [CrossRef]
- Signorelli, S.S.; Anzaldi, M.; Libra, M.; Navolanic, P.M.; Malaponte, G.; Mangano, K.; Quattrocchi, C.; Marco, D.R.; Fiore, V.; Neri, S. Plasma Levels of Inflammatory Biomarkers in Peripheral Arterial Disease: Results of a Cohort Study. Angiology 2016, 67, 870–874. [Google Scholar] [CrossRef]
- Unlu, Y.; Karapolat, S.; Karaca, Y.; Kiziltunc, A.; Kızıltunç, A. Comparison of Levels of Inflammatory Markers and Hemostatic Factors in the Patients with and without Peripheral Arterial Disease. Thromb. Res. 2006, 117, 357–364. [Google Scholar] [CrossRef]
- Barani, J.; Nilsson, J.-A.; Mattiasson, I.; Lindblad, B.; Gottsäter, A. Inflammatory Mediators Are Associated with 1-Year Mortality in Critical Limb Ischemia. J. Vasc. Surg. 2005, 42, 75–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schillinger, M.; Exner, M.; Mlekusch, W.; Haumer, M.; Rumpold, H.; Ahmadi, R.; Sabeti, S.; Wagner, O.; Minar, E. Endovascular Revascularization Below the Knee: 6-month Results and Predictive Value of C-reactive Protein Level. Radiology 2003, 227, 419–425. [Google Scholar] [CrossRef]
- Lin, C.-W.; Hsu, L.-A.; Chen, C.-C.; Yeh, J.-T.; Sun, J.-H.; Lin, C.-H.; Chen, S.-T.; Hsu, B.R.-S.; Huang, Y.-Y. C-Reactive Protein as an Outcome Predictor for Percutaneous Transluminal Angioplasty in Diabetic Patients with Peripheral Arterial Disease and Infected Foot Ulcers. Diabetes Res. Clin. Pr. 2010, 90, 167–172. [Google Scholar] [CrossRef]
- Bleda, S.; De Haro, J.; Acin, F.; Varela, C.; Esparza, L.; De Maturana, I.L. Inflammatory Burden Predicts Long-Term Outcomes in Endovascular Therapy in Peripheral Arterial Disease. Ann. Vasc. Surg. 2013, 27, 459–466. [Google Scholar] [CrossRef] [PubMed]
- Stone, P.A.; Schlarb, H.; Campbell, J.E.; Williams, D.; Thompson, S.N.; John, M.; Campbell, J.R.; Aburahma, A.F. C-Reactive Protein and Brain Natriuretic Peptide as Predictors of Adverse Events after Lower Extremity Endovascular Revascularization. J. Vasc. Surg. 2014, 60, 652–660. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Straface, G.; Biscetti, F.; Pitocco, D.; Bertoletti, G.; Misuraca, M.; Vincenzoni, C.; Snider, F.; Arena, V.; Stigliano, E.; Angelini, F.; et al. Assessment of the Genetic Effects of Polymorphisms in the Osteoprotegerin Gene, TNFRSF11B, on Serum Osteoprotegerin Levels and Carotid Plaque Vulnerability. Stroke 2011, 42, 3022–3028. [Google Scholar] [CrossRef] [PubMed]
- Augoulea, A.; Vrachnis, N.; Lambrinoudaki, I.; Dafopoulos, K.; Iliodromiti, Z.; Daniilidis, A.; Varras, M.; Alexandrou, A.; Deligeoroglou, E.; Creatsas, G. Osteoprotegerin as a Marker of Atherosclerosis in Diabetic Patients. Int. J. Endocrinol. 2013, 2013, 1–6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biscetti, F.; Porreca, C.F.; Bertucci, F.; Straface, G.; Santoliquido, A.; Tondi, P.; Angelini, F.; Pitocco, D.; Santoro, L.; Gasbarrini, A.; et al. TNFRSF11B Gene Polymorphisms Increased Risk of Peripheral Arterial Occlusive Disease and Critical Limb Ischemia in Patients with Type 2 Diabetes. Acta Diabetol. 2014, 51, 1025–1032. [Google Scholar] [CrossRef] [PubMed]
- Giovannini, S.; Tinelli, G.; Biscetti, F.; Straface, G.; Angelini, F.; Pitocco, D.; Mucci, L.; Landolfi, R.; Flex, A. Serum High Mobility Group Box-1 and Osteoprotegerin Levels Are Associated with Peripheral Arterial Disease and Critical Limb Ischemia in Type 2 Diabetic Subjects. Cardiovasc. Diabetol. 2017, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Protogerou, A.; Zampeli, E.; Fragiadaki, K.; Stamatelopoulos, K.; Papamichael, C.; Sfikakis, P. A Pilot Study of Endothelial Dysfunction and Aortic Stiffness after Interleukin-6 Receptor Inhibition in Rheumatoid Arthritis. Atherosclerosis 2011, 219, 734–736. [Google Scholar] [CrossRef]
