Molecular Determinants of the Kinetic Binding Properties of Antihistamines at the Histamine H1 Receptors
Abstract
:1. Introduction
2. Results and Discussion
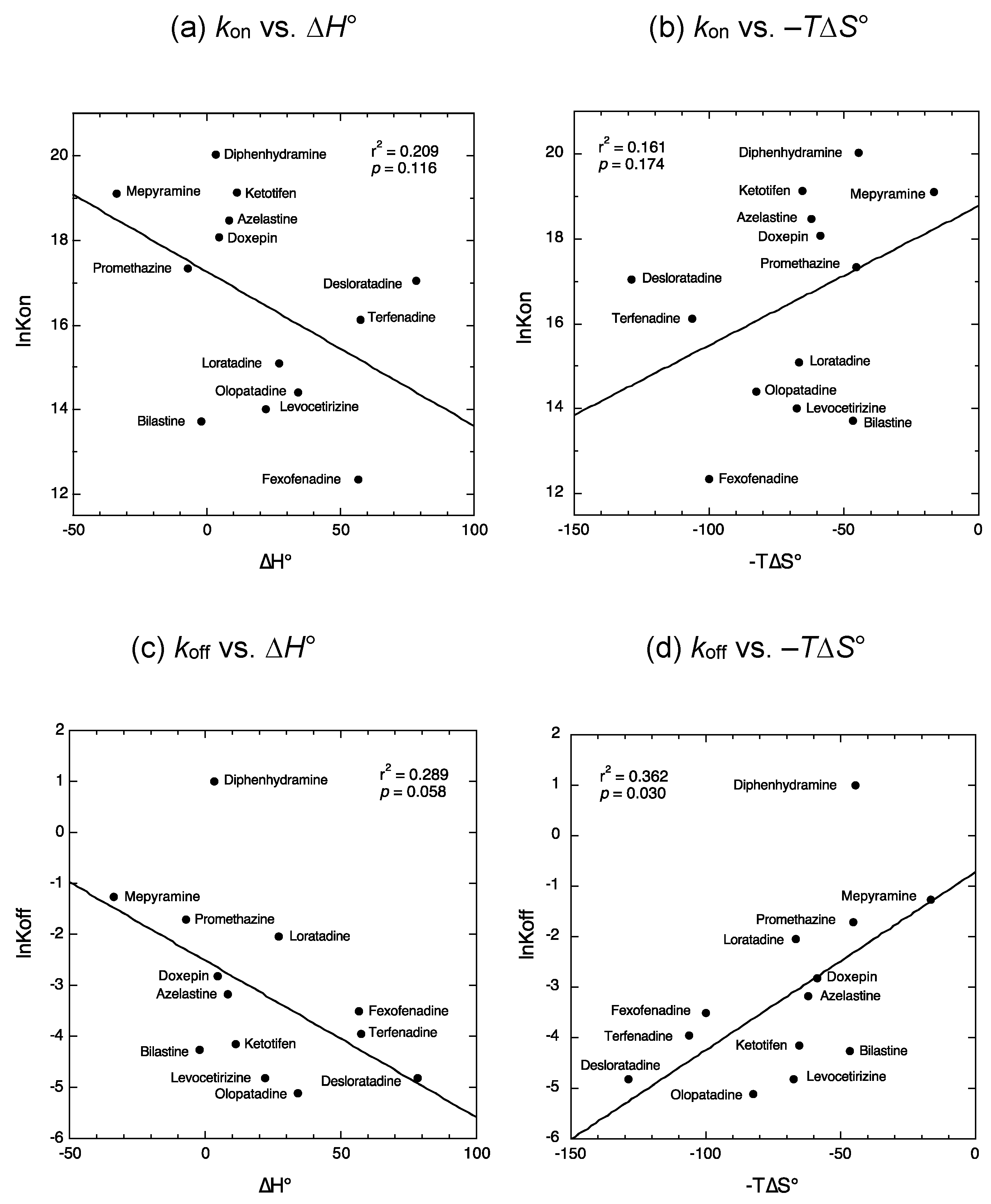
2.1. Relationship between the Kinetic and Thermodynamic Binding Parameters of Antihistamines
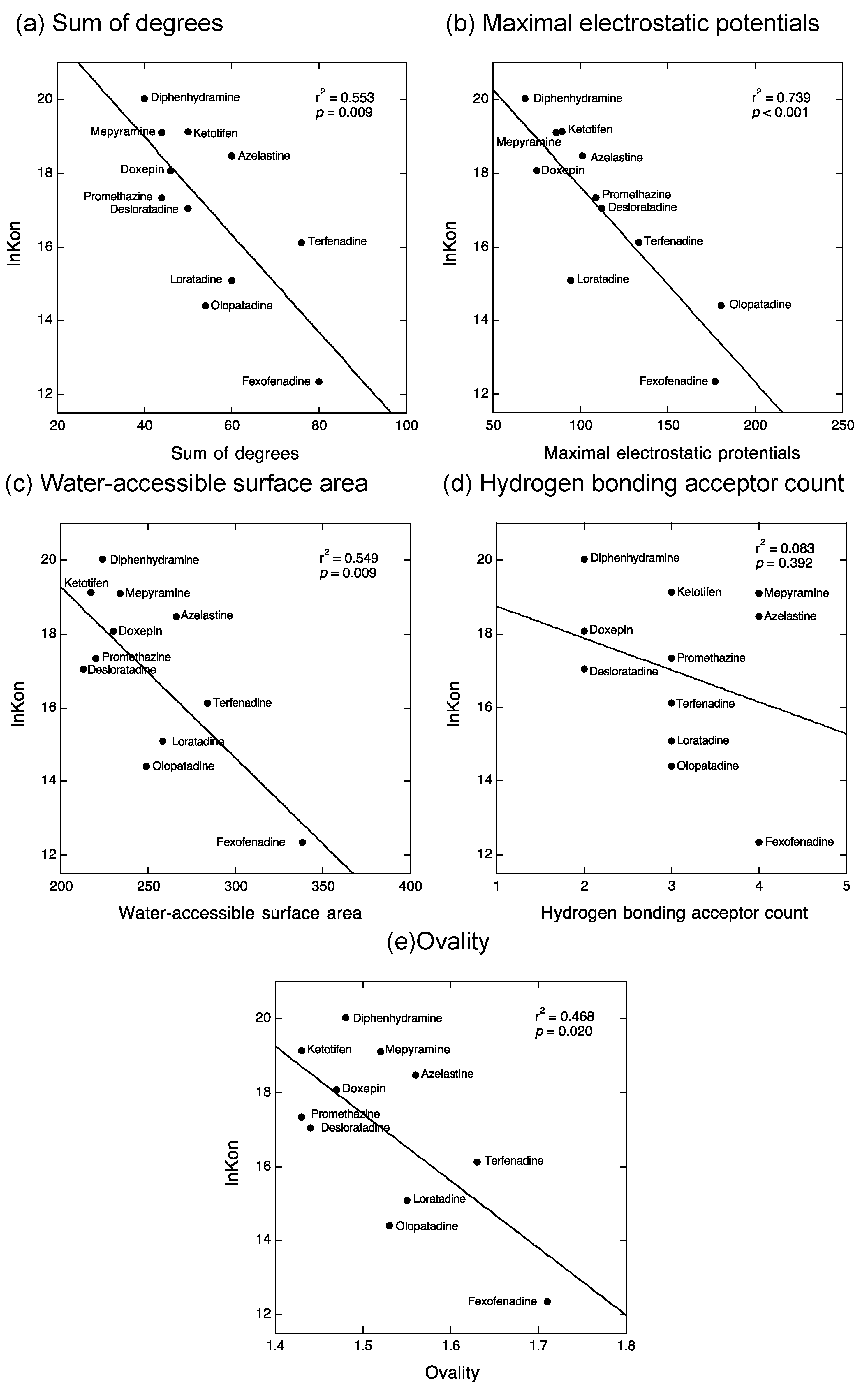
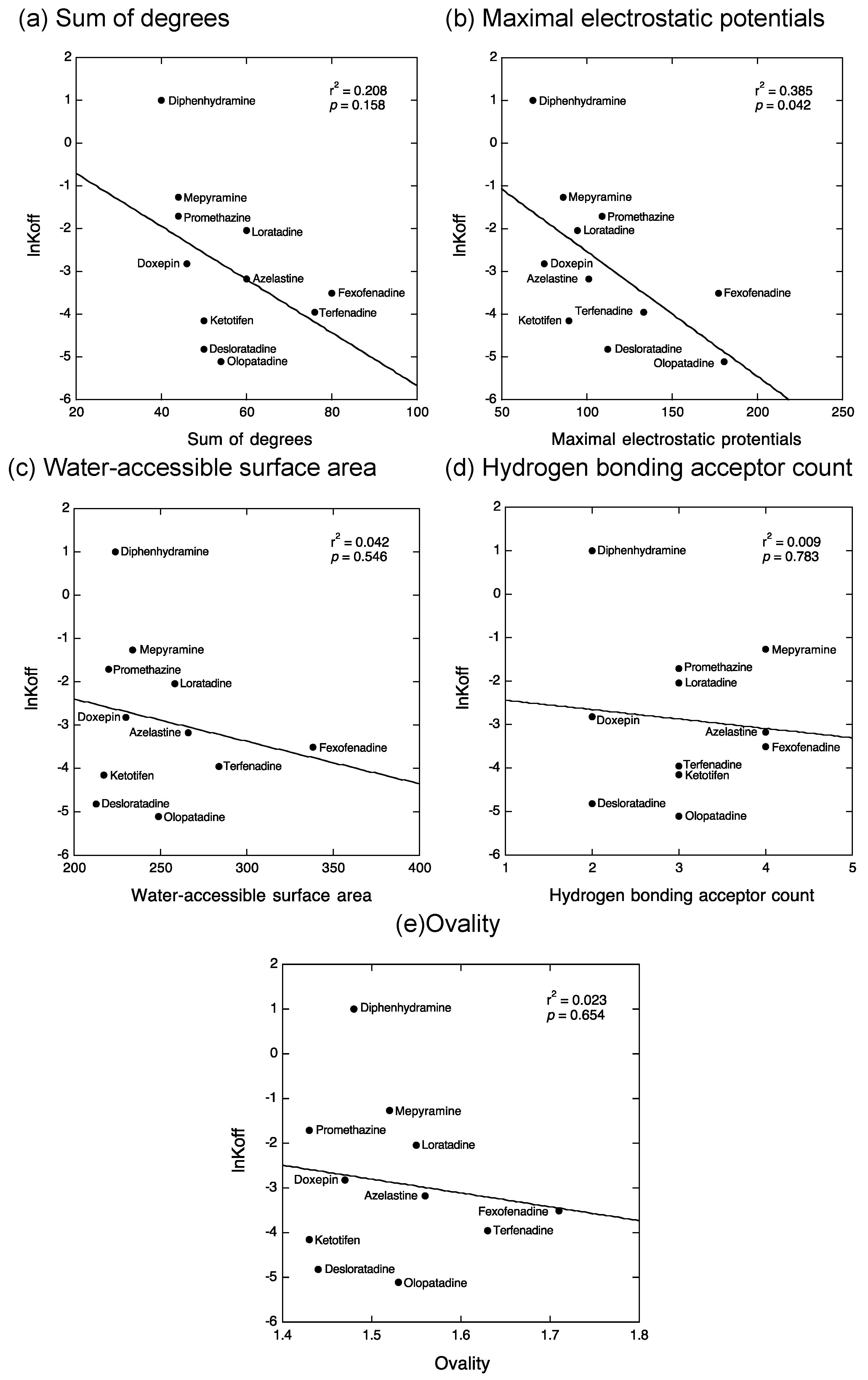
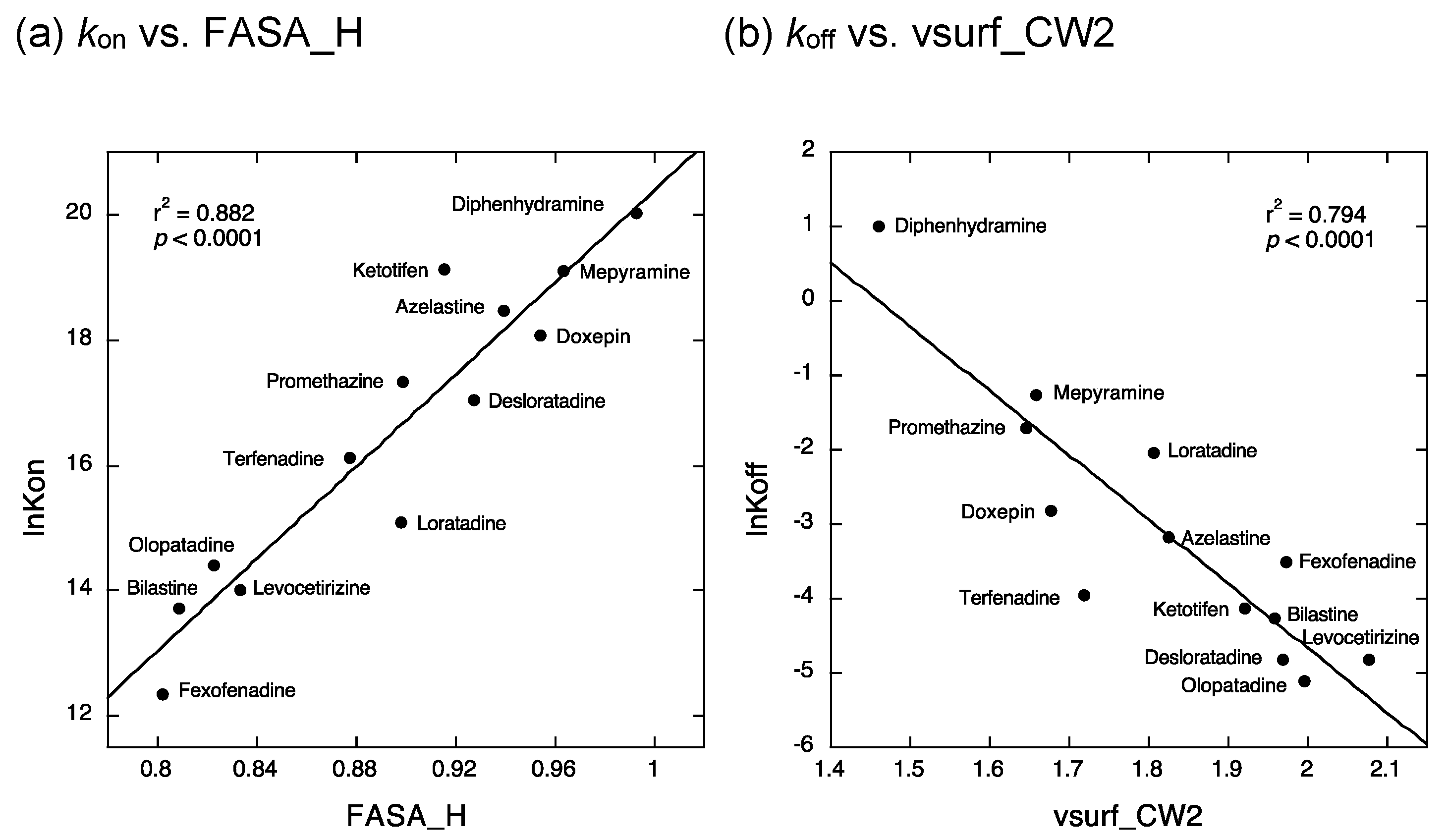
2.2. QSAR Analyses to Estimate the Structural Determinants of the Kinetic Binding Properties of Antihistamines
3. Materials and Methods
3.1. Relationship between the Kinetic and Thermodynamic Binding Parameters of Antihistamines
3.2. QSAR Analyses to Estimate the Structural Determinants of the Kinetic Binding Properties of Antihistamines
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Simons, F.E.; Simons, K.J. Histamine and H1-antihistamines: Celebrating a century of progress. J. Allergy Clin. Immunol. 2011, 128, 1139–1150. [Google Scholar] [CrossRef] [PubMed]
- Church, M.K. Allergy, histamine and antihistamines. Handb. Exp. Pharmacol. 2017, 241, 321–331. [Google Scholar] [PubMed]
- Holgate, S.T.; Canonica, G.W.; Simons, F.E.; Taglialatela, M.; Tharp, M.; Timmerman, H.; Yanai, K. Consensus group on new-generation antihistamines (CONGA): Present status and recommendations. Clin. Exp. Allergy 2003, 33, 1305–1324. [Google Scholar] [CrossRef] [Green Version]
