Synthesis of New Biscoumarin Derivatives, In Vitro Cholinesterase Inhibition, Molecular Modelling and Antiproliferative Effect in A549 Human Lung Carcinoma Cells
Abstract
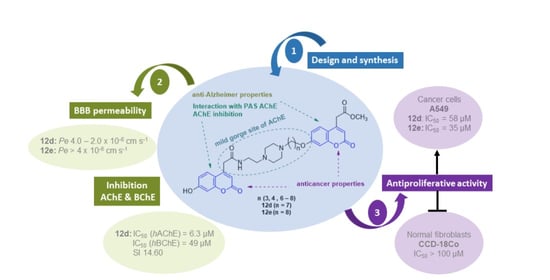
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Profil of Biscoumarine Derivatives as Potential Drugs for Treatment AD
2.2.1. Evaluation of hAChE and hBChE Inhibitory Activity
2.2.2. Molecular Modelling Studies
2.2.3. In Vitro BBB Permeation Assays
2.3. In Vitro Antiproliferative Activity and Intracellular Localization of Analyzed Compounds
2.3.1. Determination of Metabolic Activity and IC50 Values
2.3.2. Quantification of Cell Number and Viability
2.3.3. Cell Cycle Analysis
2.3.4. Evaluation of Clonogenic Survival
2.3.5. Intracellular Localization and Mitotic Activity of A549 Cells
3. Materials and Methods
3.1. Experimental Part
3.2. Synthesis of Biscoumarin Derivatives 12a–e
3.3. Spectroscopic Data
3.4. Tests of Anti-AD Action of Biscoumarine Derivatives
3.4.1. In Vitro Anti-Cholinesterase Assay
3.4.2. Molecular Modelling Studies
3.4.3. Determination of In Vitro BBB Permeation
3.5. In Vitro Antiproliferative Activity and Intracellular Localization
3.5.1. Cell Culture and Treatment
3.5.2. MTT Assay
3.5.3. Quantification of Cell Number and Viability
3.5.4. Colony-Forming Assay
3.5.5. Cell Cycle Analysis
3.5.6. Immunofluorescence Labelling
3.5.7. Confocal Microscopy
3.5.8. Image Analyses
3.5.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Peng, X.-M.; Damu, G.L.V.; Zhou, C.-H. Current developments of coumarin compounds in medicinal chemistry. Curr. Pharm. Des. 2013, 19, 3884–3930. [Google Scholar] [CrossRef]
- Gómez-Outes, A.; Suárez-Gea, M.L.; Calvo-Rojas, G.; Lecumberri, R.; Rocha, E.; Pozo-Hernández, C.; Terleira-Fernández, A.I.; Vargas-Castrillón, E. Discovery of anticoagulant drugs: A historical perspective. Curr. Drug Discov. Technol. 2012, 9, 83–104. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Singh, B.; Singh, N. A Review on coumarins as acetylcholinesterase inhibitors for Alzheimer’s disease. Bioorg. Med. Chem. 2012, 20, 1175–1180. [Google Scholar] [CrossRef] [PubMed]
- Kostova, I.; Bhatia, S.; Grigorov, P.; Balkansky, S.; Parmar, V.S.; Prasad, A.K.; Saso, L. Coumarins as antioxidants. Curr. Med. Chem. 2011, 18, 3929–3951. [Google Scholar] [CrossRef] [PubMed]
- Riveiro, M.E.; De Kimpe, N.; Moglioni, A.; Vázquez, R.; Monczor, F.; Shayo, C.; Davio, C. Coumarins: Old compounds with novel promising therapeutic perspectives. Curr. Med. Chem. 2010, 17, 1325–1338. [Google Scholar] [CrossRef]
- Wu, L.; Wang, X.; Xu, W.; Farzaneh, F.; Xu, R. The structure and pharmacological functions of coumarins and their derivatives. Curr. Med. Chem. 2009, 16, 4236–4260. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Nano, G.M.; Palmisano, S.G.; Tagliapietra, S. The Chemistry of Coumarin Derivatives, Part XIII. The Reactivity of 4-Hydroxycoumarin under Heterogenous High Intensity Sonochemical Conditions. Synthesis 2003, 34, 1286–1291. [Google Scholar]
- Shabbir, M.; Sultani, S.Z.; Jabbar, A.; Choudhary, M.I. Cinnamates and coumarins from the leaves of Murraya paniculata. Phytochemistry 1997, 44, 683–685. [Google Scholar]
- Al-Amiery, A.A.; Al-Bayati, R.I.H.; Saour, K.Y.; Radi, M.F. Cytotoxicity, antioxidant, and antimicrobial activities of novel 2-quinolone derivatives derived from coumarin. Res. Chem. Intermed. 2012, 38, 559–569. [Google Scholar] [CrossRef]