- Li, L.; Renier, G. The Connection Between C-Reactive Protein (CRP) and Diabetic Vasculopathy. Focus on Preclinical Findings. Curr. Diabetes Rev. 2010, 6, 27–34. [Google Scholar] [CrossRef]
- Mai, J.; Virtue, A.; Shen, J.; Wang, H.; Yang, X.-F. An Evolving New Paradigm: Endothelial Cells–Conditional Innate Immune Cells. J. Hematol. Oncol. 2013, 6, 61. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Erlandsson, H.H.; Andersson, U. Mini-Review: The Nuclear Protein hmgb1 as a Proinflammatory Mediator. Eur. J. Immunol. 2004, 34, 1503–1512. [Google Scholar] [CrossRef] [PubMed]
- Kalinina, N.; Agrotis, A.; Tararak, E.; Antropova, Y.; Kanellakis, P.; Ilyinskaya, O.; Bobik, A. Increased Expression of the Dna-Binding Cytokine HMBG1 in Human Atherosclerotic Lesions: Role of Activated Macrophages and Cytokines. Cardiovasc. Pathol. 2004, 13, 97–98. [Google Scholar] [CrossRef] [Green Version]
- Biscetti, F.; Straface, G.; De Cristofaro, R.; Lancellotti, S.; Rizzo, P.; Arena, V.; Stigliano, E.; Pecorini, G.; Egashira, K.; De Angelis, G.; et al. High-Mobility Group Box-1 Protein Promotes Angiogenesis After Peripheral Ischemia in Diabetic Mice Through a VEGF-Dependent Mechanism. Diabetes 2010, 59, 1496–1505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oozawa, S.; Sano, S.; Nishibori, M. Usefulness of High Mobility Group Box 1 Protein as a Plasma Biomarker in Patient with Peripheral Artery Disease. Acta Med. Okayama 2014, 68, 157–162. [Google Scholar] [PubMed]
- Biscetti, F.; Nardella, E.; Cecchini, A.L.; Flex, A.; Landolfi, R. Biomarkers of Vascular Disease in Diabetes: The Adipose-Immune System Cross Talk. Intern. Emerg. Med. 2020, 15, 381–393. [Google Scholar] [CrossRef]
- Hajer, G.R.; Van Haeften, T.W.; Visseren, F.L.J. Adipose Tissue Dysfunction in Obesity, Diabetes, and Vascular Diseases. Eur. Hear. J. 2008, 29, 2959–2971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biscetti, F.; Nardella, E.; Bonadia, N.; Angelini, F.; Pitocco, D.; Santoliquido, A.; Filipponi, M.; Landolfi, R.; Flex, A. Association between Plasma Omentin-1 Levels in Type 2 Diabetic Patients and Peripheral Artery Disease. Cardiovasc. Diabetol. 2019, 18, 74–77. [Google Scholar] [CrossRef]
- Biscetti, F.; Nardella, E.; Rando, M.M.; Cecchini, A.L.; Angelini, F.; Cina, A.; Iezzi, R.; Filipponi, M.; Santoliquido, A.; Pitocco, D.; et al. Association between Omentin-1 and Major Cardiovascular Events after Lower Extremity Endovascular Revascularization in DI-Abetic Patients: A Prospective Cohort Study. Cardiovasc. Diabetol. 2020, 19, 1–11. [Google Scholar] [CrossRef]
- Patel, K.M.; Strong, A.; Tohyama, J.; Jin, X.; Morales, C.R.; Billheimer, J.; Millar, J.; Kruth, H.; Rader, D.J. Macrophage Sortilin Promotes LDL Uptake, Foam Cell Formation, and Atherosclerosis. Circ. Res. 2015, 116, 789–796. [Google Scholar] [CrossRef] [Green Version]
- Ogawa, K.; Ueno, T.; Iwasaki, T.; Kujiraoka, T.; Ishihara, M.; Kunimoto, S.; Takayama, T.; Kanai, T.; Hirayama, A.; Hattori, H. Soluble Sortilin Is Released by Activated Platelets and Its Circulating Levels Are Associated with Cardiovascular Risk Factors. Atherosclerosis 2016, 249, 110–115. [Google Scholar] [CrossRef]
- Goettsch, C.; Kjolby, M.; Aikawa, E. Sortilin and Its Multiple Roles in Cardiovascular and Metabolic Diseases. Arter. Thromb. Vasc. Biol. 2018, 38, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Biscetti, F.; Bonadia, N.; Santini, F.; Angelini, F.; Nardella, E.; Pitocco, D.; Santoliquido, A.; Filipponi, M.; Landolfi, R.; Flex, A. Sortilin Levels Are Associated with Peripheral Arterial Disease in Type 2 Diabetic Subjects. Cardiovasc. Diabetol. 