- Kalpaklioglu, F.; Baccioglu, A. Efficacy and safety of H1-antihistamines: An update. Antiinflamm. Antiallergy Agents Med. Chem. 2012, 11, 230–237. [Google Scholar] [CrossRef]
- Yanai, K.; Yoshikawa, T.; Yanai, A.; Nakamura, T.; Iida, T.; Leurs, R.; Tashiro, M. The clinical pharmacology of non-sedating antihistamines. Pharmacol. Ther. 2017, 178, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Kawauchi, H.; Yanai, K.; Wang, D.Y.; Itahashi, K.; Okubo, K. Antihistamines for allergic rhinitis treatment from the viewpoint of nonsedative properties. Int. J. Mol. Sci. 2019, 20, 213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gillard, M.; Van der Perren, C.; Moguilevsky, N.; Massingham, R.; Chatelain, P. Binding characteristics of cetirizine and levocetirizine to human H1 histamine receptors: Contribution of Lys191 and Thr194. Mol. Pharmacol. 2002, 61, 391–399. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Slack, R.; Hart, A.; Luttmann, M.; Clark, K.; Begg, M. In vitro characterisation of the duration of action of the histamine-1 receptor antagonist azelastine. Eur. J. Pharmacol. 2011, 670, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Kanamitsu, K.; Nozaki, Y.; Nagaya, Y.; Sugiyama, Y.; Kusuhara, H. Quantitative prediction of histamine H1 receptor occupancy by the sedative and non-sedative antagonists in the human central nervous system based on systemic exposure and preclinical data. Drug Metab. Pharmacokinet. 2017, 32, 135–144. [Google Scholar] [CrossRef]
- Bosma, R.; Witt, G.; Vaas, L.A.I.; Josimovic, I.; Gribbon, P.; Vischer, H.F.; Gul, S.; Leurs, R. The target residence time of antihistamines determines their antagonism of the G protein-coupled histamine H1 receptor. Front. Pharmacol. 2017, 8, 667. [Google Scholar] [CrossRef]
- Bosma, R.; Bor, J.; Vischer, H.F.; Labeaga, L.; Leurs, R. The long duration of action of the second generation antihistamine bilastine coincides with its long residence time at the histamine H1 receptor. Eur. J. Pharmacol. 2018, 838, 107–111. [Google Scholar] [CrossRef] [PubMed]
- Stoddart, L.A.; Vernall, A.J.; Bouzo-Lorenzo, M.; Bosma, R.; Kooistra, A.J.; de Graaf, C.; Vischer, H.F.; Leurs, R.; Briddon, S.J.; Kellam, B.; et al. Development of novel fluorescent histamine H1-receptor antagonists to study ligand-binding kinetics in living cells. Sci. Rep. 2018, 8, 1572. [Google Scholar] [CrossRef] [PubMed]
- Bosma, R.; Moritani, R.; Leurs, R.; Vischer, H.F. BRET-based β-arrestin2 recruitment to the histamine H1 receptor for investigating antihistamine binding kinetics. Pharmacol. Res. 2016, 111, 679–687. [Google Scholar] [CrossRef] [PubMed]
