Small Immunomodulatory Molecules as Potential Therapeutics in Experimental Murine Models of Acute Lung Injury (ALI)/Acute Respiratory Distress Syndrome (ARDS)
Abstract
:1. Introduction
2. Results
2.1. Structure of AVR-48
2.2. Safety Profile of AVR-48 in Adult Rats
2.3. PK Profile of AVR-48 in Rat Plasma and BALF
2.4. Effect of AVR-25 and AVR-48 on Pro-Inflammatory Cytokines in the Lung
2.5. Effect of AVR-25 and AVR-48 on the Anti-Inflammatory Cytokine IL-10 in BALF
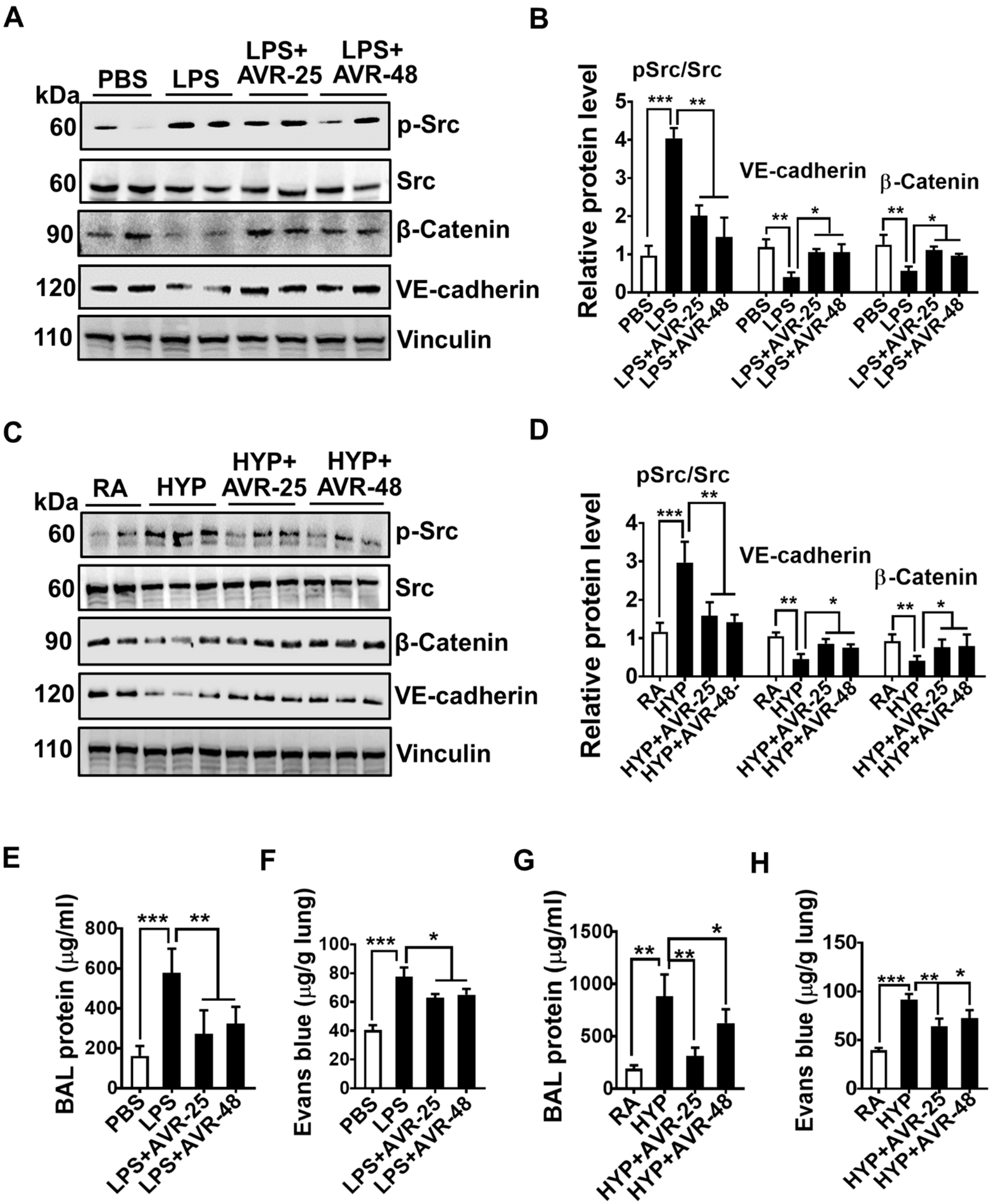
2.6. Effect of AVR-25 and AVR-48 on Pulmonary Vascular Leakage in ALI Mice
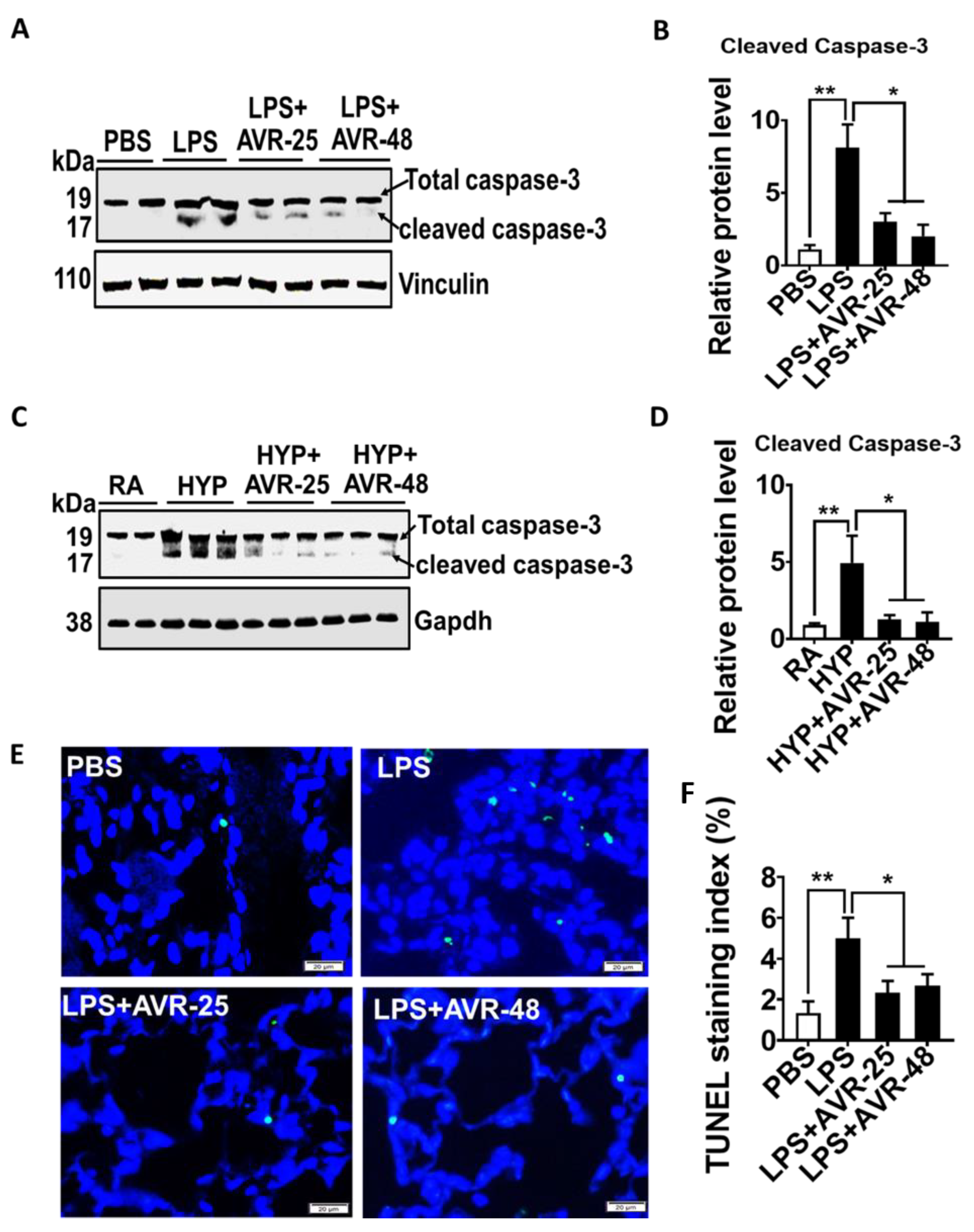
2.7. Effect of AVR-25 and AVR-48 on Lung Cell Death in ALI Mice
2.8. Effect of AVR-25 and AVR-48 on LPS-and Hyperoxia-Induced Lung Histopathology
2.9. Effect of AVR-48 on CLP-Induced Survival and Lung Histopathology
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Chemicals and Reagents
4.3. Formulations
4.3.1. Formulation for Mouse Efficacy Studies
4.3.2. Formulation for Rat PK/Tox Studies
4.4. Toxicology Study
4.5. Pharmacokinetic (PK) Study
4.6. Murine Model of Acute Lung Injury (ALI)
4.6.1. Experimental Protocol
4.6.2. Bronchoalveolar Lavage Fluid (BALF) Analysis
4.6.3. Assessment of Lung Endothelial Cell Permeability
4.6.4. Enzyme-Linked Immunosorbent Assay (ELISA)
4.7. Western Blotting
4.8. Terminal Deoxynucleotidy1 Transferase-Mediated dUTP Nick End-Labeling (TUNEL) Assay
4.9. Histological Analysis
4.10. Lung Injury Scoring
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salim, A.; Martin, M.; Constantinou, C.; Sangthong, B.; Brown, C.; Kasotakis, G.; Demetriades, D.; Belzberg, H. Acute respiratory distress syndrome in the trauma intensive care unit: Morbid but not mortal. Arch Surg. 2006, 141, 655–658. [Google Scholar] [CrossRef]
- Matthay, M.A.; Ware, L.B.; Zimmerman, G.A. The acute respiratory distress syndrome. J. Clin. Investig. 2012, 122, 2731–2740. [Google Scholar] [CrossRef] [Green Version]
