Post-Transcriptional Regulation of Gnrhr: A Checkpoint for Metabolic Control of Female Reproduction
Abstract
:1. Introduction
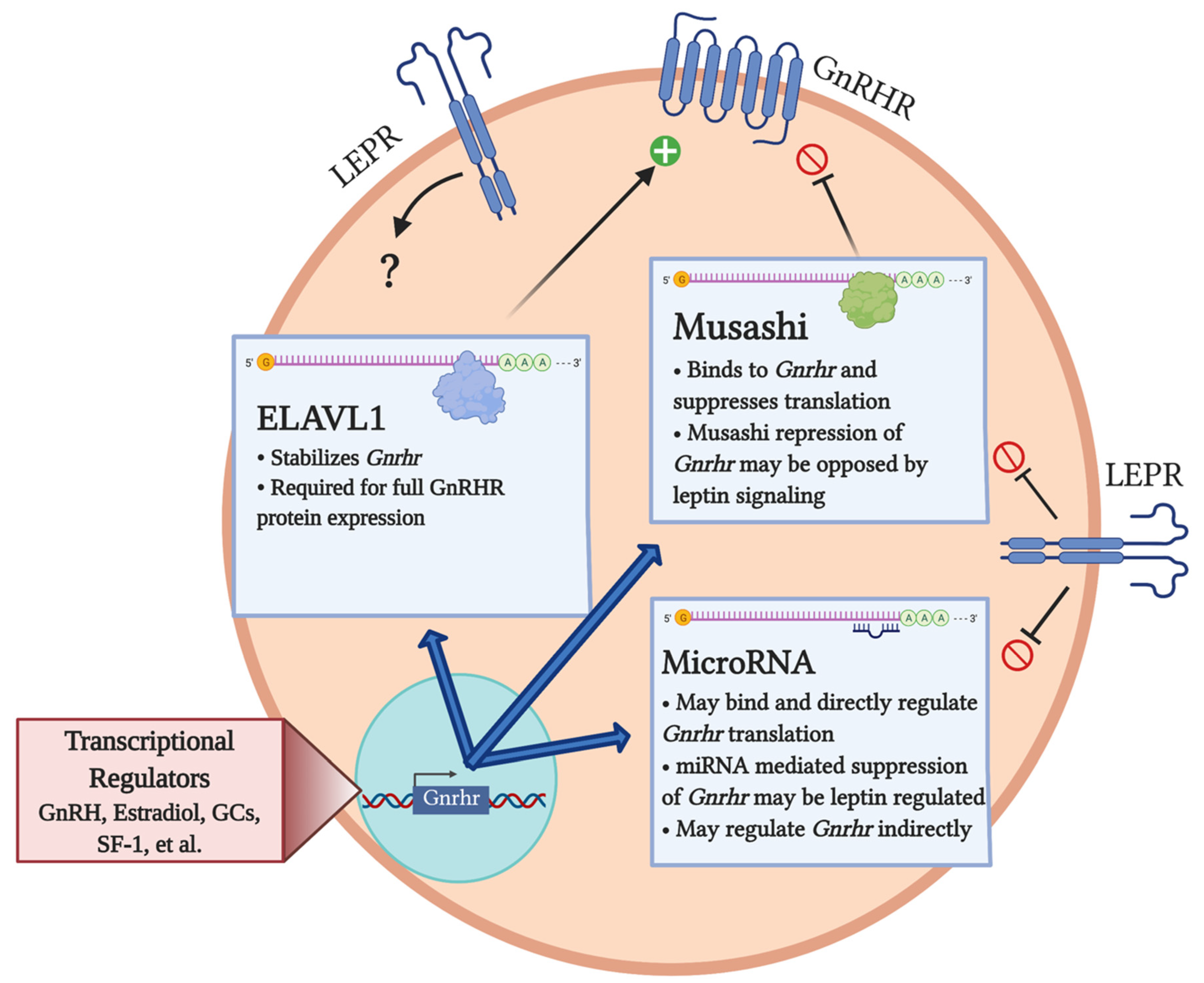
2. Transcriptional Regulation of Gnrhr
3. Post-Transcriptional Regulation of Gnrhr
3.1. miRNA Regulation of Gnrhr
3.2. Post-Transcriptional Regulation of Gnrhr mRNA by Musashi
3.3. Post-Transcriptional Regulation of Gnrhr by ELAVL1
4. Metabolic Influences on the Post-Transcriptional Regulation of GnRHR Levels: A Role for Leptin
5. Future Directions: Developmental Regulation of GnRHR
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bull, J.R.; Rowland, S.P.; Scherwitzl, E.B.; Scherwitzl, R.; Danielsson, K.G.; Harper, J. Real-world menstrual cycle characteristics of more than 600,000 menstrual cycles. NPJ Digit. Med. 2019, 2, 83. [Google Scholar] [CrossRef] [Green Version]
- Allen, E. The oestrous cycle in the mouse. Am. J. Anat. 1922, 30, 297. [Google Scholar] [CrossRef] [Green Version]
- Long, J.A.; Evans, H.M. The Oestrous Cycle in the Rat and its Associated Phenomena; University of California Press: Berkeley, CA, USA, 1922. [Google Scholar]
- Odle, A.K.; Akhter, N.; Syed, M.M.; Allensworth-James, M.L.; Benes, H.; Melgar Castillo, A.I.; MacNicol, M.C.; MacNicol, A.M.; Childs, G.V. Leptin regulation of gonadotrope gonadotropin-releasing hormone receptors as a metabolic checkpoint and gateway to reproductive competence. Front. Endocrinol. 2017, 8, 367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coss, D. Regulation of reproduction via tight control of gonadotropin hormone levels. Mol. Cell. Endocrinol. 2018, 463, 116–130. [Google Scholar] [CrossRef]
- Bédécarrats, G.Y.; Kaiser, U.B. Differential regulation of gonadotropin subunit gene promoter activity by pulsatile gonadotropin-releasing hormone (GnRH) in perifused L beta T2 cells: Role of GnRH receptor concentration. Endocrinology 2003, 144, 1802–1811. [Google Scholar] [CrossRef] [Green Version]
- Belchetz, P.E.; Plant, T.M.; Nakai, Y.; Keogh, E.J.; Knobil, E. Hypophysial responses to continuous and intermittent delivery of hypothalamic gonadotropin-releasing hormone. Science 1978, 202, 631–633. [Google Scholar] [CrossRef]
- Clayton, R.N. Gonadotrophin-releasing hormone: Its actions and receptors. J. Endocrinol. 1989, 120, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Crowley, W.F., Jr.; Filicori, M.; Spratt, D.I.; Santoro, N.F. The physiology of gonadotropin-releasing hormone (GnRH) secretion in men and women. Recent Prog. Horm. Res. 1985, 41, 473–531. [Google Scholar] [PubMed]
- Jobin, R.M.; Tomic, M.; Zheng, L.; Stojilkovic, S.S.; Catt, K.J. Gonadotropin-releasing hormone-induced sensitization of calcium-dependent exocytosis in pituitary gonadotrophs. Endocrinology 1995, 136, 3398–3405. [Google Scholar] [CrossRef]
- Lloyd, J.M.; Childs, G.V. Changes in the number of GnRH-receptive cells during the rat estrous cycle: Biphasic effects of estradiol. Neuroendocrinology 1988, 48, 138–146. [Google Scholar] [CrossRef]
- Schang, A.L.; Quérat, B.; Simon, V.; Garrel, G.; Bleux, C.; Counis, R.; Cohen-Tannoudji, J.; Laverrière, J.N. Mechanisms underlying the tissue-specific and regulated activity of the Gnrhr promoter in mammals. Front. Endocrinol. 2012, 3, 162. [Google Scholar] [CrossRef] [Green Version]
- Conn, P.M.; Knollman, P.E.; Brothers, S.P.; Janovick, J.A. Protein folding as posttranslational regulation: Evolution of a mechanism for controlled plasma membrane expression of a G protein-coupled receptor. Mol. Endocrinol. 2006, 20, 3035–3041. [Google Scholar] [CrossRef] [Green Version]
- Savoy-Moore, R.T.; Schwartz, N.B.; Duncan, J.A.; Marshall, J.C. Pituitary gonadotropin-releasing hormone receptors during the rat estrous cycle. Science 1980, 209, 942–944. [Google Scholar] [CrossRef]
