The Attenuated Secretion of Hyaluronan by UVA-Exposed Human Fibroblasts Is Associated with Up- and Downregulation of HYBID and HAS2 Expression via Activated and Inactivated Signaling of the p38/ATF2 and JAK2/STAT3 Cascades
Abstract
:1. Introduction
2. Results
2.1. Effects of UVA on the Cytotoxic Properties of Human Dermal Fibroblasts (HDFs)
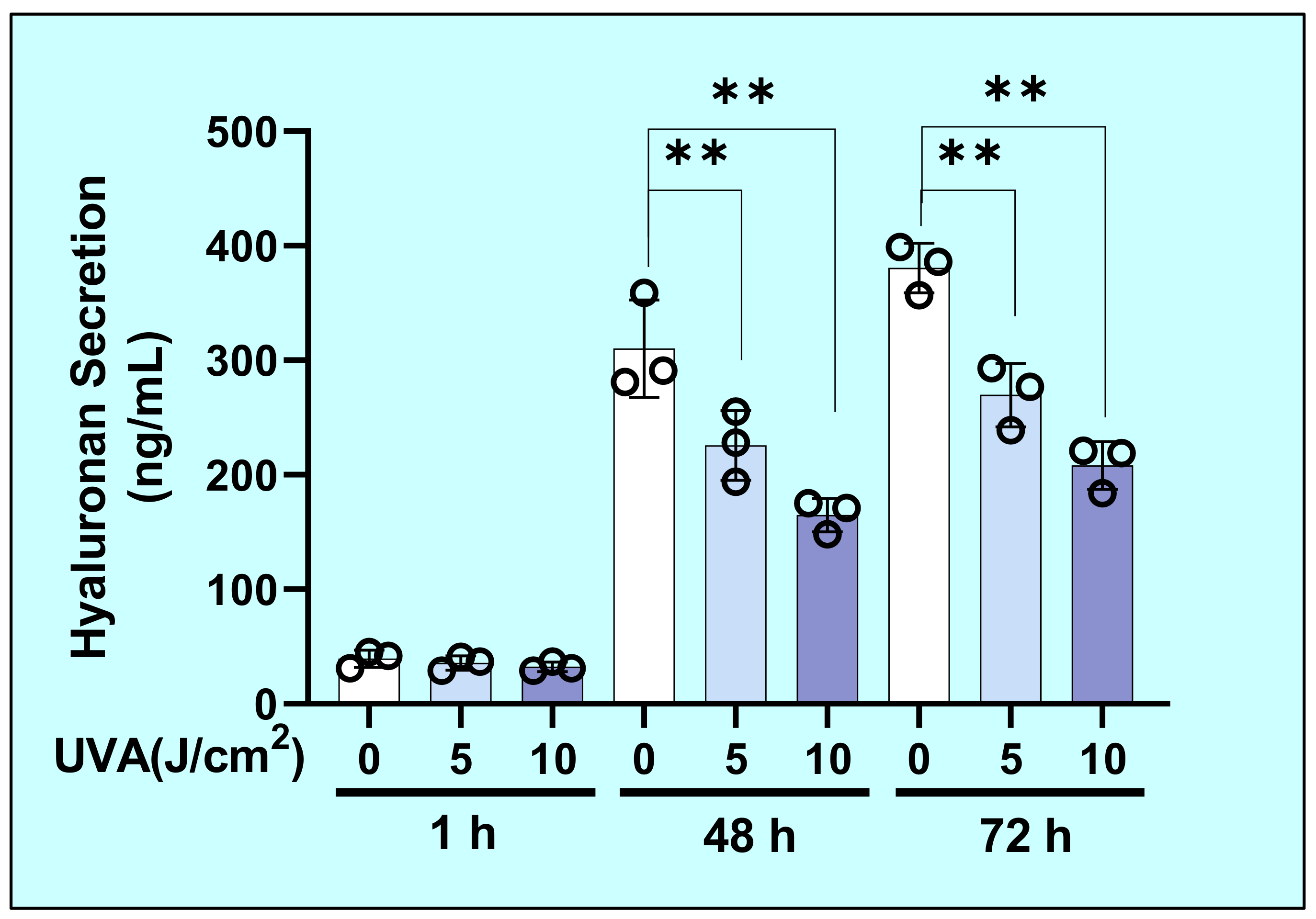
2.2. Effects of UVA on the Secretion of Hyaluronan (HA) by HDFs
2.3. Molecular Mass Distribution of Secreted HA
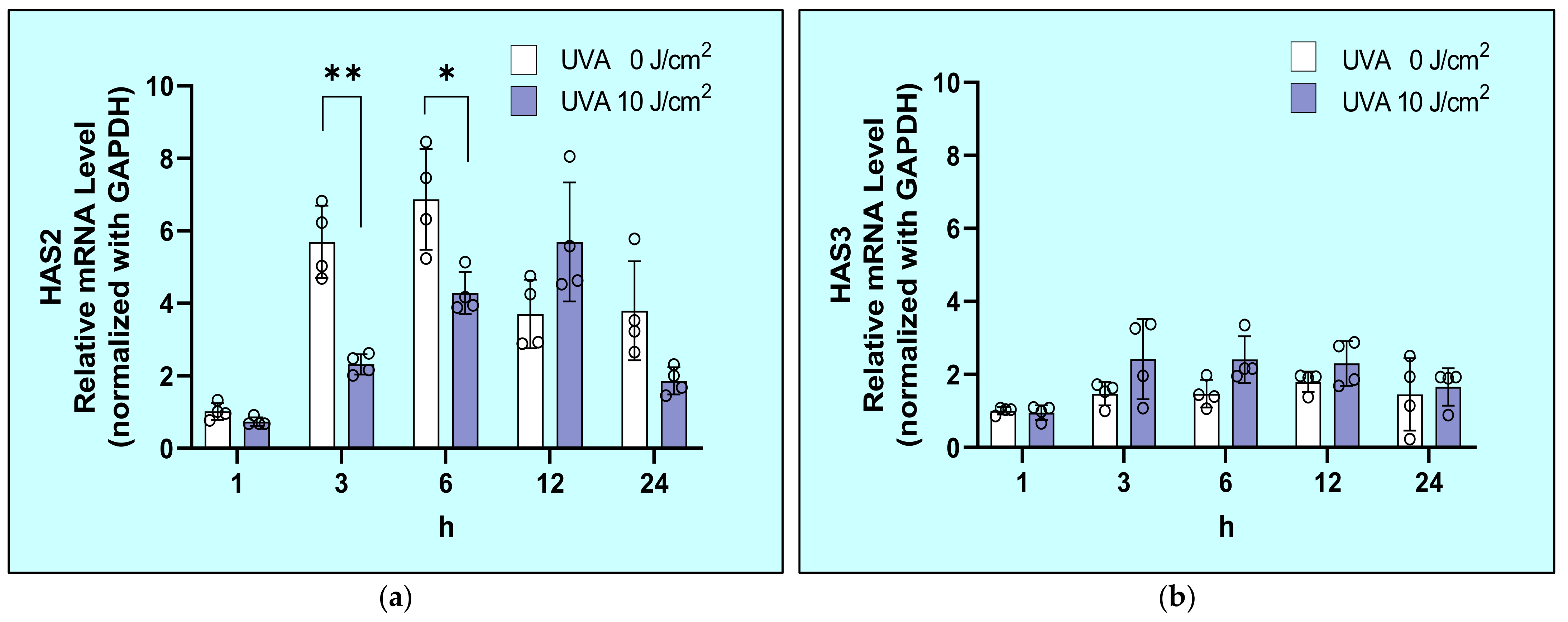
2.4. Effects of UVA on mRNA Expression Levels of HAS1/2/3 in HDFs
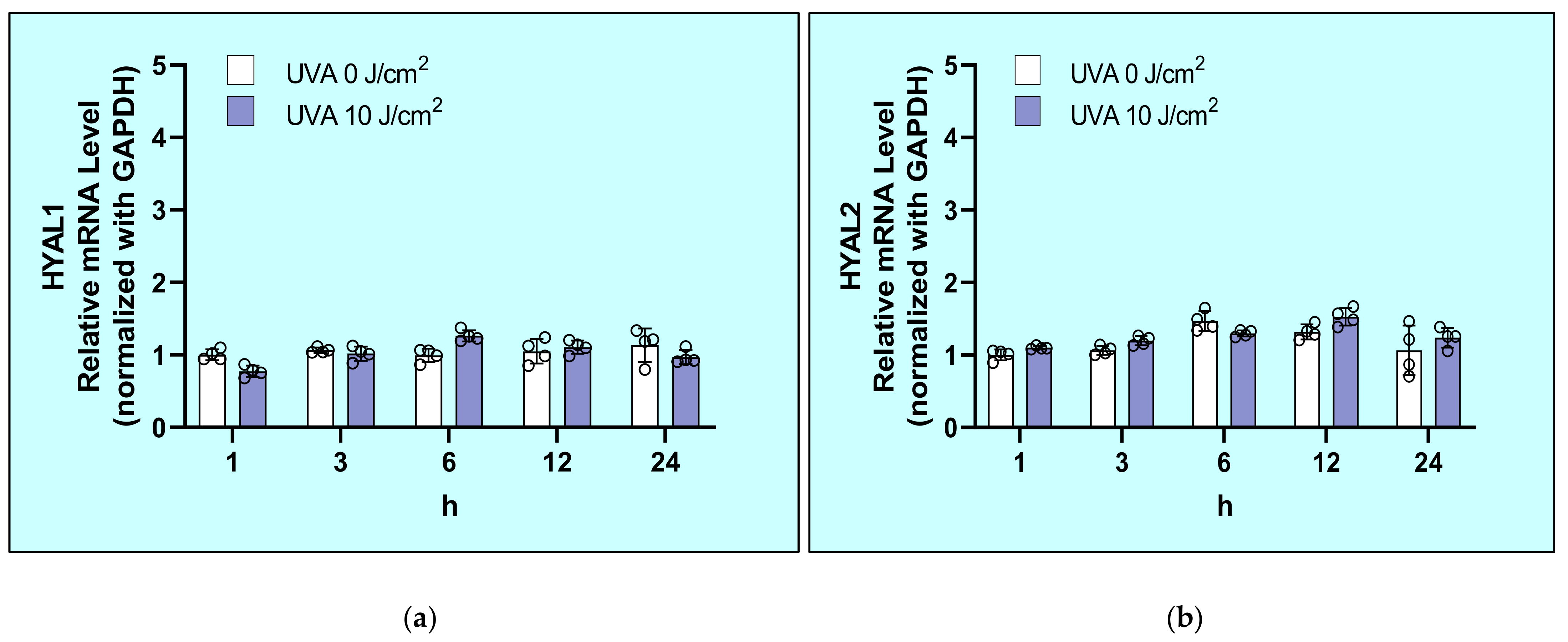
2.5. Effects of UVA on mRNA Levels of Hyaluronidases (HYAL1/2/3) in HDFs
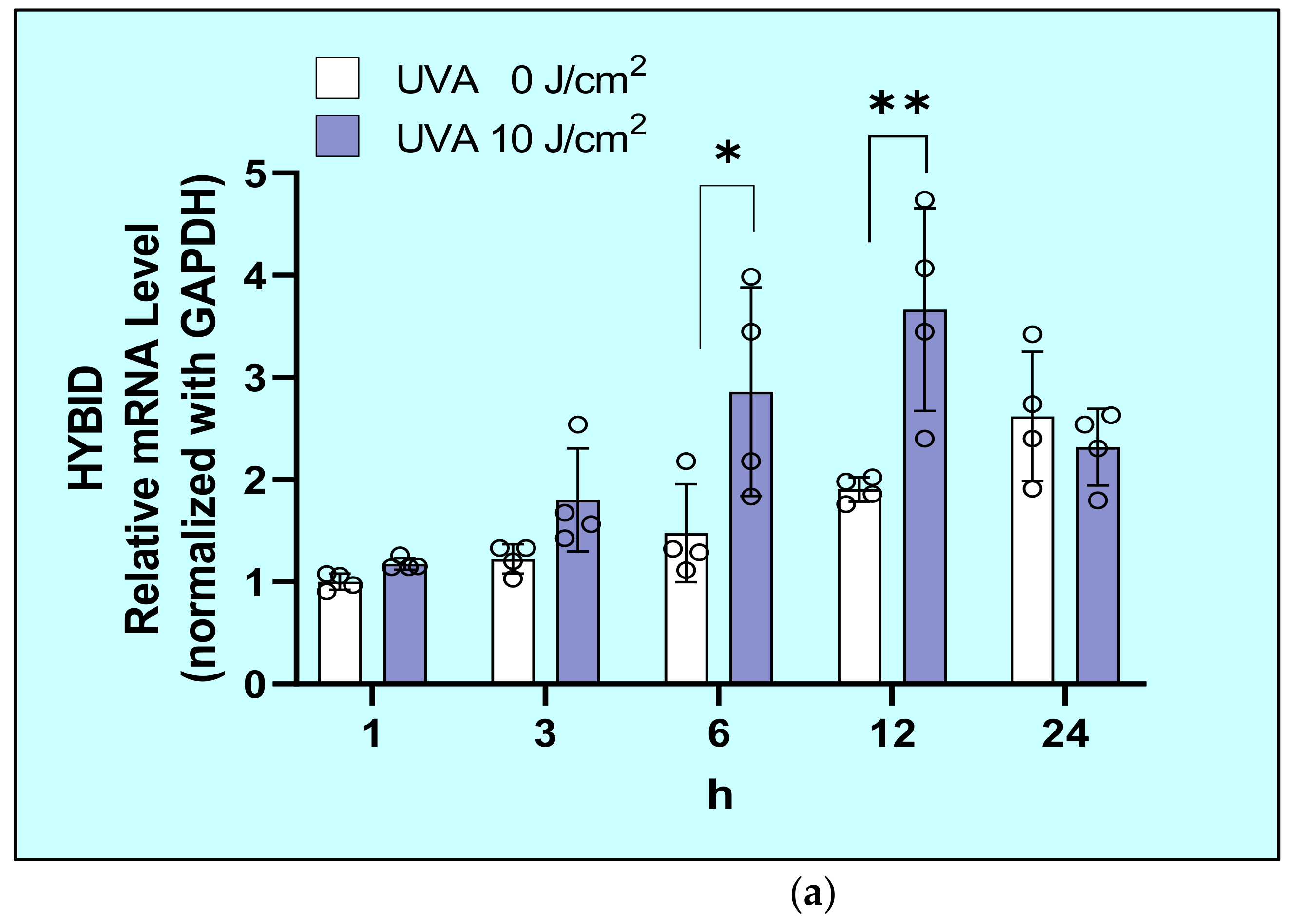
2.6. Effects of UVA on mRNA and Protein Levels of HYaluronan Binding Protein Involved in HA Depolymerization (HYBID) in HDFs
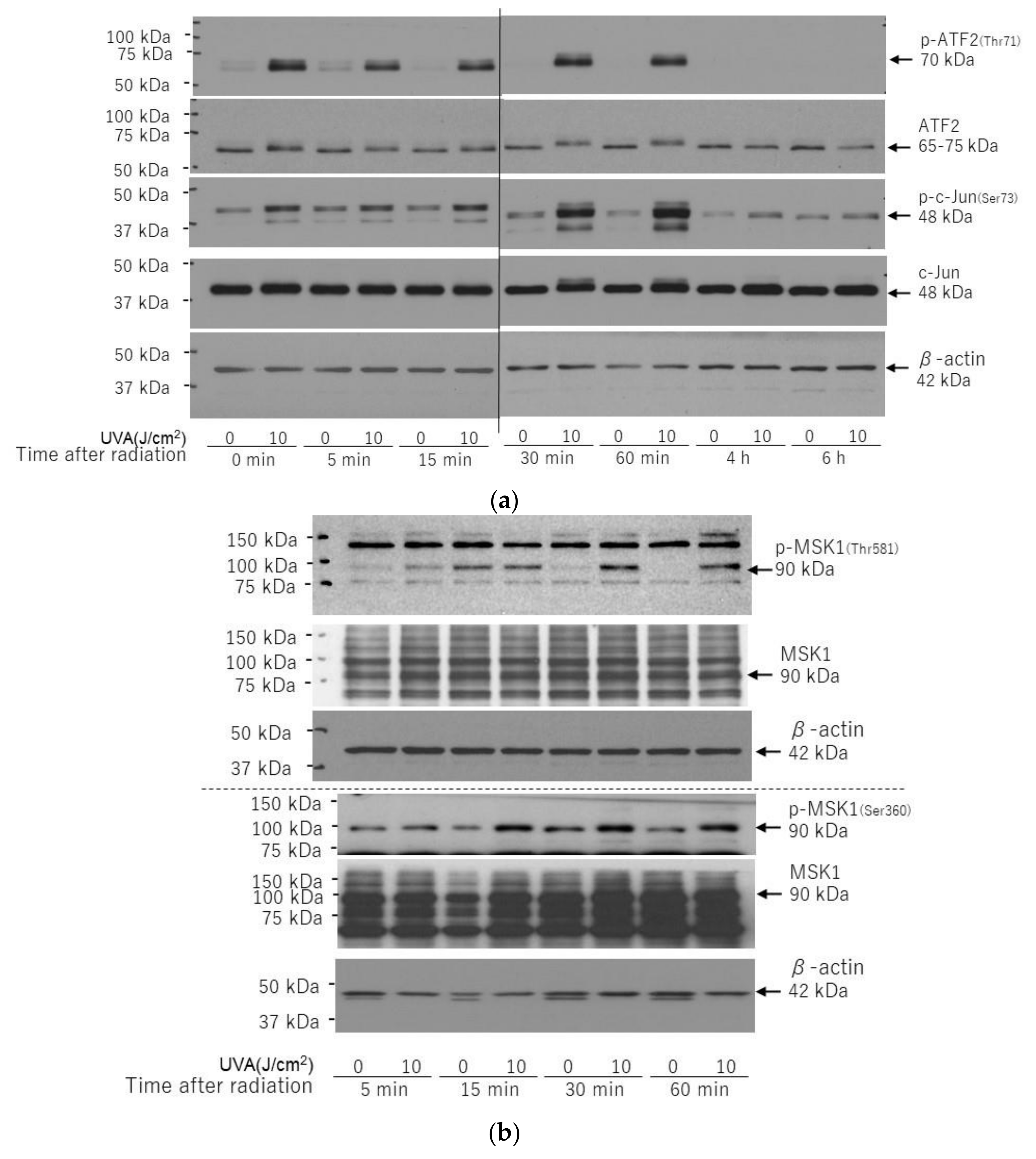
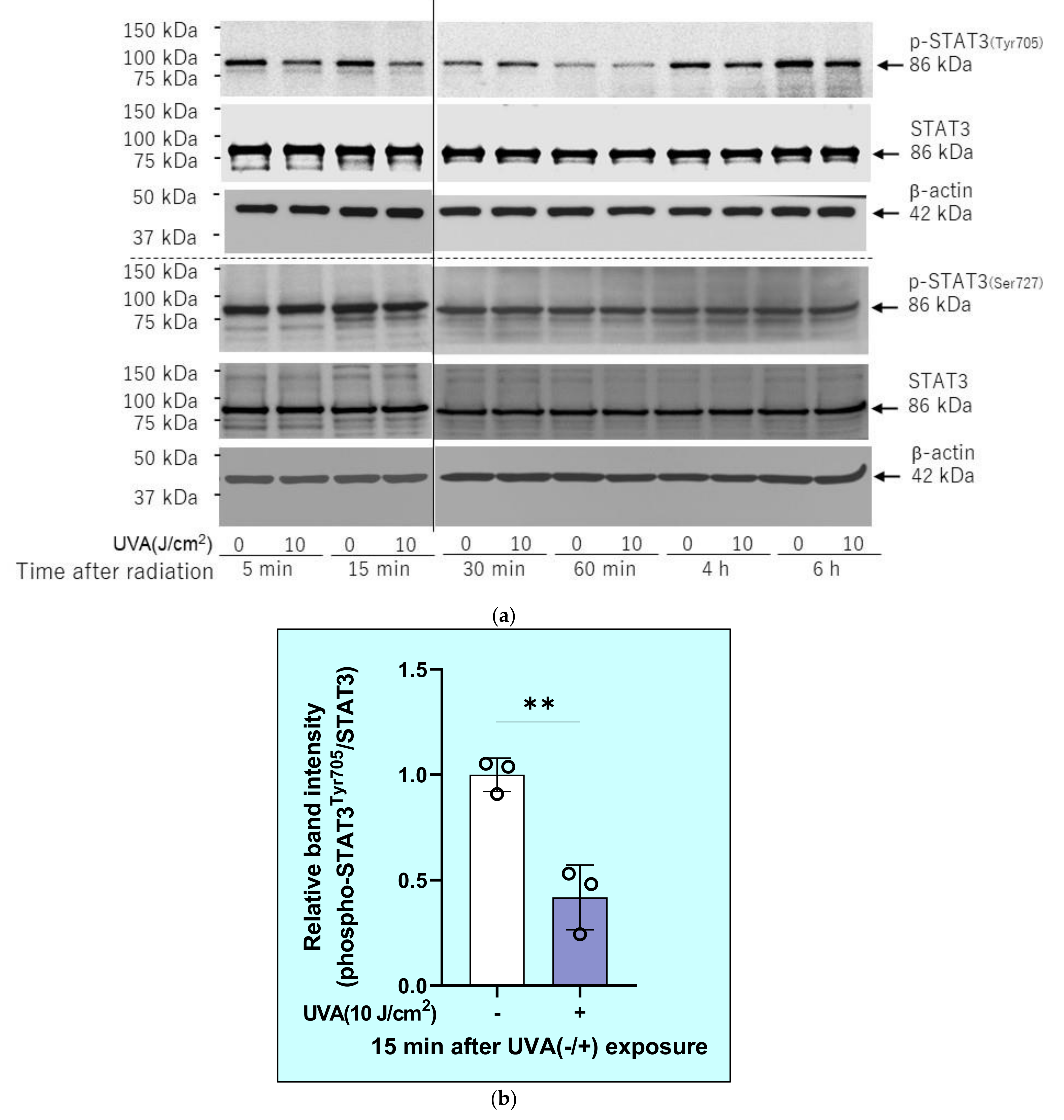
2.7. Effects of UVA on Intracellular Signaling Pathways in HDFs
2.8. Effects of Signaling Inhibitors on mRNA Expression Levels of HAS2 and HYBID
2.9. Effects of Transfection of c-Fos or ATF-2 siRNAs on the UVA-Induced Increase in HYBID Protein Level in HDFs
2.10. Effects of Inhibitors of Protein Phosphatases on the UVA-Induced Decrease in the Phosphorylation of STAT3 in HDFs
2.11. Effect of Sodium Orthovanadate on the UVA-Decreased mRNA Expression of HAS2 in HDFs
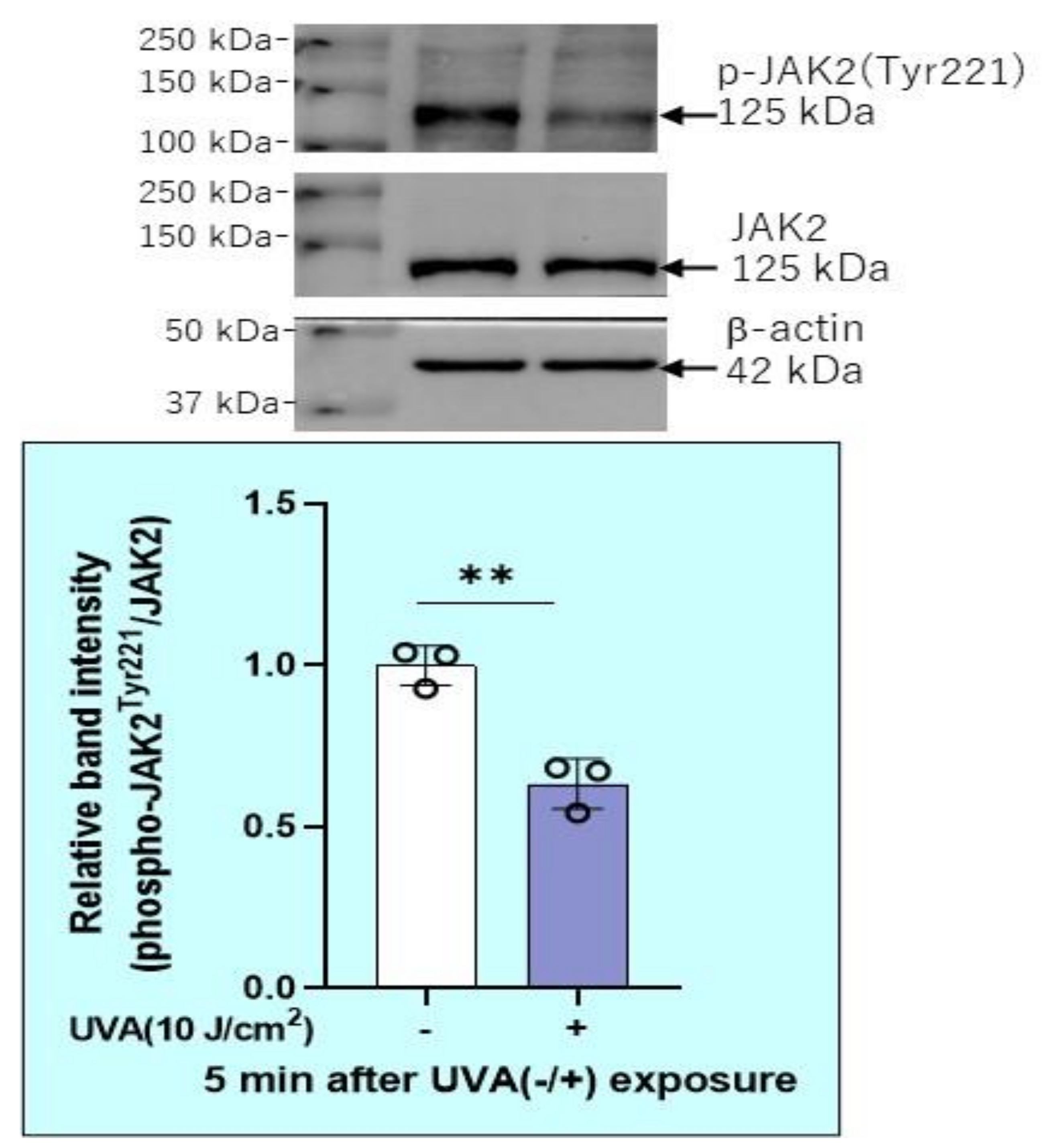
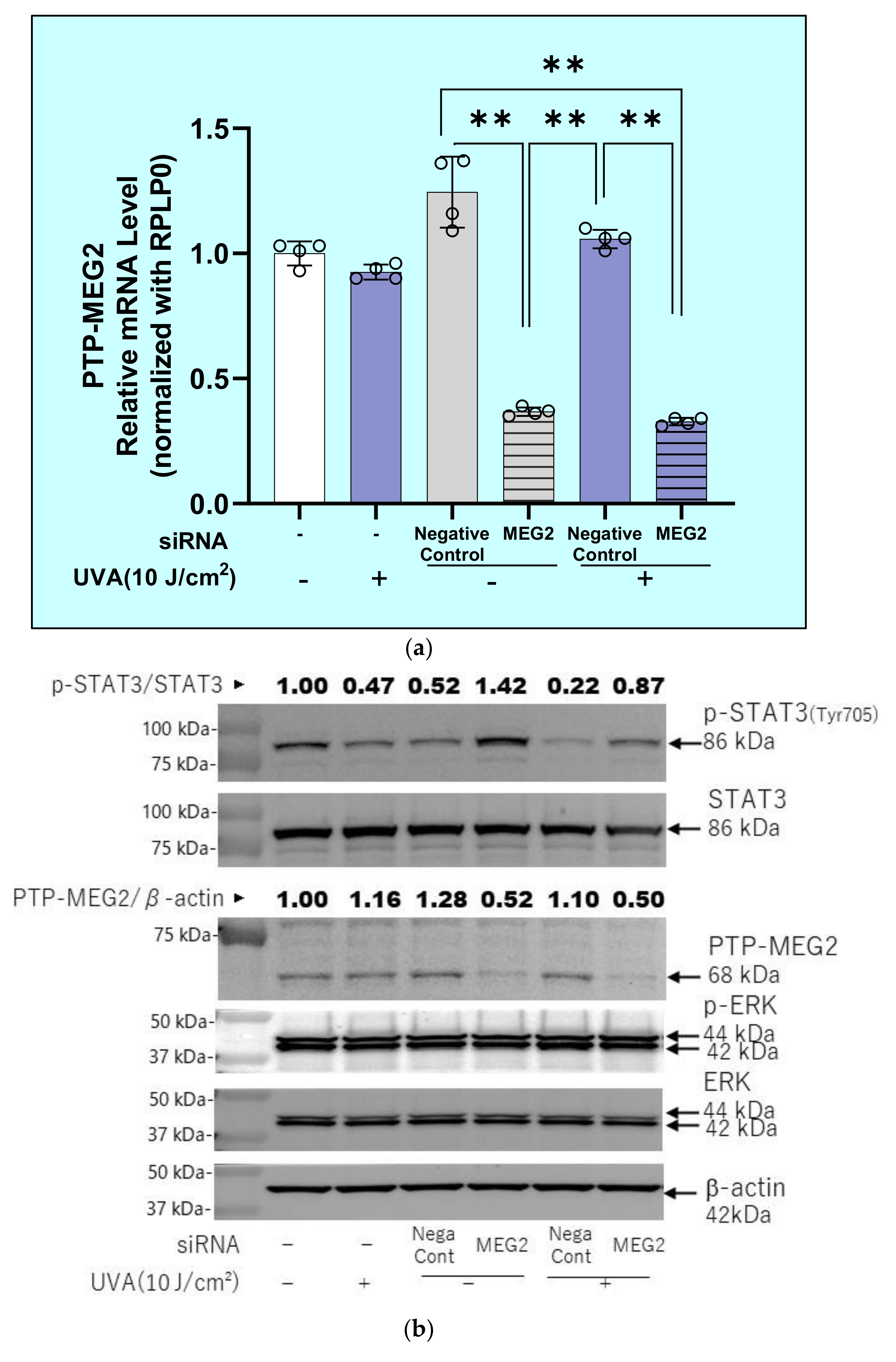
2.12. Effects of Transfection of a Protein Tyrosine Phosphatase (PTPMEG2) siRNA on the UVA-Induced Decrease in the Phosphorylation of STAT3 at Tyr 705 and JAK at Tyr 221 in HDFs
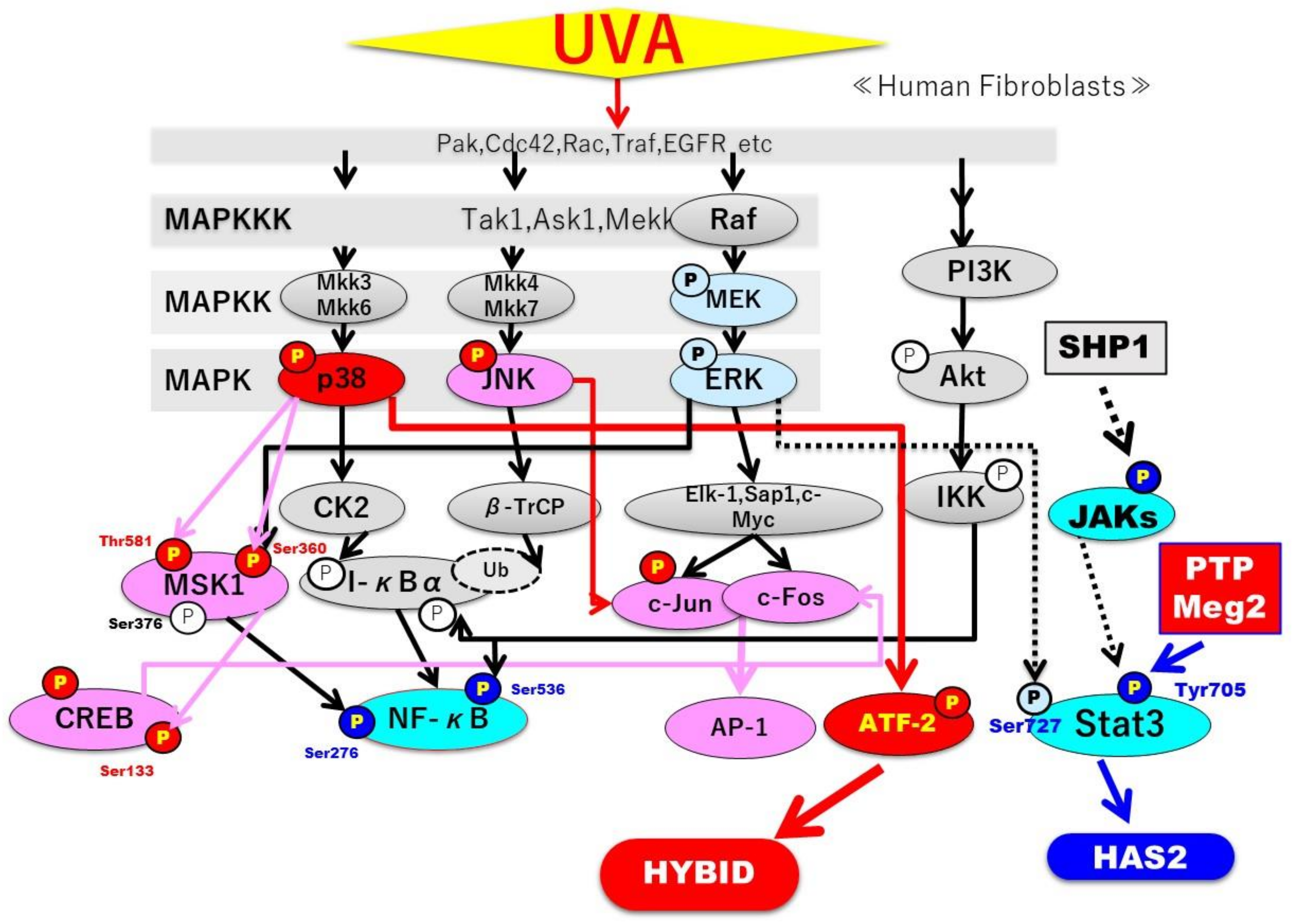
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Cultures
4.3. Cell Viability Assay
4.4. Measurement of HA
4.5. Real Time qRT-PCR
4.6. siRNA Transfection
4.7. Western Blotting
4.8. UVA Irradiation Procedures
4.9. Characterization of HA Molecular Mass Distribution
4.10. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| HA | hyaluronan |
| HDFs | human dermal fibroblast |
| HYBID | HYaluronan Binding protein Involved in HA Depolymerization |
| MMP | matrix-metalloprotease |
| HAS | hyaluronan synthases |
| HYAL | hyaluronidase |
| UV | ultraviolet |
References