- Rubenfeld, G.D.; Caldwell, E.; Peabody, E.; Weaver, J.; Martin, D.P.; Neff, M.; Stern, E.J.; Hudson, L.D. Incidence and outcomes of acute lung injury. N. Engl. J. Med. 2005, 353, 1685–1693. [Google Scholar] [CrossRef] [Green Version]
- Mora-Rillo, M.; Arsuaga, M.; Ramirez-Olivencia, G.; de la Calle, F.; Borobia, A.M.; Sanchez-Seco, P.; Lago, M.; Figueira, J.C.; Fernandez-Puntero, B.; Viejo, A.; et al. Acute respiratory distress syndrome after convalescent plasma use: Treatment of a patient with Ebola virus disease contracted in Madrid, Spain. Lancet Respir. Med. 2015, 3, 554–562. [Google Scholar] [CrossRef]
- Villar, J.; Blanco, J.; Kacmarek, R.M. Current incidence and outcome of the acute respiratory distress syndrome. Curr. Opin. Crit. Care 2016, 22, 1–6. [Google Scholar] [CrossRef]
- Matthay, M.A.; McAuley, D.F.; Ware, L.B. Clinical trials in acute respiratory distress syndrome: Challenges and opportunities. Lancet Respir. Med. 2017, 5, 524–534. [Google Scholar] [CrossRef]
- Zhang, Z.; Chen, L.; Ni, H. The effectiveness of Corticosteroids on mortality in patients with acute respiratory distress syndrome or acute lung injury: A secondary analysis. Sci. Rep. 2015, 5, 17654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bernard, G.R.; Wheeler, A.P.; Russell, J.A.; Schein, R.; Summer, W.R.; Steinberg, K.P.; Fulkerson, W.J.; Wright, P.E.; Christman, B.W.; Dupont, W.D.; et al. The effects of ibuprofen on the physiology and survival of patients with sepsis. The Ibuprofen in Sepsis Study Group. N. Engl. J. Med. 1997, 336, 912–918. [Google Scholar] [CrossRef]
- Chen, M.; Lu, J.; Chen, Q.; Cheng, L.; Geng, Y.; Jiang, H.; Wang, X. Statin in the treatment of ALI/ARDS: A systematic review and Meta-analysis based on international databases. Zhonghua Wei Zhong Bing Ji Jiu Yi Xue 2017, 29, 51–56. [Google Scholar]
- Afshari, A.; Brok, J.; Moller, A.M.; Wetterslev, J. Inhaled nitric oxide for acute respiratory distress syndrome (ARDS) and acute lung injury in children and adults. Cochrane Database Syst. Rev. 2010, Cd002787. [Google Scholar]