- Marian, J.; Cooper, R.L.; Conn, P.M. Regulation of the rat pituitary gonadotropin-releasing hormone receptor. Mol. Pharmacol. 1981, 19, 399–405. [Google Scholar] [PubMed]
- Papavasiliou, S.S.; Zmeili, S.; Khoury, S.; Landefeld, T.D.; Chin, W.W.; Marshall, J.C. Gonadotropin-releasing hormone differentially regulates expression of the genes for luteinizing hormone alpha and beta subunits in male rats. Proc. Natl. Acad. Sci. USA 1986, 83, 4026–4029. [Google Scholar] [CrossRef] [Green Version]
- Peng, C.; Fan, N.C.; Ligier, M.; Väänänen, J.; Leung, P.C. Expression and regulation of gonadotropin-releasing hormone (GnRH) and GnRH receptor messenger ribonucleic acids in human granulosa-luteal cells. Endocrinology 1994, 135, 1740–1746. [Google Scholar] [CrossRef]
- Minaretzis, D.; Jakubowski, M.; Mortola, J.F.; Pavlou, S.N. Gonadotropin-releasing hormone receptor gene expression in human ovary and granulosa-lutein cells. J. Clin. Endocrinol. Metab. 1995, 80, 430–434. [Google Scholar] [CrossRef] [Green Version]
- Bull, P.; Morales, P.; Huyser, C.; Socías, T.; Castellón, E.A. Expression of GnRH receptor in mouse and rat testicular germ cells. Mol. Hum. Reprod. 2000, 6, 582–586. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, L.S.; Roberts, V.J.; Yen, S.S. Expression of human gonadotropin-releasing hormone receptor gene in the placenta and its functional relationship to human chorionic gonadotropin secretion. J. Clin. Endocrinol. Metab. 1995, 80, 580–585. [Google Scholar] [CrossRef] [PubMed]
- Quintanar, J.L.; Salinas, E.; González, R. Gonadotropin-releasing hormone receptor in spinal cord neurons of embryos and adult rats. Neurosci. Lett. 2009, 461, 21–24. [Google Scholar] [CrossRef]
- Leblanc, P.; Crumeyrolle, M.; Latouche, J.; Jordan, D.; Fillion, G.; L’Heritier, A.; Kordon, C.; Dussaillant, M.; Rostène, W.; Haour, F. Characterization and distribution of receptors for gonadotropin-releasing hormone in the rat hippocampus. Neuroendocrinology 1988, 48, 482–488. [Google Scholar] [CrossRef]
- Ban, E.; Crumeyrolle-Arias, M.; Latouche, J.; Leblanc, P.; Heurtier, J.F.; Drieu, K.; Fillion, G.; Haour, F. GnRH receptors in rat brain, pituitary and testis; modulation following surgical and gonadotropin-releasing hormone agonist-induced castration. Mol. Cell. Endocrinol. 1990, 70, 99–107. [Google Scholar] [CrossRef]
- Wilson, A.C.; Salamat, M.S.; Haasl, R.J.; Roche, K.M.; Karande, A.; Meethal, S.V.; Terasawa, E.; Bowen, R.L.; Atwood, C.S. Human neurons express type I GnRH receptor and respond to GnRH I by increasing luteinizing hormone expression. J. Endocrinol. 2006, 191, 651–663. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Badr, M.; Pelletier, G. Characterization and autoradiographic localization of LHRH receptors in the rat brain. Synapse 1987, 1, 567–571. [Google Scholar] [CrossRef]
- Wen, S.; Götze, I.N.; Mai, O.; Schauer, C.; Leinders-Zufall, T.; Boehm, U. Genetic identification of GnRH receptor neurons: A new model for studying neural circuits underlying reproductive physiology in the mouse brain. Endocrinology 2011, 152, 1515–1526. [Google Scholar] [CrossRef] [Green Version]
- Hapgood, J.P.; Sadie, H.; van Biljon, W.; Ronacher, K. Regulation of expression of mammalian gonadotrophin-releasing hormone receptor genes. J. Neuroendocrinol. 2005, 17, 619–638. [Google Scholar] [CrossRef] [PubMed]
- Janjic, M.M.; Stojilkovic, S.S.; Bjelobaba, I. Intrinsic and regulated gonadotropin-releasing hormone receptor gene transcription in mammalian pituitary gonadotrophs. Front. Endocrinol. 2017, 8, 221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, V.H.; Lee, L.T.; Chow, B.K. Gonadotropin-releasing hormone: Regulation of the GnRH gene. FEBS J. 2008, 275, 5458–5478. [Google Scholar] [CrossRef]
