Interleukin-13 Propagates Prothrombin Kringle-2-Induced Neurotoxicity in Hippocampi In Vivo via Oxidative Stress
Abstract
:1. Introduction
2. Results
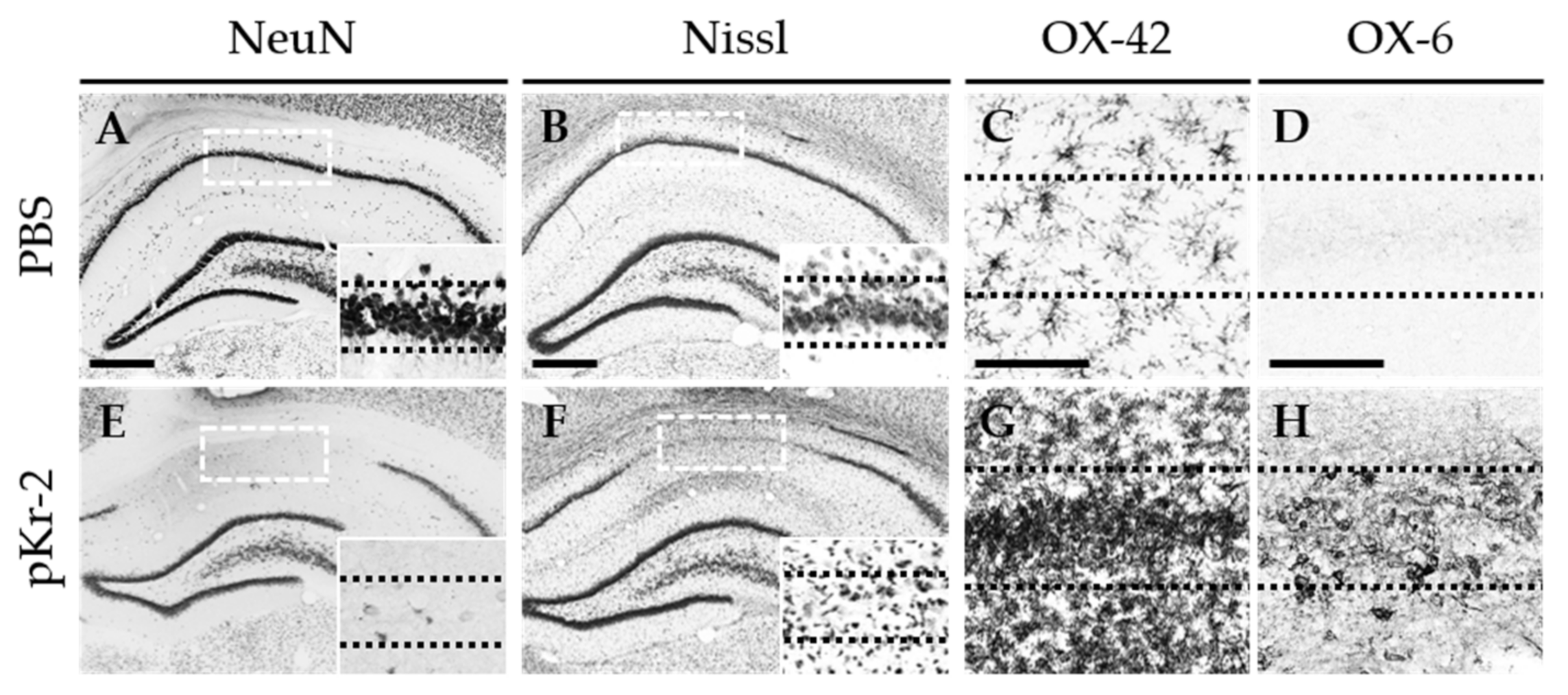
2.1. pKr-2 Induces Degeneration of Hippocampal Neurons and Activation of Microglia/Macrophages and Neutrophils in the Hippocampus In Vivo
2.2. pKr-2-Induced IL-13 Expression Is Localized within Activated Microglia/Macrophages and Neutrophils in the Hippocampus In Vivo
2.3. Neurotoxic Action of IL-13 on Degeneration of Hippocampal Neurons via iNOS and MPO in the CA1 Layer of Hippocampus In Vivo
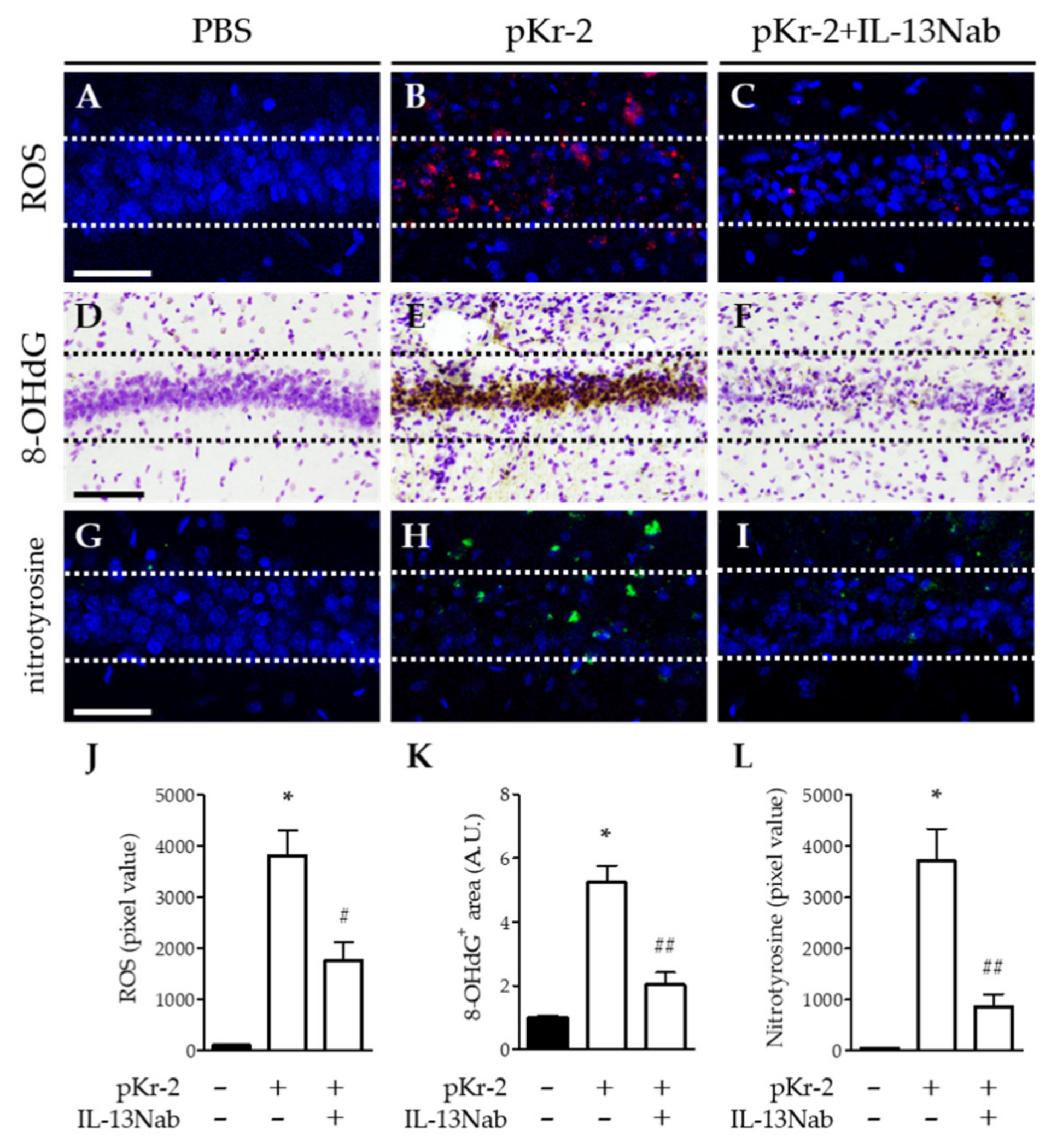
2.4. IL-13 Is Associated with Oxidative/Nitrosative Stress in the CA1 Layer of pKr-2-Injected Hippocampus In Vivo
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Intrahippocampal Microinjection
4.3. Immunohistochemistry (IHC) and Nissl Staining
4.4. Immunofluorescence (IF) Staining
4.5. Hydroethidine Histochemistry for Detecting O2− and O2− -Derived Oxidants
4.6. Image J Analysis
4.7. Statistical Anaylsis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Di Meo, S.; Reed, T.T.; Venditti, P.; Victor, V.M. Role of ROS and RNS sources in physiological and pathological conditions. Oxidative Med. Cell Longev. 2016, 2016, 1245049. [Google Scholar] [CrossRef]
- Kurutas, E.B. The importance of antioxidants which play the role in cellular response against oxidative/nitrosative stress: Current state. Nutr. J. 2016, 15, 71. [Google Scholar] [CrossRef] [Green Version]
- Liochev, S.I. Reactive oxygen species and the free radical theory of aging. Free Radic. Biol. Med. 2013, 60, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Cobb, C.A.; Cole, M.P. Oxidative and nitrative stress in neurodegeneration. Neurobiol. Dis. 2015, 84, 4–21. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chung, Y.C.; Ko, H.W.; Bok, E.; Park, E.S.; Huh, S.H.; Nam, J.H.; Jin, B.K. The role of neuroinflammation on the pathogenesis of Parkinson’s disease. BMB Rep. 2010, 43, 225–232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dasuri, K.; Zhang, L.; Keller, J.N. Oxidative stress, neurodegeneration, and the balance of protein degradation and protein synthesis. Free Radic. Biol. Med. 2013, 62, 170–185. [Google Scholar] [CrossRef]