- Imokawa, G.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging I: Reduced skin elasticity, highly associated with enhanced dermal elastase activity, triggers wrinkling and sagging. Int. J. Mol. Sci. 2015, 16, 7753–7775. [Google Scholar] [CrossRef] [Green Version]
- Margelin, D.; Medaisko, C.; Lombard, D.; Picard, J.; Fourtanier, A. Hyaluronic acid and dermatan sulfate are selectively stimulated by retinoic acid in irradiated and nonirradiated hairless mouse skin. J. Investig. Dermatol. 1996, 106, 505–509. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, Y.; Ishikawa, O.; Okada, K.; Ohnishi, K.; Miyachi, Y. Disaccharide analysis of the skin glycosaminoglycans in chronically ultraviolet light-irradiated hairless mice. J. Dermatol. Sci. 1995 10, 139–144. [CrossRef]
- Fisher, G.J.; Datta, S.C.; Talwar, H.S.; Wang, Z.Q.; Varani, J.; Kang, S.; Voorhees, J.J. Molecular basis of sun-induced premature skin ageing and retinoid antagonism. Nature 1996, 379, 335–339. [Google Scholar] [CrossRef]
- Fisher, G.J.; Quan, T.; Purohit, T.; Shao, Y.; Cho, M.K.; He, T.; Varani, J.; Kang, S.; Voorhees, J.J. Collagen fragmentation promotes oxidative stress and elevates matrix metalloproteinase-1 in fibroblasts in aged human skin. Am. J. Pathol. 2009, 174, 101–114. [Google Scholar] [CrossRef] [Green Version]
- Tsukahara, K.; Takema, Y.; Moriwaki, S.; Tsuji, N.; Suzuki, Y.; Fujimura, T.; Imokawa, G. Selective inhibition of skin fibroblast elastase elicits a concentration-dependent prevention of ultraviolet B-induced wrinkle formation. J. Investig. Dermatol. 2001, 117, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Tsuji, N.; Moriwaki, S.; Suzuki, Y.; Takema, Y.; Imokawa, G. The role of elastases secreted by fibroblasts in wrinkle formation: Implication through selective inhibition of elastase activity. Photochem. Photobiol. 2001, 74, 283–290. [Google Scholar] [CrossRef]
- Tsukahara, K.; Nakagawa, H.; Moriwaki, S.; Kakuo, S.; Ohuchi, A.; Takema, Y.; Imokawa, G. Ovariectomy is sufficient to accelerate spontaneous skin ageing and to stimulate ultraviolet irradiation-induced photoageing of murine skin. Br. J. Dermatol. 2004, 151, 984–994. [Google Scholar] [CrossRef]
- Morisaki, N.; Moriwaki, S.; Sugiyama-Nakagiri, Y.; Haketa, K.; Takema, Y.; Imokawa, G. Neprilysin is identical to skin fibroblast elastase: Its role in skin aging and UV responses. J. Biol. Chem. 2010, 285, 39819–39827. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakajima, H.; Ezaki, Y.; Nagai, T.; Yoshioka, R.; Imokawa, G. Epithelial-mesenchymal interaction during UVB-induced up-regulation of neutral endopeptidase. Biochem. J. 2012, 443, 297–305. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imokawa, G.; Nakajima, H.; Ishida, K. Biological mechanisms underlying the ultraviolet radiation-induced formation of skin wrinkling and sagging II: Over-expression of neprilysin plays an essential role. Int. J. Mol. Sci. 2015, 16, 7776–7795. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imokawa, G. Epithelial-mesenchymal interaction mechanisms leading to the over-expression of neprilysin are involved in the UVB-induced formation of wrinkles in the skin. Exp. Dermatol. 2016, 25 (Suppl. 3), 2–13. [Google Scholar] [CrossRef] [Green Version]
- Akazaki, S.; Imokawa, G. Mechanical methods for evaluating skin surface architecture in relation to wrinkling. J. Dermatol. Sci. 2001, 27 (Suppl. 1), S5–S10. [Google Scholar] [CrossRef]
- Akazaki, S.; Nakagawa, H.; Kazama, H.; Osanai, O.; Kawai, M.; Takema, Y.; Imokawa, G. Age-related changes in skin wrinkles assessed by a novel three-dimensional morphometric analysis. Br. J. Dermatol. 2002, 147, 689–695. [Google Scholar] [CrossRef]
- Takema, Y.; Yorimoto, Y.; Kawai, M. The relationship between age-related changes in the physical properties and development of wrinkles in human facial skin. J. Soc. Cosmet. Chem. 1995, 46, 163–173. [Google Scholar]
- Yoshida, H.; Nagaoka, A.; Komiya, A.; Aoki, M.; Nakamura, S.; Morikawa, T.; Ohtsuki, R.; Sayo, T.; Okada, Y.; Takahashi, Y. Reduction of hyaluronan and increased expression of HYBID (alias CEMIP and KIAA1199) correlate with clinical symptoms in photoaged skin. Br. J. Dermatol. 2018, 179, 136–144. [Google Scholar] [CrossRef]
- Yoshida, H.; Komiya, A.; Ohtsuki, R.; Kusaka-Kikushima, A.; Sakai, S.; Kawabata, K.; Kobayashi, M.; Nakamura, S.; Nagaoka, A.; Sayo, T.; et al. Relationship of hyaluronan and HYBID (KIAA1199) expression with roughness parameters of photoaged skin in Caucasian women. Skin. Res. Technol. 2018, 24, 562–569. [Google Scholar] [CrossRef]
- Itano, N.; Kimata, K. Molecular cloning of human hyaluronan synthase. Biochem. Biophys. Res. Commun. 1996, 222, 816–820. [Google Scholar] [CrossRef] [PubMed]
- Stern, R. Hyaluronan catabolism: A new metabolic pathway. Eur. J. Cell. Biol. 2004, 83, 317–325. [Google Scholar] [CrossRef] [PubMed]