2019, 18, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biscetti, F.; Nardella, E.; Rando, M.M.; Cecchini, A.L.; Bonadia, N.; Bruno, P.; Angelini, F.; Di Stasi, C.; Contegiacomo, A.; Santoliquido, A.; et al. Sortilin Levels Correlate with Major Cardiovascular Events of Diabetic Patients with Peripheral Artery Disease Following Revas-Cularization: A Prospective Study. Cardiovasc. Diabetol. 2020, 19, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Venermo, M.; Sprynger, M.; Desormais, I.; Björck, M.; Brodmann, M.; Cohnert, T.; De Carlo, M.; Espinola-Klein, C.; Kownator, S.; Mazzolai, L.; et al. Follow-up of Patients after Revascularisation for Peripheral Arterial Diseases: A Consensus Document from the European Socie-Ty of Cardiology Working Group on Aorta and Peripheral Vascular Diseases and the European Society for Vascular Surgery. Eur. J. Prev. Cardiol. 2019, 26, 1971–1984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Humphries, M.D.; Pevec, W.C.; Laird, J.R.; Yeo, K.K.; Hedayati, N.; Dawson, D.L. Early Duplex Scanning after Infrainguinal Endovascular Therapy. J. Vasc. Surg. 2011, 53, 353–358. [Google Scholar] [CrossRef] [Green Version]
- Baril, D.T.; Rhee, R.Y.; Kim, J.; Makaroun, M.S.; Chaer, R.A.; Marone, L.K. Duplex Criteria for Determination of in-Stent Stenosis after Angioplasty and Stenting of the Superficial Femoral Artery. J. Vasc. Surg. 2009, 49, 133–139. [Google Scholar] [CrossRef] [Green Version]
- Saarinen, E.; Laukontaus, S.; Albäck, A.; Venermo, M. Duplex Surveillance After Endovascular Revascularisation for Critical Limb Ischaemia. Eur. J. Vasc. Endovasc. Surg. 2014, 47, 418–421. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CAPRIE Steering Committee. A Randomised, Blinded, Trial of Clopidogrel versus Aspirin in Patients at Risk of Ischaemic Events (Caprie). Caprie Steering Committee. Lancet 1996, 348, 1329–1339. [Google Scholar] [CrossRef]
- Bhatt, D.L.; Fox, K.A.; Hacke, W.; Berger, P.B.; Black, H.R.; Boden, W.E.; Cacoub, P.; Cohen, E.A.; Creager, M.A.; Easton, J.D.; et al. Clopidogrel and Aspirin versus Aspirin Alone for the Prevention of Atherothrombotic Events. N. Engl. J. Med. 2006, 354, 1706–1717. [Google Scholar] [CrossRef] [Green Version]
- Cassar, K.; Ford, I.; Greaves, M.; Bachoo, P.; Brittenden, J. Randomized Clinical Trial of the Antiplatelet Effects of Aspirin-Clopidogrel Combination versus Aspirin Alone after Lower Limb Angioplasty. Br. J. Surg. 2005, 92, 159–165. [Google Scholar] [CrossRef]
- Tepe, G.; Bantleon, R.; Brechtel, K.F.M.; Schmehl, J.-M.; Zeller, T.; Claussen, C.D.; Strobl, F.F.X. Management of Peripheral Arterial Interventions with Mono or Dual Antiplatelet Therapy—the MIRROR Study: A Randomised and Double-Blinded Clinical Trial. Eur. Radiol. 2012, 22, 1998–2006. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biscetti, F.; Nardella, E.; Rando, M.M.; Cecchini, A.L.; Gasbarrini, A.; Massetti, M.; Flex, A. Outcomes of Lower Extremity Endovascular Revascularization: Potential Predictors and Prevention Strategies. Int. J. Mol. Sci. 2021, 22, 2002. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22042002
Biscetti F, Nardella E, Rando MM, Cecchini AL, Gasbarrini A, Massetti M, Flex A. Outcomes of Lower Extremity Endovascular Revascularization: Potential Predictors and Prevention Strategies. International Journal of Molecular Sciences. 2021; 22(4):2002. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22042002
Chicago/Turabian StyleBiscetti, Federico, Elisabetta Nardella, Maria Margherita Rando, Andrea Leonardo Cecchini, Antonio Gasbarrini, Massimo Massetti, and Andrea Flex. 2021. "Outcomes of Lower Extremity Endovascular Revascularization: Potential Predictors and Prevention Strategies" International Journal of Molecular Sciences 22, no. 4: 2002. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22042002