- Yasin, M.; Dalkin, A.C.; Haisenleder, D.J.; Kerrigan, J.R.; Marshall, J.C. Gonadotropin-releasing hormone (GnRH) pulse pattern regulates GnRH receptor gene expression: Augmentation by estradiol. Endocrinology 1995, 136, 1559–1564. [Google Scholar] [CrossRef] [Green Version]
- Norwitz, E.R.; Cardona, G.R.; Jeong, K.H.; Chin, W.W. Identification and characterization of the gonadotropin-releasing hormone response elements in the mouse gonadotropin-releasing hormone receptor gene. J. Biol. Chem. 1999, 274, 867–880. [Google Scholar] [CrossRef] [Green Version]
- Albarracin, C.T.; Kaiser, U.B.; Chin, W.W. Isolation and characterization of the 5′-flanking region of the mouse gonadotropin-releasing hormone receptor gene. Endocrinology 1994, 135, 2300–2306. [Google Scholar] [CrossRef] [PubMed]
- Norwitz, E.R.; Xu, S.; Jeong, K.H.; Bédécarrats, G.Y.; Winebrenner, L.D.; Chin, W.W.; Kaiser, U.B. Activin A augments GnRH-mediated transcriptional activation of the mouse GnRH receptor gene. Endocrinology 2002, 143, 985–997. [Google Scholar] [CrossRef]
- Norwitz, E.R.; Xu, S.; Xu, J.; Spiryda, L.B.; Park, J.S.; Jeong, K.H.; McGee, E.A.; Kaiser, U.B. Direct binding of AP-1 (Fos/Jun) proteins to a SMAD binding element facilitates both gonadotropin-releasing hormone (GnRH)- and activin-mediated transcriptional activation of the mouse GnRH receptor gene. J. Biol. Chem. 2002, 277, 37469–37478. [Google Scholar] [CrossRef] [Green Version]
- Fortin, J.; Ongaro, L.; Li, Y.; Tran, S.; Lamba, P.; Wang, Y.; Zhou, X.; Bernard, D.J. Minireview: Activin signaling in gonadotropes: What does the FOX say … to the SMAD? Mol. Endocrinol. 2015, 29, 963–977. [Google Scholar] [CrossRef]
- Kumar, T.R.; Agno, J.; Janovick, J.A.; Conn, P.M.; Matzuk, M.M. Regulation of FSHbeta and GnRH receptor gene expression in activin receptor II knockout male mice. Mol. Cell. Endocrinol. 2003, 212, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Nathwani, P.S.; Kang, S.K.; Cheng, K.W.; Choi, K.C.; Leung, P.C. Regulation of gonadotropin-releasing hormone and its receptor gene expression by 17beta-estradiol in cultured human granulosa-luteal cells. Endocrinology 2000, 141, 1754–1763. [Google Scholar] [CrossRef]
- Maya-Núñez, G.; Conn, P.M. Transcriptional regulation of the GnRH receptor gene by glucocorticoids. Mol. Cell. Endocrinol. 2003, 200, 89–98. [Google Scholar] [CrossRef]
- Ngan, E.S.; Cheng, P.K.; Leung, P.C.; Chow, B.K. Steroidogenic factor-1 interacts with a gonadotrope-specific element within the first exon of the human gonadotropin-releasing hormone receptor gene to mediate gonadotrope-specific expression. Endocrinology 1999, 140, 2452–2462. [Google Scholar] [CrossRef] [PubMed]
- Duval, D.L.; Nelson, S.E.; Clay, C.M. A binding site for steroidogenic factor-1 is part of a complex enhancer that mediates expression of the murine gonadotropin-releasing hormone receptor gene. Biol. Reprod. 1997, 56, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Pincas, H.; Laverrière, J.N.; Counis, R. Pituitary adenylate cyclase-activating polypeptide and cyclic adenosine 3’,5’-monophosphate stimulate the promoter activity of the rat gonadotropin-releasing hormone receptor gene via a bipartite response element in gonadotrope-derived cells. J. Biol. Chem. 2001, 276, 23562–23571. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schang, A. Inside and outside the pituitary: Comparative analysis of Gnrhr expression provides insight into the mechanisms underlying the evolution of gene expression. J. Neuroendocrinol. 2015, 27, 177–186. [Google Scholar] [CrossRef] [PubMed]
- Loumaye, E.; Catt, K.J. Homologous regulation of gonadotropin-releasing hormone receptors in cultured pituitary cells. Science 1982, 215, 983–985. [Google Scholar] [CrossRef]