- Termeer, C.; Benedix, F.; Sleeman, J.; Fieber, C.; Voith, U.; Ahrens, T.; Miyake, K.; Freudenberg, M.; Galanos, C.; Simon, J.C. Oligosaccharides of Hyaluronan activate dendritic cells via toll-like receptor 4. J. Exp. Med. 2002, 195, 99–111. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, D.; Liang, J.; Fan, J.; Yu, S.; Chen, S.; Luo, Y.; Prestwich, G.D.; Mascarenhas, M.M.; Garg, H.G.; Quinn, D.A.; et al. Regulation of lung injury and repair by Toll-like receptors and hyaluronan. Nat. Med. 2005, 11, 1173–1179. [Google Scholar] [CrossRef]
- Yoshida, H.; Nagaoka, A.; Kusaka-Kikushima, A.; Tobiishi, M.; Kawabata, K.; Sayo, T.; Sakai, S.; Sugiyama, Y.; Enomoto, H.; Okada, Y.; et al. KIAA1199, a deafness gene of unknown function, is a new hyaluronan binding protein involved in hyaluronan depolymerization. Proc. Natl. Acad. Sci. USA 2013, 110, 5612–5617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suganuma, K.; Nakajima, H.; Ohtsuki, M.; Imokawa, G. Astaxanthin attenuates the UVA-induced up-regulation of matrix-metalloproteinase-1 and skin fibroblast elastase in human dermal fibroblasts. J. Dermatol. Sci. 2010, 58, 136–142. [Google Scholar] [CrossRef] [PubMed]
- Schmut, O.; Horwath-Winter, J.; Rieger, G.; Winkler, R.; Trummer, G.; Spitzenberger, H.; Wachswender, C. Iodide protection from UVB irradiation-induced degradation of hyaluronate and against UVB-damage of human conjunctival fibroblasts. Graefes Arch. Clin. Exp. Ophthalmol. 2004, 242, 279–283. [Google Scholar] [CrossRef]
- Averbeck, M.; Gebhardt, C.A.; Voigt, S.; Beilharz, S.; Anderegg, U.; Termeer, C.C.; Sleeman, J.P.; Simon, J.C. Differential regulation of hyaluronan metabolism in the epidermal and dermal compartments of human skin by UVB irradiation. J. Investig. Dermatol. 2007, 127, 687–697. [Google Scholar] [CrossRef] [Green Version]
- Dai, G.; Freudenberger, T.; Zipper, P.; Melchior, A.; Grether-Beck, S.; Rabausch, B.; de Groot, J.; Twarock, S.; Hanenberg, H.; Homey, B.; et al. Chronic ultraviolet B irradiation causes loss of hyaluronic acid from mouse dermis because of down-regulation of hyaluronic acid synthases. Am. J. Pathol. 2007, 171, 1451–1461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rock, K.; Grandoch, M.; Majora, M.; Krutmann, J.; Fischer, J.W. Collagen fragments inhibit hyaluronan synthesis in skin fibroblasts in response to ultraviolet B (UVB): New insights into mechanisms of matrix remodeling. J. Biol. Chem. 2011, 286, 18268–18276. [Google Scholar] [CrossRef] [Green Version]