- Bosma, R.; Wang, Z.; Kooistra, A.J.; Bushby, N.; Kuhne, S.; van den Bor, J.; Waring, M.J.; de Graaf, C.; de Esch, I.J.; Vischer, H.F.; et al. Route to prolonged residence time at the histamine H1 receptor: Growing from desloratadine to rupatadine. J. Med. Chem. 2019, 62, 6630–6644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hitzemann, R. Thermodynamics aspects of drug–receptor interactions. Trends Pharm. Sci. 1988, 9, 408–411. [Google Scholar] [CrossRef]
- Borea, P.A.; Dalpiaz, A.; Varani, K.; Gilli, P.; Gilli, G. Can thermodynamic measurements of receptor binding yield information on drug affinity and efficacy? Biochem. Pharmacol. 2000, 60, 1549–1556. [Google Scholar] [CrossRef]
- Wittmann, H.J.; Seifert, R.; Strasser, A. Contribution of binding enthalpy and entropy to affinity of antagonist and agonist binding at human and guinea pig histamine H1-receptor. Mol. Pharmacol. 2009, 76, 25–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hishinuma, S.; Sugawara, K.; Uesawa, Y.; Fukui, H.; Shoji, M. Differential thermodynamic driving force of first- and second-generation antihistamines to determine their binding affinity for human H1 receptors. Biochem. Pharmacol. 2014, 91, 231–241. [Google Scholar] [CrossRef] [PubMed]