- Katt, J.A.; Duncan, J.A.; Herbon, L.; Barkan, A.; Marshall, J.C. The frequency of gonadotropin-releasing hormone stimulation determines the number of pituitary gonadotropin-releasing hormone receptors. Endocrinology 1985, 116, 2113–2115. [Google Scholar] [CrossRef]
- Tsutsumi, M.; Laws, S.C.; Sealfon, S.C. Homologous up-regulation of the gonadotropin-releasing hormone receptor in alpha T3-1 cells is associated with unchanged receptor messenger RNA (mRNA) levels and altered mRNA activity. Mol. Endocrinol. 1993, 7, 1625–1633. [Google Scholar] [CrossRef] [Green Version]
- Tsutsumi, M.; Laws, S.C.; Rodic, V.; Sealfon, S.C. Translational regulation of the gonadotropin-releasing hormone receptor in alpha T3-1 cells. Endocrinology 1995, 136, 1128–1136. [Google Scholar] [CrossRef] [PubMed]
- Yuen, T.; Ruf, F.; Chu, T.; Sealfon, S.C. Microtranscriptome regulation by gonadotropin-releasing hormone. Mol. Cell. Endocrinol. 2009, 302, 12–17. [Google Scholar] [CrossRef] [Green Version]
- Godoy, J.; Nishimura, M.; Webster, N.J. Gonadotropin-releasing hormone induces miR-132 and miR-212 to regulate cellular morphology and migration in immortalized LbetaT2 pituitary gonadotrope cells. Mol. Endocrinol. 2011, 25, 810–820. [Google Scholar] [CrossRef]
- Lannes, J.; L’Hôte, D.; Garrel, G.; Laverrière, J.N.; Cohen-Tannoudji, J.; Quérat, B. Rapid communication: A microRNA-132/212 pathway mediates GnRH activation of FSH expression. Mol. Endocrinol. 2015, 29, 364–372. [Google Scholar] [CrossRef] [Green Version]
- Lannes, J.; L’Hôte, D.; Fernandez-Vega, A.; Garrel, G.; Laverrière, J.N.; Cohen-Tannoudji, J.; Quérat, B. A regulatory loop between miR-132 and miR-125b involved in gonadotrope cells desensitization to GnRH. Sci. Rep. 2016, 6, 31563. [Google Scholar] [CrossRef]
- Han, D.X.; Sun, X.L.; Xu, M.Q.; Chen, C.Z.; Jiang, H.; Gao, Y.; Yuan, B.; Zhang, J.B. Roles of differential expression of microRNA-21-3p and microRNA-433 in FSH regulation in rat anterior pituitary cells. Oncotarget 2017, 8, 36553–36565. [Google Scholar] [CrossRef]
- Han, D.X.; Xiao, Y.; Wang, C.J.; Jiang, H.; Gao, Y.; Yuan, B.; Zhang, J.B. Regulation of FSH expression by differentially expressed miR-186-5p in rat anterior adenohypophyseal cells. PLoS ONE 2018, 13, e0194300. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, R.S.; Xi, Q.Y.; Qi, Q.; Cheng, X.; Chen, T.; Li, H.; Kallon, S.; Shu, G.; Wang, S.B.; Jiang, Q.Y.; et al. Differentially expressed miRNAs after GnRH treatment and their potential roles in FSH regulation in porcine anterior pituitary cell. PLoS ONE 2013, 8, e57156. [Google Scholar] [CrossRef]
- Zhang, L.; Cai, Z.; Wei, S.; Zhou, H.; Zhou, H.; Jiang, X.; Xu, N. MicroRNA expression profiling of the porcine developing hypothalamus and pituitary tissue. Int. J. Mol. Sci. 2013, 14, 20326–20339. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Hastings, R.; Miller, W.L.; Kumar, T.R. Fshb-iCre mice are efficient and specific Cre deleters for the gonadotrope lineage. Mol. Cell. Endocrinol. 2016, 419, 124–138. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, K.; LaPierre, M.P.; Gasser, E.; Denzler, R.; Yang, Y.; Rülicke, T.; Kero, J.; Latreille, M.; Stoffel, M. Loss of microRNA-7a2 induces hypogonadotropic hypogonadism and infertility. J. Clin. Investig. 2017, 127, 1061–1074. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Graham, I.; Hastings, R.; Gunewardena, S.; Brinkmeier, M.L.; Conn, P.M.; Camper, S.A.; Kumar, T.R. Gonadotrope-specific deletion of Dicer results in severely suppressed gonadotropins and fertility defects. J. Biol. Chem. 2015, 290, 2699–2714. [Google Scholar] [CrossRef] [PubMed]
- Bernstein, E.; Caudy, A.A.; Hammond, S.M.; Hannon, G.J. Role for a bidentate ribonuclease in the initiation step of RNA interference. Nature 2001, 409, 363–366. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, V.; Bell, G.W.; Nam, J.W.; Bartel, D.P. Predicting effective microRNA target sites in mammalian mRNAs. eLife 2015, 4. [Google Scholar] [CrossRef]