- Su, F.; Ren, F.; Rong, Y.; Wang, Y.; Geng, Y.; Wang, Y.; Feng, M.; Ju, Y.; Li, Y.; Zhao, Z.J.; et al. Protein tyrosine phosphatase Meg2 dephosphorylates signal transducer and activator of transcription 3 and suppresses tumor growth in breast cancer. Breast Cancer Res. 2012, 14, R38. [Google Scholar] [CrossRef] [Green Version]
- Tebbutt, N.C.; Giraud, A.S.; Inglese, M.; Jenkins, B.; Waring, P.; Clay, F.J.; Malki, S.; Alderman, B.M.; Grail, D.; Hollande, F.; et al. Reciprocal regulation of gastrointestinal homeostasis by SHP2 and STAT-mediated trefoil gene activation in gp130 mutant mice. Nat. Med. 2002, 8, 1089–1097. [Google Scholar] [CrossRef] [PubMed]
- Oh, J.H.; Joo, Y.H.; Karadeniz, F.; Ko, J.; Kong, C.S. Syringaresinol Inhibits UVA-Induced MMP-1 Expression by Suppression of MAPK/AP-1 Signaling in HaCaT Keratinocytes and Human Dermal Fibroblasts. Int. J. Mol. Sci. 2020, 21, 3981. [Google Scholar] [CrossRef]
- Hseu, Y.C.; Korivi, M.; Lin, F.Y.; Li, M.L.; Lin, R.W.; Wu, J.J.; Yang, H.L. Trans-cinnamic acid attenuates UVA-induced photoaging through inhibition of AP-1 activation and induction of Nrf2-mediated antioxidant genes in human skin fibroblasts. J. Dermatol. Sci. 2018, 90, 123–134. [Google Scholar] [CrossRef] [Green Version]
- Kocturk, S.; Yuksel Egrilmez, M.; Aktan, S.; Oktay, G.; Resmi, H.; Simsek Keskin, H.; Sert Serdar, B.; Erkmen, T.; Guner Akdogan, G.; Ozkan, S. Melatonin attenuates the detrimental effects of UVA irradiation in human dermal fibroblasts by suppressing oxidative damage and MAPK/AP-1 signal pathway in vitro. Photodermatol. Photoimmunol. Photomed. 2019, 35, 221–231. [Google Scholar]
- Ryu, J.; Kwon, M.J.; Nam, T.J. Nrf2 and NF-kappaB Signaling Pathways Contribute to Porphyra-334-Mediated Inhibition of UVA-Induced Inflammation in Skin Fibroblasts. Mar. Drugs 2015, 13, 4721–4732. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Q.; Hou, W.; Zheng, Y.; Liu, C.; Gong, Z.; Lu, C.; Lai, W.; Maibach, H.I. Ultraviolet A-induced cathepsin K expression is mediated via MAPK/AP-1 pathway in human dermal fibroblasts. PLoS ONE 2014, 9, e102732. [Google Scholar] [CrossRef] [Green Version]
- Syed, D.N.; Afaq, F.; Mukhtar, H. Differential activation of signaling pathways by UVA and UVB radiation in normal human epidermal keratinocytes. Photochem. Photobiol. 2012, 88, 1184–1190. [Google Scholar] [CrossRef] [PubMed]