- Butovsky, O.; Weiner, H.L. Microglial signatures and their role in health and disease. Nat. Rev. Neurosci. 2018, 19, 622–635. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Barres, B.A. Microglia and macrophages in brain homeostasis and disease. Nat. Rev. Immunol. 2018, 18, 225–242. [Google Scholar] [CrossRef]
- Yin, J.; Valin, K.L.; Dixon, M.L.; Leavenworth, J.W. The role of microglia and macrophages in CNS homeostasis, autoimmunity, and cancer. J. Immunol. Res. 2017, 2017, 5150678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Doens, D.; Fernandez, P.L. Microglia receptors and their implications in the response to amyloid beta for Alzheimer’s disease pathogenesis. J. Neuroinflamm. 2014, 11, 48. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Izquierdo, P.; Attwell, D.; Madry, C. Ion channels and receptors as determinants of microglial function. Trends Neurosci. 2019, 42, 278–292. [Google Scholar] [CrossRef] [PubMed]
- Galloway, D.A.; Phillips, A.E.M.; Owen, D.R.J.; Moore, C.S. Phagocytosis in the brain: Homeostasis and disease. Front. Immunol. 2019, 10, 790. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hickman, S.; Izzy, S.; Sen, P.; Morsett, L.; El Khoury, J. Microglia in neurodegeneration. Nat. Neurosci. 2018, 21, 1359–1369. [Google Scholar] [CrossRef]
- Wolf, S.A.; Boddeke, H.W.; Kettenmann, H. Microglia in physiology and disease. Annu. Rev. Physiol. 2017, 79, 619–643. [Google Scholar] [CrossRef]
- Simpson, D.S.A.; Oliver, P.L. ROS generation in microglia: Understanding oxidative stress and inflammation in neurodegenerative disease. Antioxidants 2020, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Jeong, J.Y.; Jin, B.K. Interleukin-4-mediated oxidative stress is harmful to hippocampal neurons of prothrombin kringle-2-lesioned rat in vivo. Antioxidants 2020, 9, 1068. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Chung, Y.C.; Jin, B.K. Interleukin-4 and interleukin-13 exacerbate neurotoxicity of prothrombin kringle-2 in cortex in vivo via oxidative stress. Int. J. Mol. Sci. 2019, 20, 1927. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.R.; Chung, E.S.; Bok, E.; Baik, H.H.; Chung, Y.C.; Won, S.Y.; Joe, E.; Kim, T.H.; Kim, S.S.; Jin, M.Y.; et al. Prothrombin kringle-2 induces death of mesencephalic dopaminergic neurons in vivo and in vitro via microglial activation. J. Neurosci. Res. 2010, 88, 1537–1548. [Google Scholar] [CrossRef] [PubMed]