- Hishinuma, S.; Tamura, Y.; Kobayashi, C.; Akatsu, C.; Shoji, M. Differential regulation of thermodynamic binding forces of levocetirizine and (S)-cetirizine by Lys191 in human histamine H1 receptors. Int. J. Mol. Sci. 2018, 19, 4067. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, C.; Tanaka, A.; Yasuda, T.; Hishinuma, S. Roles of Lys191 and Lys179 in regulating thermodynamic binding forces of ligands to determine their binding affinity for human histamine H1 receptors. Biochem. Pharmacol. 2020, 180, 114185. [Google Scholar] [CrossRef]
- Kurosaki, K.; Wu, R.; Uesawa, Y. A toxicity prediction tool for potential agonist/antagonist activities in molecular initiating events based on chemical structures. Int. J. Mol. Sci. 2020, 21, 7853. [Google Scholar] [CrossRef] [PubMed]
- Hansch, C.; Fujita, T. Rho-sigma-pi analysis; method for the correlation of biological activity and chemical structure. J. Am. Chem. Soc. 1964, 86, 1616–1626. [Google Scholar] [CrossRef]

| Antihistamines | kon (106 min−1·M−1) | koff (min−1) | References 1 |
|---|---|---|---|
| Loratadine | 3.6 | 0.13 | Gillard et al. [7] |
| Terfenadine | 10 | 0.019 | Gillard et al. [7] |
| Azelastine | 106 | 0.042 | Slack et al. [8] |
| Ketotifen | 204 | 0.016 | Kanamitsu et al. [9] |