- Odle, A.K.; Benes, H.; Melgar Castillo, A.; Akhter, N.; Syed, M.; Haney, A.; Allensworth-James, M.; Hardy, L.; Winter, B.; Manoharan, R.; et al. Association of Gnrhr mRNA with the stem cell determinant Musashi: A mechanism for leptin-mediated modulation of GnRHR expression. Endocrinology 2018, 159, 883–894. [Google Scholar] [CrossRef]
- Fox, R.G.; Park, F.D.; Koechlein, C.S.; Kritzik, M.; Reya, T. Musashi signaling in stem cells and cancer. Annu. Rev. Cell Dev. Biol. 2015, 31, 249–267. [Google Scholar] [CrossRef] [Green Version]
- Allensworth-James, M.; Banik, J.; Odle, A.; Hardy, L.; Lagasse, A.; Moreira, A.R.S.; Bird, J.; Thomas, C.L.; Avaritt, N.; Kharas, M.G.; et al. Control of the anterior pituitary cell lineage regulator POU1F1 by the stem cell determinant Musashi. Endocrinology 2021, 162. [Google Scholar] [CrossRef]
- Terasaka, T.; Kim, T.; Dave, H.; Gangapurkar, B.; Nicholas, D.A.; Muñoz, O.; Terasaka, E.; Li, D.; Lawson, M.A. The RNA-binding protein ELAVL1 regulates GnRH receptor expression and the response to GnRH. Endocrinology 2019, 160, 1999–2014. [Google Scholar] [CrossRef] [PubMed]
- Hinman, M.N.; Lou, H. Diverse molecular functions of Hu proteins. Cell. Mol. Life Sci. CMLS 2008, 65, 3168–3181. [Google Scholar] [CrossRef] [Green Version]
- Brothers, K.J.; Wu, S.; DiVall, S.A.; Messmer, M.R.; Kahn, C.R.; Miller, R.S.; Radovick, S.; Wondisford, F.E.; Wolfe, A. Rescue of obesity-induced infertility in female mice due to a pituitary-specific knockout of the insulin receptor. Cell Metab. 2010, 12, 295–305. [Google Scholar] [CrossRef] [Green Version]
- Wu, S.; Divall, S.; Nwaopara, A.; Radovick, S.; Wondisford, F.; Ko, C.; Wolfe, A. Obesity-induced infertility and hyperandrogenism are corrected by deletion of the insulin receptor in the ovarian theca cell. Diabetes 2014, 63, 1270–1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Childs, G.; Odle, A.; MacNicol, M.; MacNicol, A. Leptin and its importance to reproduction. Endocrinology 2020. In Press. [Google Scholar]
- Schwartz, M.W.; Peskind, E.; Raskind, M.; Boyko, E.J.; Porte, D., Jr. Cerebrospinal fluid leptin levels: Relationship to plasma levels and to adiposity in humans. Nat. Med. 1996, 2, 589–593. [Google Scholar] [CrossRef]
- Frederich, R.C.; Hamann, A.; Anderson, S.; Lollmann, B.; Lowell, B.B.; Flier, J.S. Leptin levels reflect body lipid content in mice: Evidence for diet-induced resistance to leptin action. Nat. Med. 1995, 1, 1311–1314. [Google Scholar] [CrossRef]
- Grodstein, F.; Goldman, M.B.; Cramer, D.W. Body mass index and ovulatory infertility. Epidemiology 1994, 5, 247–250. [Google Scholar] [CrossRef]
- Rich-Edwards, J.W.; Goldman, M.B.; Willett, W.C.; Hunter, D.J.; Stampfer, M.J.; Colditz, G.A.; Manson, J.E. Adolescent body mass index and infertility caused by ovulatory disorder. Am. J. Obstet. Gynecol. 1994, 171, 171–177. [Google Scholar] [CrossRef]
- Zaadstra, B.M.; Seidell, J.C.; Van Noord, P.A.; te Velde, E.R.; Habbema, J.D.; Vrieswijk, B.; Karbaat, J. Fat and female fecundity: Prospective study of effect of body fat distribution on conception rates. BMJ 1993, 306, 484–487. [Google Scholar] [CrossRef] [Green Version]
- Sir-Petermann, T.; Piwonka, V.; Pérez, F.; Maliqueo, M.; Recabarren, S.E.; Wildt, L. Are circulating leptin and luteinizing hormone synchronized in patients with polycystic ovary syndrome? Hum. Reprod. 1999, 14, 1435–1439. [Google Scholar] [CrossRef]
- Charlton, H.M. Mouse mutants as models in endocrine research. Q. J. Exp. Physiol. 1984, 69, 655–676. [Google Scholar] [CrossRef] [Green Version]