- Nam, J.H.; Park, K.W.; Park, E.S.; Lee, Y.B.; Lee, H.G.; Baik, H.H.; Kim, Y.S.; Maeng, S.; Park, J.; Jin, B.K. Interleukin-13/-4-induced oxidative stress contributes to death of hippocampal neurons in abeta1-42-treated hippocampus in vivo. Antioxid. Redox Signal. 2012, 16, 1369–1383. [Google Scholar] [CrossRef]
- Won, S.Y.; Choi, S.H.; Jin, B.K. Prothrombin kringle-2-induced oxidative stress contributes to the death of cortical neurons in vivo and in vitro: Role of microglial NADPH oxidase. J. Neuroimmunol. 2009, 214, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Berzin, T.M.; Zipser, B.D.; Rafii, M.S.; Kuo-Leblanc, V.; Yancopoulos, G.D.; Glass, D.J.; Fallon, J.R.; Stopa, E.G. Agrin and microvascular damage in Alzheimer’s disease. Neurobiol. Aging 2000, 21, 349–355. [Google Scholar] [CrossRef]
- Shin, W.H.; Jeon, M.T.; Leem, E.; Won, S.Y.; Jeong, K.H.; Park, S.J.; McLean, C.; Lee, S.J.; Jin, B.K.; Jung, U.J.; et al. Induction of microglial toll-like receptor 4 by prothrombin kringle-2: A potential pathogenic mechanism in Parkinson’s disease. Sci. Rep. 2015, 5, 14764. [Google Scholar] [CrossRef] [Green Version]
- Junttila, I.S. Tuning the cytokine responses: An update on interleukin (IL)-4 and IL-13 receptor complexes. Front. Immunol. 2018, 9, 888. [Google Scholar] [CrossRef]
- McKenzie, A.N.J.; Spits, H.; Eberl, G. Innate lymphoid cells in inflammation and immunity. Immunity 2014, 41, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Mori, S.; Maher, P.; Conti, B. Neuroimmunology of the interleukins 13 and 4. Brain Sci. 2016, 6, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quarta, A.; Berneman, Z.; Ponsaerts, P. Neuroprotective modulation of microglia effector functions following priming with interleukin 4 and 13: Current limitations in understanding their mode-of-action. Brain Behav. Immun. 2020, 88, 856–866. [Google Scholar] [CrossRef]