| Desloratadine | 25 | 0.008 | Bosma et al. [10] |
| Doxepin | 70 | 0.06 | Bosma et al. [10] |
| Levocetirizine | 1.2 | 0.008 | Bosma et al. [10] |
| Mepyramine | 200 | 0.28 | Bosma et al. [10] |
| Olopatadine | 1.8 | 0.006 | Bosma et al. [10] |
| Bilastine | 0.9 | 0.014 | Bosma et al. [11] |
| Diphenhydramine | 500 | 2.70 | Bosma et al. [11] |
| Fexofenadine | 0.23 | 0.03 | Bosma et al. [11] |
| Promethazine | 33.7 | 0.18 | Stoddart et al. [12] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Akimoto, H.; Uesawa, Y.; Hishinuma, S. Molecular Determinants of the Kinetic Binding Properties of Antihistamines at the Histamine H1 Receptors. Int. J. Mol. Sci. 2021, 22, 2400. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22052400
Akimoto H, Uesawa Y, Hishinuma S. Molecular Determinants of the Kinetic Binding Properties of Antihistamines at the Histamine H1 Receptors. International Journal of Molecular Sciences. 2021; 22(5):2400. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22052400
Chicago/Turabian StyleAkimoto, Hayato, Yoshihiro Uesawa, and Shigeru Hishinuma. 2021. "Molecular Determinants of the Kinetic Binding Properties of Antihistamines at the Histamine H1 Receptors" International Journal of Molecular Sciences 22, no. 5: 2400. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22052400