- Panda, S.K.; Kumar, S.; Tupperwar, N.C.; Vaidya, T.; George, A.; Rath, S.; Bal, V.; Ravindran, B. Chitohexaose activates macrophages by alternate pathway through TLR4 and blocks endotoxemia. PLoS Pathog. 2012, 8, e1002717. [Google Scholar] [CrossRef] [Green Version]
- Martinez, F.O.; Helming, L.; Gordon, S. Alternative activation of macrophages: An immunologic functional perspective. Annu. Rev. Immunol. 2009, 27, 451–483. [Google Scholar] [CrossRef] [Green Version]
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Acharya, S.; Das, P.; Agarwal, B. Novel Immunodulating Small Molecules. US Patent 20200022995, 23 January 2020. [Google Scholar]
- Das, P.; Panda, S.K.; Agarwal, B.; Behera, S.; Ali, S.M.; Pulse, M.E.; Solomkin, J.S.; Opal, S.M.; Bhandari, V.; Acharya, S. Novel Chitohexaose Analog Protects Young and Aged mice from CLP Induced Polymicrobial Sepsis. Sci. Rep. 2019, 9, 2904. [Google Scholar] [CrossRef]
- Iscimen, R.; Cartin-Ceba, R.; Yilmaz, M.; Khan, H.; Hubmayr, R.D.; Afessa, B.; Gajic, O. Risk factors for the development of acute lung injury in patients with septic shock: An observational cohort study. Crit. Care Med. 2008, 36, 1518–1522. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, L.; Millar, A.B. Relative production of tumour necrosis factor alpha and interleukin 10 in adult respiratory distress syndrome. Thorax 1997, 52, 442–446. [Google Scholar] [CrossRef] [Green Version]
- Bi, M.H.; Wang, B.E.; Zheng, X.X.; Li, M.; Mayer, K.; Zhang, S.W. The effect of recombinant interleukin-10/Fc fusion protein on lipopolysaccharide-induced acute lung injury in mice. Zhongguo Wei Zhong Bing Ji Jiu Yi Xue 2008, 20, 461–464. [Google Scholar]
- Inoue, G. Effect of interleukin-10 (IL-10) on experimental LPS-induced acute lung injury. J. Infect. Chemother. 2000, 6, 51–60. [Google Scholar] [CrossRef]
- Hiroshima, Y.; Hsu, K.; Tedla, N.; Chung, Y.M.; Chow, S.; Herbert, C.; Geczy, C.L. S100A8 induces IL-10 and protects against acute lung injury. J. Immunol. 2014, 192, 2800–2811. [Google Scholar] [CrossRef] [PubMed]