- Ingalls, A.M.; Dickie, M.M.; Snell, G.D. Obese, a new mutation in the house mouse. J. Hered. 1950, 41, 317–318. [Google Scholar] [CrossRef] [PubMed]
- Swerdloff, R.S.; Batt, R.A.; Bray, G.A. Reproductive hormonal function in the genetically obese (ob/ob) mouse. Endocrinology 1976, 98, 1359–1364. [Google Scholar] [CrossRef] [PubMed]
- Barash, I.A.; Cheung, C.C.; Weigle, D.S.; Ren, H.; Kabigting, E.B.; Kuijper, J.L.; Clifton, D.K.; Steiner, R.A. Leptin is a metabolic signal to the reproductive system. Endocrinology 1996, 137, 3144–3147. [Google Scholar] [CrossRef] [Green Version]
- Chehab, F.F.; Lim, M.E.; Lu, R. Correction of the sterility defect in homozygous obese female mice by treatment with the human recombinant leptin. Nat. Genet. 1996, 12, 318–320. [Google Scholar] [CrossRef]
- Karlsson, C.; Lindell, K.; Svensson, E.; Bergh, C.; Lind, P.; Billig, H.; Carlsson, L.M.; Carlsson, B. Expression of functional leptin receptors in the human ovary. J. Clin. Endocrinol. Metab. 1997, 82, 4144–4148. [Google Scholar] [CrossRef] [PubMed]
- Ratra, D.V.; Elias, C.F. Chemical identity of hypothalamic neurons engaged by leptin in reproductive control. J. Chem. Neuroanat. 2014, 61–62, 233–238. [Google Scholar] [CrossRef] [Green Version]
- Smith, J.T.; Acohido, B.V.; Clifton, D.K.; Steiner, R.A. KiSS-1 neurones are direct targets for leptin in the ob/ob mouse. J. Neuroendocrinol. 2006, 18, 298–303. [Google Scholar] [CrossRef]
- Akhter, N.; CarlLee, T.; Syed, M.M.; Odle, A.K.; Cozart, M.A.; Haney, A.C.; Allensworth-James, M.L.; Benes, H.; Childs, G.V. Selective deletion of leptin receptors in gonadotropes reveals activin and GnRH-binding sites as leptin targets in support of fertility. Endocrinology 2014, 155, 4027–4042. [Google Scholar] [CrossRef] [PubMed]
- Landrier, J.F.; Derghal, A.; Mounien, L. MicroRNAs in obesity and related metabolic disorders. Cells 2019, 8, 859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Derghal, A.; Astier, J.; Sicard, F.; Couturier, C.; Landrier, J.F.; Mounien, L. Leptin modulates the expression of miRNAs-targeting POMC mRNA by the JAK2-STAT3 and PI3K-akt pathways. J. Clin. Med. 2019, 8, 2213. [Google Scholar] [CrossRef] [Green Version]
- Meerson, A.; Yehuda, H. Leptin and insulin up-regulate miR-4443 to suppress NCOA1 and TRAF4, and decrease the invasiveness of human colon cancer cells. BMC Cancer 2016, 16, 882. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, W.H.; Chang, A.C.; Wang, S.W.; Wang, S.J.; Chang, Y.S.; Chang, T.M.; Hsu, S.K.; Fong, Y.C.; Tang, C.H. Leptin promotes VEGF-C production and induces lymphangiogenesis by suppressing miR-27b in human chondrosarcoma cells. Sci. Rep. 2016, 6, 28647. [Google Scholar] [CrossRef] [Green Version]
- Zhai, X.; Cheng, F.; Ji, L.; Zhu, X.; Cao, Q.; Zhang, Y.; Jia, X.; Zhou, Q.; Guan, W.; Zhou, Y. Leptin reduces microRNA-122 level in hepatic stellate cells in vitro and in vivo. Mol. Immunol. 2017, 92, 68–75. [Google Scholar] [CrossRef]
- Allensworth-James, M.L.; Odle, A.K.; Lim, J.; LaGasse, A.N.; Miles, T.K.; Hardy, L.L.; Haney, A.C.; MacNicol, M.C.; MacNicol, A.M.; Childs, G.A.-O. Metabolic signalling to somatotrophs: Transcriptional and post-transcriptional mediators. J. Endocrinol. 2020, 32, e12883. [Google Scholar] [CrossRef]
- Hasuwa, H.; Ueda, J.; Ikawa, M.; Okabe, M. miR-200b and miR-429 function in mouse ovulation and are essential for female fertility. Science 2013, 341, 71–73. [Google Scholar] [CrossRef]
- Eisenberg, I.; Nahmias, N.; Novoselsky Persky, M.; Greenfield, C.; Goldman-Wohl, D.; Hurwitz, A.; Haimov-Kochman, R.; Yagel, S.; Imbar, T. Elevated circulating micro-ribonucleic acid (miRNA)-200b and miRNA-429 levels in anovulatory women. Fertil. Steril. 2017, 107, 269–275. [Google Scholar] [CrossRef] [Green Version]