- Negi, N.; Ochi, A.; Kurosawa, M.; Ushijima, K.; Kitaguchi, Y.; Kusakabe, E.; Okasho, F.; Kimachi, T.; Teshima, N.; Ju-Ichi, M.; et al. Two new dimeric coumarins isolated from Murraya exotica. Chem. Pharm. Bull. 2005, 53, 1180–1182. [Google Scholar] [CrossRef] [Green Version]
- Tan, G.; Yao, Y.; Gu, Y.; Li, S.; Lv, M.; Wang, K.; Chen, H.; Li, X. Cytotoxicity and DNA binding property of the dimers of triphenylethylene- coumarin hybrid with one amino side chain. Bioorganic Med. Chem. Lett. 2014, 24, 2825–2830. [Google Scholar] [CrossRef]
- Kurt, B.Z.; Dag, A.; Doğan, B.; Durdagi, S.; Angeli, A.; Nocentini, A.; Supuran, C.T.; Sonmez, F. Synthesis, biological activity and multiscale molecular modeling studies of bis-coumarins as selective carbonic anhydrase IX and XII inhibitors with effective cytotoxicity against hepatocellular carcinoma. Bioorg. Chem. 2019, 87, 838–850. [Google Scholar] [CrossRef]
- Umar, M.I.; Saeed, A.; Ejaz, S.A.; Sévigny, J.; Iqbal, J.; Ibrar, A.; Lecka, J. Expanding the alkaline phosphatase inhibition, cytotoxic and proapoptotic profile of biscoumarin-iminothiazole and coumarin-triazolothiadiazine conjugates. ChemistrySelect 2018, 3, 13377–13386. [Google Scholar]
- Morsy, S.A.; Farahat, A.A.; Nasr, M.N.A.; Tantawy, A.S. Synthesis, Molecular modeling and anticancer activity of new coumarin containing compounds. Saudi Pharm. J. 2017, 25, 873–883. [Google Scholar] [CrossRef]
- Xu, J.; Ai, J.; Liu, S.; Peng, X.; Yu, L.; Geng, M.; Nan, F. Design and synthesis of 3,3′-biscoumarin-based c-Met inhibitors. Org. Biomol. Chem. 2014, 12, 3721–3734. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xie, S.S.; Wang, X.; Jiang, N.; Yu, W.; Wang, K.D.G.; Lan, J.S.; Li, Z.R.; Kong, L.Y. Multi-target tacrine-coumarin hybrids: Cholinesterase and monoamine oxidase b inhibition properties against Alzheimer’s disease. Eur. J. Med. Chem. 2015, 95, 153–165. [Google Scholar] [CrossRef]
- Joubert, J.; Foka, G.B.; Repsold, B.P.; Oliver, D.W.; Kapp, E.; Malan, S.F. Synthesis and evaluation of 7-substituted coumarin derivatives as multimodal monoamine oxidase-B and cholinesterase inhibitors for the treatment of Alzheimer’s disease. Eur. J. Med. Chem. 2017, 125, 853–864. [Google Scholar] [CrossRef] [PubMed]
- Hilgert, M.; Nöldner, M.; Chatterjee, S.S.; Klein, J. KA-672 Inhibits rat brain acetylcholinesterase in vitro but not in vivo. Neurosci. Lett. 1999, 263, 193–196. [Google Scholar] [CrossRef]
- Borumandnia, N. Worldwide Patterns in Alzheimer’s Disease and Other Dementias Prevalence from 1990 to 2017: A Growth Mixture Models Approach Analysis of Trends. Res. Sq. 2020. [Google Scholar] [CrossRef]
- Guo, J.; Cheng, J.; North, B.J.; Wei, W. Functional analyses of major cancer-related signaling pathways in Alzheimer’s disease etiology. Biochim. Biophys. Acta Rev. Cancer 2017, 1868, 341–358. [Google Scholar] [CrossRef]
- Majd, S.; Power, J.; Majd, Z. Alzheimer’s disease and cancer: When two monsters cannot be together. Front. Neurosci. 2019, 13, 155. [Google Scholar] [CrossRef] [Green Version]
- Monacelli, F.; Cea, M.; Borghi, R.; Odetti, P.; Nencioni, A. Do cancer drugs counteract neurodegeneration? Repurposing for Alzheimer’s disease. J. Alzheimer’s Dis. 2017, 55, 1295–1306. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Adem, A.; Sabbagh, M. New acetylcholinesterase inhibitors for alzheimer’s disease. Int. J. Alzheimer’s Dis. 2012, 2012, 8. [Google Scholar] [CrossRef]