- Densmore, J.C.; Signorino, P.R.; Ou, J.; Hatoum, O.A.; Rowe, J.J.; Shi, Y.; Kaul, S.; Jones, D.W.; Sabina, R.E.; Pritchard, K.A.; et al. Endothelium-derived microparticles induce endothelial dysfunction and acute lung injury. Shock 2006, 26, 464–471. [Google Scholar] [CrossRef]
- Chien, J.-Y.; Hsueh, P.-R.; Cheng, W.-C.; Yu, C.-J.; Yang, P.-C. Temporal changes in cytokine/chemokine profiles and pulmonary involvement in severe acute respiratory syndrome. Respirology 2006, 11, 715–722. [Google Scholar] [CrossRef]
- Conti, P.; Ronconi, G.; Caraffa, A.; Gallenga, C.E.; Ross, R.; Frydas, I.; Kritas, S.K. Induction of pro-inflammatory cytokines (IL-1 and IL-6) and lung inflammation by Coronavirus-19 (COVI-19 or SARS-CoV-2): Anti-inflammatory strategies. J. Biol. Regul. Homeost Agents 2020, 34, 1. [Google Scholar] [PubMed]
- Shakoory, B.; Carcillo, J.A.; Chatham, W.W.; Amdur, R.L.; Zhao, H.; Dinarello, C.A.; Cron, R.Q.; Opal, S.M. Interleukin-1 Receptor Blockade Is Associated With Reduced Mortality in Sepsis Patients With Features of Macrophage Activation Syndrome: Reanalysis of a Prior Phase III Trial. Crit. Care Med. 2016, 44, 275–281. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, K.W.; Rousset, F.; Banchereau, J. Evolving principles in immunopathology: Interleukin 10 and its relationship to Epstein-Barr virus protein BCRF1. Springer Semin. Immunopathol. 1991, 13, 157–166. [Google Scholar] [CrossRef]
- Kapur, R.; Kim, M.; Rebetz, J.; Rondina, M.T.; Porcelijn, L.; Semple, J.W. Low levels of interleukin-10 in patients with transfusion-related acute lung injury. Ann. Transl. Med. 2017, 5, 339. [Google Scholar] [CrossRef] [Green Version]
- Arai, T.; Abe, K.; Matsuoka, H.; Yoshida, M.; Mori, M.; Goya, S.; Kida, H.; Nishino, K.; Osaki, T.; Tachibana, I.; et al. Introduction of the interleukin-10 gene into mice inhibited bleomycin-induced lung injury in vivo. Am. J. Physiol. Lung Cell Mol. Physiol. 2000, 278, L914–L922. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reinhart, P.G.; Gupta, S.K.; Bhalla, D.K. Attenuation of ozone-induced lung injury by interleukin-10. Toxicol. Lett. 1999, 110, 35–42. [Google Scholar] [CrossRef]