- Granger, A.; Ngo-Muller, V.; Bleux, C.; Guigon, C.; Pincas, H.; Magre, S.; Daegelen, D.; Tixier-Vidal, A.; Counis, R.; Laverriere, J.N. The promoter of the rat gonadotropin-releasing hormone receptor gene directs the expression of the human placental alkaline phosphatase reporter gene in gonadotrope cells in the anterior pituitary gland as well as in multiple extrapituitary tissues. Endocrinology 2004, 145, 983–993. [Google Scholar] [CrossRef]
- Wen, S.; Ai, W.; Alim, Z.; Boehm, U. Embryonic gonadotropin-releasing hormone signaling is necessary for maturation of the male reproductive axis. Proc. Natl. Acad. Sci. USA 2010, 107, 16372–16377. [Google Scholar] [CrossRef] [Green Version]
- Pointis, G.; Mahoudeau, J.A. [Study of Leydig cells and gonadotropin activity in 14–18 days old fetal mouse (author’s transl)]. Ann. D’endocrinol. 1979, 40, 431–432. [Google Scholar]
- Herbison, A.E. Control of puberty onset and fertility by gonadotropin-releasing hormone neurons. Nat. Rev. Endocrinol. 2016, 12, 452–466. [Google Scholar] [CrossRef] [PubMed]
- Bjelobaba, I.; Janjic, M.M.; Kucka, M.; Stojilkovic, S.S. Cell type-specific sexual dimorphism in rat pituitary gene expression during maturation. Biol. Reprod. 2015, 93, 21. [Google Scholar] [CrossRef] [Green Version]
- Ahima, R.S.; Prabakaran, D.; Flier, J.S. Postnatal leptin surge and regulation of circadian rhythm of leptin by feeding. Implications for energy homeostasis and neuroendocrine function. J. Clin. Investig. 1998, 101, 1020–1027. [Google Scholar] [CrossRef] [Green Version]
- Jaquet, D.; Leger, J.; Levy-Marchal, C.; Oury, J.F.; Czernichow, P. Ontogeny of leptin in human fetuses and newborns: Effect of intrauterine growth retardation on serum leptin concentrations. J. Clin. Endocrinol. Metab. 1998, 83, 1243–1246. [Google Scholar] [CrossRef] [PubMed]
- Delahaye, F.; Breton, C.; Risold, P.Y.; Enache, M.; Dutriez-Casteloot, I.; Laborie, C.; Lesage, J.; Vieau, D. Maternal perinatal undernutrition drastically reduces postnatal leptin surge and affects the development of arcuate nucleus proopiomelanocortin neurons in neonatal male rat pups. Endocrinology 2008, 149, 470–475. [Google Scholar] [CrossRef]
- Lopez-Gallardo, M.; Anton-Fernandez, A.; Llorente, R.; Mela, V.; Llorente-Berzal, A.; Prada, C.; Viveros, M.P. Neonatal treatment with a pegylated leptin antagonist induces sexually dimorphic effects on neurones and glial cells, and on markers of synaptic plasticity in the developing rat hippocampal formation. J. Neuroendocrinol. 2015, 27, 658–669. [Google Scholar] [CrossRef] [PubMed]
- Coupe, B.; Amarger, V.; Grit, I.; Benani, A.; Parnet, P. Nutritional programming affects hypothalamic organization and early response to leptin. Endocrinology 2010, 151, 702–713. [Google Scholar] [CrossRef]
- Attig, L.; Larcher, T.; Gertler, A.; Abdennebi-Najar, L.; Djiane, J. Postnatal leptin is necessary for maturation of numerous organs in newborn rats. Organogenesis 2011, 7, 88–94. [Google Scholar] [CrossRef]
- Mela, V.; Diaz, F.; Vazquez, M.J.; Argente, J.; Tena-Sempere, M.; Viveros, M.P.; Chowen, J.A. Interaction between neonatal maternal deprivation and serum leptin levels on metabolism, pubertal development, and sexual behavior in male and female rats. Biol. Sex. Differ. 2016, 7, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mela, V.; Diaz, F.; Lopez-Rodriguez, A.B.; Vazquez, M.J.; Gertler, A.; Argente, J.; Tena-Sempere, M.; Viveros, M.P.; Chowen, J.A. Blockage of the neonatal leptin surge affects the gene expression of growth factors, glial proteins, and neuropeptides involved in the control of metabolism and reproduction in peripubertal male and female rats. Endocrinology 2015, 156, 1571–1581. [Google Scholar] [CrossRef]
- Chehab, F.F.; Mounzih, K.; Lu, R.; Lim, M.E. Early onset of reproductive function in normal female mice treated with leptin. Science 1997, 275, 88–90. [Google Scholar] [CrossRef]