- Won, S.Y.; Kim, S.R.; Maeng, S.; Jin, B.K. Interleukin-13/Interleukin-4-induced oxidative stress contributes to death of prothrombinkringle-2 (pKr-2)-activated microglia. J. Neuroimmunol. 2013, 265, 36–42. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.S.; Ji, K.A.; Jeon, S.B.; Jin, B.K.; Kim, S.U.; Jou, I.; Joe, E. Interleukin-13 enhances cyclooxygenase-2 expression in activated rat brain microglia: Implications for death of activated microglia. J. Immunol. 2006, 177, 1323–1329. [Google Scholar] [CrossRef] [Green Version]
- Park, K.W.; Baik, H.H.; Jin, B.K. IL-13-induced oxidative stress via microglial NADPH oxidase contributes to death of hippocampal neurons in vivo. J. Immunol. 2009, 183, 4666–4674. [Google Scholar] [CrossRef]
- Shin, W.H.; Lee, D.Y.; Park, K.W.; Kim, S.U.; Yang, M.S.; Joe, E.H.; Jin, B.K. Microglia expressing interleukin-13 undergo cell death and contribute to neuronal survival in vivo. Glia 2004, 46, 142–152. [Google Scholar] [CrossRef]
- Rossi, S.; Mancino, R.; Bergami, A.; Mori, F.; Castelli, M.; De Chiara, V.; Studer, V.; Mataluni, G.; Sancesario, G.; Parisi, V.; et al. Potential role of IL-13 in neuroprotection and cortical excitability regulation in multiple sclerosis. Mult. Scler. 2011, 17, 1301–1312. [Google Scholar] [CrossRef]
- Kawahara, K.; Suenobu, M.; Yoshida, A.; Koga, K.; Hyodo, A.; Ohtsuka, H.; Kuniyasu, A.; Tamamaki, N.; Sugimoto, Y.; Nakayama, H. Intracerebral microinjection of interleukin-4/interleukin-13 reduces beta-amyloid accumulation in the ipsilateral side and improves cognitive deficits in young amyloid precursor protein 23 mice. Neuroscience 2012, 207, 243–260. [Google Scholar] [CrossRef] [PubMed]
- Morrison, B.E.; Marcondes, M.C.; Nomura, D.K.; Sanchez-Alavez, M.; Sanchez-Gonzalez, A.; Saar, I.; Kim, K.S.; Bartfai, T.; Maher, P.; Sugama, S.; et al. Cutting edge: IL-13Ralpha1 expression in dopaminergic neurons contributes to their oxidative stress-mediated loss following chronic peripheral treatment with lipopolysaccharide. J. Immunol. 2012, 189, 5498–5502. [Google Scholar] [CrossRef]
- Brown, D.R. Role of microglia in age-related changes to the nervous system. Sci. World J. 2009, 9, 1061–1071. [Google Scholar] [CrossRef]
- Kim, S.U.; de Vellis, J. Microglia in health and disease. J. Neurosci. Res. 2005, 81, 302–313. [Google Scholar] [CrossRef]
- Kaur, C.; Rathnasamy, G.; Ling, E.A. Roles of activated microglia in hypoxia induced neuroinflammation in the developing brain and the retina. J. Neuroimmune Pharmacol. 2013, 8, 66–78. [Google Scholar] [CrossRef]