- Terazawa, S.; Mori, S.; Nakajima, H.; Yasuda, M.; Imokawa, G. The UVB-Stimulated Expression of Transglutaminase 1 Is Mediated Predominantly via the NFkappaB Signaling Pathway: New Evidence of Its Significant Attenuation through the Specific Interruption of the p38/MSK1/NFkappaBp65 Ser276 Axis. PLoS ONE 2015, 10, e0136311. [Google Scholar]
- Terazawa, S.; Imokawa, G. Signaling Cascades Activated by UVB in Human Melanocytes Lead to the Increased Expression of Melanocyte Receptors, Endothelin B Receptor and c-KIT. Photochem. Photobiol. 2018, 94, 421–431. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, H.; Shimizu, T.; Nishihira, J.; Abe, R.; Nakayama, T.; Taniguchi, M.; Sabe, H.; Ishibashi, T.; Shimizu, H. Ultraviolet A-induced production of matrix metalloproteinase-1 is mediated by macrophage migration inhibitory factor (MIF) in human dermal fibroblasts. J. Biol. Chem. 2004, 279, 1676–1683. [Google Scholar] [CrossRef] [Green Version]
- Xu, Q.F.; Zheng, Y.; Chen, J.; Xu, X.Y.; Gong, Z.J.; Huang, Y.F.; Lu, C.; Maibach, H.I.; Lai, W. Ultraviolet A Enhances Cathepsin L Expression and Activity via JNK Pathway in Human Dermal Fibroblasts. Chin. Med. J. 2016, 129, 2853–2860. [Google Scholar] [CrossRef] [PubMed]
- Terazawa, S.; Nakano, M.; Yamamoto, A.; Imokawa, G. Mycosporine-like amino acids stimulate hyaluronan secretion by up-regulating hyaluronan synthase 2 via activation of the p38/MSK1/CREB/c-Fos/AP-1 axis. J. Biol. Chem. 2020, 295, 7274–7288. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saavalainen, K.; Pasonen-Seppanen, S.; Dunlop, T.W.; Tammi, R.; Tammi, M.I.; Carlberg, C. The human hyaluronan synthase 2 gene is a primary retinoic acid and epidermal growth factor responding gene. J. Biol. Chem. 2005, 280, 14636–14644. [Google Scholar] [CrossRef] [Green Version]
- Saavalainen, K.; Tammi, M.I.; Bowen, T.; Schmitz, M.L.; Carlberg, C. Integration of the activation of the human hyaluronan synthase 2 gene promoter by common cofactors of the transcription factors retinoic acid receptor and nuclear factor kappaB. J. Biol. Chem. 2007, 282, 11530–11539. [Google Scholar] [CrossRef] [Green Version]
- Monslow, J.; Williams, J.D.; Guy, C.A.; Price, I.K.; Craig, K.J.; Williams, H.J.; Williams, N.M. Identification and analysis of the promoter region of the human hyaluronan synthase 2 gene. J. Biol. Chem. 2004, 279, 20576–20581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Monslow, J.; Williams, J.D.; Fraser, D.J.; Michael, D.R.; Foka, P.; Kift-Morgan, A.P.; Luo, D.D.; Fielding, C.A.; Craig, K.J.; Topley, N.; et al. Sp1 and Sp3 mediate constitutive transcription of the human hyaluronan synthase 2 gene. J. Biol. Chem. 2006, 281, 18043–18050. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makkonen, K.M.; Pasonen-Seppanen, S.; Torronen, K.; Tammi, M.I.; Carlberg, C. Regulation of the hyaluronan synthase 2 gene by convergence in cyclic AMP response element-binding protein and retinoid acid receptor signaling. J. Biol. Chem. 2009, 284, 18270–18281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jokela, T.A.; Karna, R.; Makkonen, K.M.; Laitinen, J.T.; Tammi, R.H.; Tammi, M.I. Extracellular UDP-glucose activates P2Y14 Receptor and Induces Signal Transducer and Activator of Transcription 3 (STAT3) Tyr705 phosphorylation and binding to hyaluronan synthase 2 (HAS2) promoter, stimulating hyaluronan synthesis of keratinocytes. J. Biol. Chem. 2014, 289, 18569–18581. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dudka, A.A.; Sweet, S.M.; Heath, J.K. Signal transducers and activators of transcription-3 binding to the fibroblast growth factor receptor is activated by receptor amplification. Cancer Res. 2010, 70, 3391–3401. [Google Scholar] [CrossRef] [Green Version]
- Mi, C.; Ma, J.; Wang, K.S.; Wang, Z.; Li, M.Y.; Li, J.B.; Li, X.; Piao, L.X.; Xu, G.H.; Jin, X.; et al. Amorfrutin A inhibits TNF-alpha induced JAK/STAT signaling, cell survival and proliferation of human cancer cells. Immunopharmacol. Immunotoxicol. 2017, 39, 338–347. [Google Scholar] [CrossRef]
- Dehkhoda, F.; Lee, C.M.M.; Medina, J.; Brooks, A.J. The Growth Hormone Receptor: Mechanism of Receptor Activation, Cell Signaling, and Physiological Aspects. Front. Endocrinol. 2018, 9, 35. [Google Scholar] [CrossRef] [Green Version]
- Johnson, K.J.; Peck, A.R.; Liu, C.; Tran, T.H.; Utama, F.E.; Sjolund, A.B.; Schaber, J.D.; Witkiewicz, A.K.; Rui, H. PTP1B suppresses prolactin activation of Stat5 in breast cancer cells. Am. J. Pathol. 2010, 177, 2971–2983. [Google Scholar] [CrossRef] [Green Version]
- Meyer, T.; Hendry, L.; Begitt, A.; John, S.; Vinkemeier, U. A single residue modulates tyrosine dephosphorylation, oligomerization, and nuclear accumulation of stat transcription factors. J. Biol. Chem. 2004, 279, 18998–19007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chughtai, N.; Schimchowitsch, S.; Lebrun, J.J.; Ali, S. Prolactin induces SHP-2 association with Stat5, nuclear translocation, and binding to the beta-casein gene promoter in mammary cells. J. Biol. Chem. 2002, 277, 31107–31114. [Google Scholar] [CrossRef] [PubMed] [Green Version]