- Ahima, R.S.; Dushay, J.; Flier, S.N.; Prabakaran, D.; Flier, J.S. Leptin accelerates the onset of puberty in normal female mice. J. Clin. Investig. 1997, 99, 391–395. [Google Scholar] [CrossRef] [PubMed]
- Cheung, C.C.; Thornton, J.E.; Kuijper, J.L.; Weigle, D.S.; Clifton, D.K.; Steiner, R.A. Leptin is a metabolic gate for the onset of puberty in the female rat. Endocrinology 1997, 138, 855–858. [Google Scholar] [CrossRef]
- Schwartz, M.W.; Seeley, R.J.; Woods, S.C.; Weigle, D.S.; Campfield, L.A.; Burn, P.; Baskin, D.G. Leptin increases hypothalamic pro-opiomelanocortin mRNA expression in the rostral arcuate nucleus. Diabetes 1997, 46, 2119–2123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akira, S.; Maeda, K. Control of RNA stability in immunity. Annu. Rev. Immunol. 2021. [Google Scholar] [CrossRef]
- Ho, J.J.; Marsden, P.A. Competition and collaboration between RNA-binding proteins and microRNAs. Wiley Interdiscip. Rev. RNA 2014, 5, 69–86. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Hjelmeland, A.B.; Nabors, L.B.; King, P.H. Anti-cancer effects of the HuR inhibitor, MS-444, in malignant glioma cells. Cancer Biol. 2019, 20, 979–988. [Google Scholar] [CrossRef] [PubMed]
- Wu, M.; Tong, C.W.S.; Yan, W.; To, K.K.W.; Cho, W.C.S. The RNA binding protein HuR: A promising drug target for anticancer therapy. Curr. Cancer Drug Targets 2019, 19, 382–399. [Google Scholar] [CrossRef]
- Muralidharan, R.; Mehta, M.; Ahmed, R.; Roy, S.; Xu, L.; Aubé, J.; Chen, A.; Zhao, Y.D.; Herman, T.; Ramesh, R.; et al. HuR-targeted small molecule inhibitor exhibits cytotoxicity towards human lung cancer cells. Sci. Rep. 2017, 7, 9694. [Google Scholar] [CrossRef] [PubMed]
- Statello, L.; Guo, C.J.; Chen, L.L.; Huarte, M. Gene regulation by long non-coding RNAs and its biological functions. Nat. Rev. Mol. Cell Biol. 2021, 22, 96–118. [Google Scholar] [CrossRef] [PubMed]
- Raue, R.; Frank, A.C.; Syed, S.N.; Brüne, B. Therapeutic targeting of MicroRNAs in the tumor microenvironment. Int. J. Mol. Sci. 2021, 22, 2210. [Google Scholar] [CrossRef] [PubMed]
| Predicted 3′ UTR Target Site | Position in Mouse Gnrhr 3′ UTR (ntds) | Conserved in Human Gnrhr 3′ UTR? | Conserved in Rat Gnrhr 3′ UTR? | miRNA Target Site Overlap with MBE? |
|---|---|---|---|---|
| miR-150-5p | 1–8 | |||
| miR-532-3p | 2–8 | Yes | ||
| miR-669d-5p | 11–18 | Yes | ||
| miR-3089-5p | 17–23 | |||
| miR-1199-5p | 19–25 | Yes | ||
| miR-3061-3p | 33–39 | Yes | ||
| miR-7223-5p | 66–72 | |||
| miR-599 | 75–81 | |||
| miR-467eh-5p/miR-668-5p | 79–85 | |||
| miR-344-3p/miR-410-3p | 83–89 | |||
| miR-1981-3p | 106–112 | Yes | ||
| miR-495-3p | 136–142 | Yes | ||
| miR-3065-5p | 137–143 | Yes | Yes | |
| miR-493-3p | 158–165 | Yes | ||
| miR-129-5p | 176–182 | |||
| miR-129-5p | 183–189 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Odle, A.K.; MacNicol, M.C.; Childs, G.V.; MacNicol, A.M. Post-Transcriptional Regulation of Gnrhr: A Checkpoint for Metabolic Control of Female Reproduction. Int. J. Mol. Sci. 2021, 22, 3312. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22073312
Odle AK, MacNicol MC, Childs GV, MacNicol AM. Post-Transcriptional Regulation of Gnrhr: A Checkpoint for Metabolic Control of Female Reproduction. International Journal of Molecular Sciences. 2021; 22(7):3312. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22073312
Chicago/Turabian StyleOdle, Angela K., Melanie C. MacNicol, Gwen V. Childs, and Angus M. MacNicol. 2021. "Post-Transcriptional Regulation of Gnrhr: A Checkpoint for Metabolic Control of Female Reproduction" International Journal of Molecular Sciences 22, no. 7: 3312. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22073312