- Lee, P.; Lee, J.; Kim, S.; Lee, M.S.; Yagita, H.; Kim, S.Y.; Kim, H.; Suk, K. NO as an autocrine mediator in the apoptosis of activated microglial cells: Correlation between activation and apoptosis of microglial cells. Brain Res. 2001, 892, 380–385. [Google Scholar] [CrossRef]
- Lucas, S.M.; Rothwell, N.J.; Gibson, R.M. The role of inflammation in CNS injury and disease. Br. J. Pharmacol. 2006, 147 (Suppl. 1), S232–S240. [Google Scholar] [CrossRef] [Green Version]
- Choi, S.H.; Lee, D.Y.; Chung, E.S.; Hong, Y.B.; Kim, S.U.; Jin, B.K. Inhibition of thrombin-induced microglial activation and NADPH oxidase by minocycline protects dopaminergic neurons in the substantia nigra in vivo. J. Neurochem. 2005, 95, 1755–1765. [Google Scholar] [CrossRef] [PubMed]
- Ji, K.A.; Yang, M.S.; Jeong, H.K.; Min, K.J.; Kang, S.H.; Jou, I.; Joe, E.H. Resident microglia die and infiltrated neutrophils and monocytes become major inflammatory cells in lipopolysaccharide-injected brain. Glia 2007, 55, 1577–1588. [Google Scholar] [CrossRef]
- Green, P.S.; Mendez, A.J.; Jacob, J.S.; Crowley, J.R.; Growdon, W.; Hyman, B.T.; Heinecke, J.W. Neuronal expression of myeloperoxidase is increased in Alzheimer’s disease. J. Neurochem. 2004, 90, 724–733. [Google Scholar] [CrossRef] [PubMed]
- Huh, S.H.; Chung, Y.C.; Piao, Y.; Jin, M.Y.; Son, H.J.; Yoon, N.S.; Hong, J.Y.; Pak, Y.K.; Kim, Y.S.; Hong, J.K.; et al. Ethyl pyruvate rescues nigrostriatal dopaminergic neurons by regulating glial activation in a mouse model of Parkinson’s disease. J. Immunol. 2011, 187, 960–969. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.; Liang, Y.; Huang, Z.; Jones, D.W.; Pritchard, K.A., Jr.; Zhang, H. Erratum to: Inhibition of myeloperoxidase oxidant production by N-acetyl lysyltyrosylcysteine amide reduces brain damage in a murine model of stroke. J. Neuroinflamm. 2016, 13, 166. [Google Scholar] [CrossRef] [Green Version]
- Pattison, D.I.; Hawkins, C.L.; Davies, M.J. Hypochlorous acid-mediated oxidation of lipid components and antioxidants present in low-density lipoproteins: Absolute rate constants, product analysis, and computational modeling. Chem. Res. Toxicol. 2003, 16, 439–449. [Google Scholar] [CrossRef]