| Primer Name | Sequence | |
|---|---|---|
| GAPDH | Forward | 5’-GCACCGTCAAGGCGAGAAC-3’ |
| Reverse | 5’-TGGTGAAGACGCCAGTGGA-3’ | |
| KIAA1199 (HYBID) | Forward | 5’-CCAGGAATGTTGAATGTCT-3’ |
| Reverse | 5’-ATTGGCTCTTGGTGAATG-3’ | |
| HAS1 | Forward | 5’-ATCCTGCATCAGCGGTCCTC-3’ |
| Reverse | 5’-CTGGTTGTACCAGGCCTCAAGAA-3’ | |
| HAS2 | Forward | 5’-TCGCAACACGTAACGCAAT-3’ |
| Reverse | 5’-ACTTCTCTTTTTCCACCCCATTT-3’ | |
| HAS3 | Forward | 5’-AACAAGTACGACTCATGGATTTCCT-3’ |
| Reverse | 5’-GCCCGCTCCACGTTGA-3’ | |
| PTP-MEG2 | Forward | 5’-TTTATGCAGCTTGGCCCTGTC-3’ |
| Reverse | 5’-ACACAGAACCCACCCAGTTTGAG-3’ | |
| HYAL1 | Forward | 5’-CGATATGGCCCAAGGCTTTAG-3 |
| Reverse | 5’-ACCACATCGAAGACACTGACAT-3’ | |
| HYAL2 | Forward | 5’-TTCTACCGCGACCGTCTAGG-3’ |
| Reverse | 5’-TGTCCGAATGTAGTGCTCCAC-3’ | |
| HYAL3 | Forward | 5’-TGCCTCTTCTTTTCCCTGCTG-3 |
| Reverse | 5’-GGGAGGGTTGACTGTAAACTG-3 | |
| RPLPO | Forward | 5’- TTCGACAATGGCAGCATCTACAA -3’ |
| Reverse | 5’- CTGCAGACAGACACTGGCAACA -3’ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terazawa, S.; Takada, M.; Sato, Y.; Nakajima, H.; Imokawa, G. The Attenuated Secretion of Hyaluronan by UVA-Exposed Human Fibroblasts Is Associated with Up- and Downregulation of HYBID and HAS2 Expression via Activated and Inactivated Signaling of the p38/ATF2 and JAK2/STAT3 Cascades. Int. J. Mol. Sci. 2021, 22, 2057. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22042057
Terazawa S, Takada M, Sato Y, Nakajima H, Imokawa G. The Attenuated Secretion of Hyaluronan by UVA-Exposed Human Fibroblasts Is Associated with Up- and Downregulation of HYBID and HAS2 Expression via Activated and Inactivated Signaling of the p38/ATF2 and JAK2/STAT3 Cascades. International Journal of Molecular Sciences. 2021; 22(4):2057. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22042057
Chicago/Turabian StyleTerazawa, Shuko, Mariko Takada, Yoriko Sato, Hiroaki Nakajima, and Genji Imokawa. 2021. "The Attenuated Secretion of Hyaluronan by UVA-Exposed Human Fibroblasts Is Associated with Up- and Downregulation of HYBID and HAS2 Expression via Activated and Inactivated Signaling of the p38/ATF2 and JAK2/STAT3 Cascades" International Journal of Molecular Sciences 22, no. 4: 2057. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22042057