- Piazzi, L.; Cavalli, A.; Colizzi, F.; Belluti, F.; Bartolini, M.; Mancini, F.; Recanatini, M.; Andrisano, V.; Rampa, A. Multi-target-directed coumarin derivatives: hAChE and BACE1 inhibitors as potential anti-alzheimer compounds. Bioorg. Med. Chem. Lett. 2008, 18, 423–426. [Google Scholar] [CrossRef] [PubMed]
- Stefanachi, A.; Leonetti, F.; Pisani, L.; Catto, M.; Carotti, A. Coumarin: A natural, privileged and versatile scaffold for bioactive compounds. Molecules 2018, 23, 250. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kancheva, V.D.; Boranova, P.V.; Nechev, J.T.; Manolov, I.I. Structure-activity relationships of new 4-hydroxy bis-coumarins as radical scavengers and chain-breaking antioxidants. Biochimie 2010, 92, 1138–1146. [Google Scholar] [CrossRef]
- Chimenti, F.; Secci, D.; Bolasco, A.; Chimenti, P.; Granese, A.; Carradori, S.; Befani, O.; Turini, P.; Alcaro, S.; Ortuso, F. Synthesis, Molecular modeling studies, and selective inhibitory activity against monoamine oxidase of N,N’-Bis[2 -oxo-2H-benzopyran]-3-carboxamides. Bioorg. Med. Chem. Lett. 2006, 16, 4135–4140. [Google Scholar] [CrossRef]
- Barta, J.A.; Powell, C.A.; Wisnivesky, J.P. Global epidemiology of lung cancer. Ann. Glob. Health 2019, 85, 8. [Google Scholar] [CrossRef] [Green Version]
- Xi, H.J.; Wu, R.P.; Liu, J.J.; Zhang, L.J.; Li, Z.S. Role of acetylcholinesterase in lung cancer. Thorac. Cancer 2015, 6, 390–398. [Google Scholar] [CrossRef]
- Friedman, J.R.; Richbart, S.D.; Merritt, J.C.; Brown, K.C.; Nolan, N.A.; Akers, A.T.; Lau, J.K.; Robateau, Z.R.; Miles, S.L.; Dasgupta, P. Acetylcholine Signaling System in Progression of Lung Cancers. Pharmacol. Ther. 2019, 194, 222–254. [Google Scholar] [CrossRef]
- Vidal, C.J. Expression of cholinesterases in brain and non-brain tumours. Chem. Biol. Interact. 2005, 157–158, 227–232. [Google Scholar] [CrossRef]
- Ballard, C.G.; Greig, N.H.; Guillozet-Bongaarts, A.L.; Enz, A.; Darvesh, S. Cholinesterases: Roles in the brain during health and disease. Curr. Alzheimer Res. 2005, 2, 307–318. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.; Moore, S.W. The Adhesion Function on Acetylcholinesterase Is Located at the Peripheral Anionic Site. Biochem. Biophys. Res. Commun. 1999, 258, 758–762. [Google Scholar] [CrossRef]
- Pérez-Aguilar, B.; Vidal, C.J.; Palomec, G.; García-Dolores, F.; Gutiérrez-Ruiz, M.C.; Bucio, L.; Gómez-Olivares, J.L.; Gómez-Quiroz, L.E. Acetylcholinesterase is associated with a decrease in cell proliferation of hepatocellular carcinoma cells. Biochim. Biophys. Acta Mol. Basis Dis. 2015, 1852, 1380–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Price, D.; Muterspaugh, R.; Clegg, B.; Williams, A.; Stephens, A.; Guthrie, J.; Heyl, D.; Evans, H.G. IGFBP-3 blocks hyaluronan-CD44 signaling, leading to increased acetylcholinesterase levels in A549 cell media and apoptosis in a p53-dependent manner. Sci. Rep. 2020, 10, 5083. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Campoy, F.J.; Vidal, C.J.; Muñoz-Delgado, E.; Montenegro, M.F.; Cabezas-Herrera, J.; Nieto-Cerón, S. Cholinergic system and cell proliferation. Chem. Biol. Interact. 2016, 259, 257–265. [Google Scholar] [CrossRef]