- Yap, Y.W.; Whiteman, M.; Cheung, N.S. Chlorinative stress: An under appreciated mediator of neurodegeneration? Cell. Signal. 2007, 19, 219–228. [Google Scholar] [CrossRef]
- Arnhold, J.; Flemmig, J. Human myeloperoxidase in innate and acquired immunity. Arch. Biochem. Biophys. 2010, 500, 92–106. [Google Scholar] [CrossRef]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Inside the neutrophil phagosome: Oxidants, myeloperoxidase, and bacterial killing. Blood 1998, 92, 3007–3017. [Google Scholar] [CrossRef] [PubMed]
- Jana, M.; Palencia, C.A.; Pahan, K. Fibrillar amyloid-beta peptides activate microglia via TLR2: Implications for Alzheimer’s disease. J. Immunol. 2008, 181, 7254–7262. [Google Scholar] [CrossRef]
- Jang, J.H.; Lee, S.H.; Jung, K.; Yoo, H.; Park, G. Inhibitory effects of myricetin on lipopolysaccharide-induced neuroinflammation. Brain Sci. 2020, 10, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marik, C.; Felts, P.A.; Bauer, J.; Lassmann, H.; Smith, K.J. Lesion genesis in a subset of patients with multiple sclerosis: A role for innate immunity? Brain 2007, 130 Pt 11, 2800–2815. [Google Scholar] [CrossRef] [Green Version]
- Tieu, K.; Ischiropoulos, H.; Przedborski, S. Nitric oxide and reactive oxygen species in Parkinson’s disease. IUBMB Life 2003, 55, 329–335. [Google Scholar] [CrossRef] [PubMed]
- Chung, Y.C.; Baek, J.Y.; Kim, S.R.; Ko, H.W.; Bok, E.; Shin, W.H.; Won, S.Y.; Jin, B.K. Capsaicin prevents degeneration of dopamine neurons by inhibiting glial activation and oxidative stress in the MPTP model of Parkinson’s disease. Exp. Mol. Med. 2017, 49, e298. [Google Scholar] [CrossRef] [PubMed]
- Pravalika, K.; Sarmah, D.; Kaur, H.; Wanve, M.; Saraf, J.; Kalia, K.; Borah, A.; Yavagal, D.R.; Dave, K.R.; Bhattacharya, P. Myeloperoxidase and neurological disorder: A crosstalk. ACS Chem. Neurosci. 2018, 9, 421–430. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Joseph, J.; Fales, H.M.; Sokoloski, E.A.; Levine, R.L.; Vasquez-Vivar, J.; Kalyanaraman, B. Detection and characterization of the product of hydroethidine and intracellular superoxide by HPLC and limitations of fluorescence. Proc. Natl. Acad. Sci. USA 2005, 102, 5727–5732. [Google Scholar] [CrossRef] [PubMed] [Green Version]



| Category | Antibody | Company | Cat. No. | Dilution Factor | Detection Target |