- Cuzzocrea, S.; Mazzon, E.; Dugo, L.; Serraino, I.; Di Paola, R.; Genovese, T.; De Sarro, A.; Caputi, A.P. Absence of endogenous interleukin-10 enhances the evolution of acute lung injury. Eur. Cytokine Netw. 2002, 13, 285–297. [Google Scholar]
- Lee, H.-S.; Kim, C.-K. Effect of recombinant IL-10 on cultured fetal rat alveolar type II cells exposed to 65%-hyperoxia. Respir. Res. 2011, 12, 68. [Google Scholar] [CrossRef] [Green Version]
- Bhandari, V. Molecular mechanisms of hyperoxia-induced acute lung injury. Front Biosci. 2008, 13, 6653–6661. [Google Scholar] [CrossRef] [Green Version]
- Li, H.D.; Zhang, Q.X.; Mao, Z.; Xu, X.J.; Li, N.Y.; Zhang, H. Exogenous interleukin-10 attenuates hyperoxia-induced acute lung injury in mice. Exp. Physiol. 2015, 100, 331–340. [Google Scholar] [CrossRef]
- Sun, Y.; Ma, J.; Li, D.; Li, P.; Zhou, X.; Li, Y.; He, Z.; Qin, L.; Liang, L.; Luo, X. Interleukin-10 inhibits interleukin-1β production and inflammasome activation of microglia in epileptic seizures. J. Neuroinflammation 2019, 16, 66. [Google Scholar] [CrossRef]
- Sheu, C.-C.; Gong, M.N.; Zhai, R.; Chen, F.; Bajwa, E.K.; Clardy, P.F.; Gallagher, D.C.; Thompson, B.T.; Christiani, D.C. Clinical Characteristics and Outcomes of Sepsis-Related vs Non-Sepsis-Related ARDS. CHEST 2010, 138, 559–567. [Google Scholar] [CrossRef] [Green Version]
- Syed, M.; Das, P.; Pawar, A.; Aghai, Z.H.; Kaskinen, A.; Zhuang, Z.W.; Ambalavanan, N.; Pryhuber, G.; Andersson, S.; Bhandari, V. Hyperoxia causes miR-34a-mediated injury via angiopoietin-1 in neonatal lungs. Nat. Commun. 2017, 8, 1173. [Google Scholar] [CrossRef]
- Shah, D.; Romero, F.; Stafstrom, W.; Duong, M.; Summer, R. Extracellular ATP mediates the late phase of neutrophil recruitment to the lung in murine models of acute lung injury. Am. J. Physiol. Lung. Cell Mol. Physiol. 2013, 306, L152–L161. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Matute-Bello, G.; Downey, G.; Moore, B.B.; Groshong, S.D.; Matthay, M.A.; Slutsky, A.S.; Kuebler, W.M.; Acute Lung Injury in Animals Study, G. An official American Thoracic Society workshop report: Features and measurements of experimental acute lung injury in animals. Am. J. Respir. Cell Mol. Biol. 2011, 44, 725–738. [Google Scholar] [CrossRef] [Green Version]
- An, X.; Sun, X.; Hou, Y.; Yang, X.; Chen, H.; Zhang, P.; Wu, J. Protective effect of oxytocin on LPS-induced acute lung injury in mice. Sci. Rep. 2019, 9, 2836. [Google Scholar] [CrossRef] [Green Version]
- Kubiak, B.D.; Albert, S.P.; Gatto, L.A.; Snyder, K.P.; Maier, K.G.; Vieau, C.J.; Roy, S.; Nieman, G.F. Peritoneal negative pressure therapy prevents multiple organ injury in a chronic porcine sepsis and ischemia/reperfusion model. Shock 2010, 34, 525–534. [Google Scholar] [CrossRef] [Green Version]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shah, D.; Das, P.; Acharya, S.; Agarwal, B.; Christensen, D.J.; Robertson, S.M.; Bhandari, V. Small Immunomodulatory Molecules as Potential Therapeutics in Experimental Murine Models of Acute Lung Injury (ALI)/Acute Respiratory Distress Syndrome (ARDS). Int. J. Mol. Sci. 2021, 22, 2573. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22052573
Shah D, Das P, Acharya S, Agarwal B, Christensen DJ, Robertson SM, Bhandari V. Small Immunomodulatory Molecules as Potential Therapeutics in Experimental Murine Models of Acute Lung Injury (ALI)/Acute Respiratory Distress Syndrome (ARDS). International Journal of Molecular Sciences. 2021; 22(5):2573. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22052573
Chicago/Turabian StyleShah, Dilip, Pragnya Das, Suchismita Acharya, Beamon Agarwal, Dale J. Christensen, Stella M. Robertson, and Vineet Bhandari. 2021. "Small Immunomodulatory Molecules as Potential Therapeutics in Experimental Murine Models of Acute Lung Injury (ALI)/Acute Respiratory Distress Syndrome (ARDS)" International Journal of Molecular Sciences 22, no. 5: 2573. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22052573