|---|---|---|---|---|---|
| Primary Antibody | NeuN | Merck | MAB377 | 1:1000 | Neurons |
| OX-42 | Bio-rad | MCA275G | 1:400 | Microglia/ Macrophages/ Neutrophils | |
| OX-6 | BD Bioscience | 554926 | 1:400 | Activated microglia | |
| 8-OHdG | Jaica | MOG-100P | 1:300 | Oxidative DNA damage | |
| Secondary Antibody | Biotin-conjugated anti-mouse IgG | Seracare | 5260-0051 | 1:400 | Mouse IgG |
| Category | Antibody | Company | Cat. No. | Dilution Factor | Detection Target |
|---|---|---|---|---|---|
| Primary Antibody | IL-13 | R&D systems | AF1945 | 1:200 | IL-13 |
| FITC-TL | Vector Laboratories | FL-1171 | 1:1000 | Microglia/ Macrophage/ Neutrophils | |
| NeuN | Merck | MAB377 | 1:1000 | Neurons | |
| GFAP | Sigma-Aldrich | G3893 | 1:500 | Astrocytes | |
| OX-42 | Bio-rad | MCA275G | 1:400 | Microglia/ Macrophage/ Neutrophils | |
| iNOS | BD Biosciences | 610333 | 1:200 | iNOS | |
| MPO | Dakocytomation | A0398 | 1:500 | MPO | |
| Nitrotyrosine | Abcam | Ab7048 | 1:50 | Oxidative protein damage | |
| Secondary Antibody | FITC-conjugated anti-mouse IgG | Sigma-Aldrich | AP124F | 1:500 | Mouse IgG |
| Fluorescein conjugated anti-mouse IgG | Vector Laboratories | FL-2000 | 1:300 | Mouse IgG | |
| Cy3-conjugated anti-rabbit IgG | Sigma-Aldrich | AP132C | 1:1000 | Rabbit IgG | |
| Alexa Fluor 594-conjugated anti-goat IgG | Invitrogen | A11058 | 1:1000 | Goat IgG |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.Y.; Wi, R.; Chung, Y.C.; Jin, B.K. Interleukin-13 Propagates Prothrombin Kringle-2-Induced Neurotoxicity in Hippocampi In Vivo via Oxidative Stress. Int. J. Mol. Sci. 2021, 22, 3486. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22073486
Jeong JY, Wi R, Chung YC, Jin BK. Interleukin-13 Propagates Prothrombin Kringle-2-Induced Neurotoxicity in Hippocampi In Vivo via Oxidative Stress. International Journal of Molecular Sciences. 2021; 22(7):3486. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22073486
Chicago/Turabian StyleJeong, Jae Yeong, Rayul Wi, Young Cheul Chung, and Byung Kwan Jin. 2021. "Interleukin-13 Propagates Prothrombin Kringle-2-Induced Neurotoxicity in Hippocampi In Vivo via Oxidative Stress" International Journal of Molecular Sciences 22, no. 7: 3486. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22073486