- Zhang, X.J.; Greenberg, D.S. Acetylcholinesterase involvement in apoptosis. Front. Mol. Neurosci. 2012, 5, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Zhang, X.; Zhang, B.; Wu, J.; Zhang, X. Synaptic acetylcholinesterase targeted by MicroRNA-212 functions as a tumor suppressor in non-small cell lung cancer. Int. J. Biochem. Cell Biol. 2013, 45, 2530–2540. [Google Scholar] [CrossRef]
- Calaf, G.M.; Parra, E.; Garrido, F. Cell proliferation and tumor formation induced by Eserine, an acetylcholinesterase inhibitor, in rat mammary gland. Oncol. Rep. 2007, 17, 25–33. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.-M.; Zhang, Z.-J.; Liu, A.-L.; LV, F.-J.; LI, S.-F. Cholinesterase and human lung cancer cells (A-549) inhibitory activity of the Cassava Peel of Euphorbiaceae in vitro. In Proceedings of the DEStech Transactions on Environment, Energy and Earth Sciences; Joint International Conference on Social Science and Environmental Science (SSES 2016) and International Conference on Food Science and Engineering (ICFSE 2016), Guangzhou, China, 15–16 October 2016; pp. 389–394. [Google Scholar]
- Nguyen, T.-H.-T.; Pham, H.-V.-T.; Pham, N.-K.-T.; Quach, N.-D.-P.; Pudhom, K.; Hansen, P.E.; Nguyen, K.-P.-P. Chemical constituents from Sonneratia ovata backer and their in vitro cytotoxicity and acetylcholinesterase inhibitory activities. Bioorg. Med. Chem. Lett. 2015, 25, 2366–2371. [Google Scholar] [CrossRef]
- Zovko, A.; Sepcic, K.; Turk, T.; Faimali, M.; Garaventa, F.; Chelossi, E.; Paleari, L.; Falugi, C.; Aluigi, M.; Angelini, C.; et al. New aspects of the relationship between acetylcholinesterase activity and cancer I: Poly-Aps experiments. WSEAS Trans. Biol. Biomed. 2009, 6, 58–69. [Google Scholar]
- Lee, J.; Sohn, E.J.; Yoon, S.W.; Kim, C.G.; Lee, S.; Kim, J.Y.; Baek, N.; Kim, S.-H. Anti-metastatic effect of dehydrocorydaline on H1299 non-small cell lung carcinoma cells via inhibition of matrix metalloproteinases and B cell lymphoma 2. Phyther. Res. 2017, 31, 441–448. [Google Scholar] [CrossRef] [PubMed]
- Deng, R.; Li, W.; Guan, Z.; Zhou, J.-M.; Wang, Y.; Mei, Y.-P.; Li, M.-T.; Feng, G.-K.; Huang, W.; Liu, Z.-C.; et al. Acetylcholinesterase expression mediated by c-Jun-NH2-terminal kinase pathway during anticancer drug-induced apoptosis. Oncogene 2006, 25, 7070–7077. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Syed, M.; Fenoglio-Preiser, C.; Skau, K.A.; Weber, G.F. Acetylcholinesterase supports anchorage independence in colon cancer. Clin. Exp. Metastasis 2008, 25, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Arafath, M.A.; Adam, F.; Al-Suede, F.S.R.; Razali, M.R.; Ahamed, M.B.K.; Abdul Majid, A.M.S.; Hassan, M.Z.; Osman, H.; Abubakar, S. Synthesis, Characterization, X-ray crystal structures of heterocyclic schiff base compounds and in vitro cholinesterase inhibition and anticancer activity. J. Mol. Struct. 2017, 1149, 216–228. [Google Scholar] [CrossRef]
- Ozmen Ozgun, D.; Gul, H.I.; Yamali, C.; Sakagami, H.; Gulcin, I.; Sukuroglu, M.; Supuran, C.T. Synthesis and bioactivities of pyrazoline benzensulfonamides as carbonic anhydrase and acetylcholinesterase inhibitors with low cytotoxicity. Bioorg. Chem. 2019, 84, 511–517. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, S.; Zhang, S.; Pei, J.; Li, Y.; Zhang, Y.; He, X.; Hu, L. Development of a multivalent acetylcholinesterase inhibitor via dynamic combinatorial chemistry. Int. J. Biol. Macromol. 2020, 150, 1184–1191. [Google Scholar] [CrossRef]
- Laskowski, S.C.; Clinton, R.O. Coumarins. II. Derivatives of coumarin-3- and -4-acetic acids. J. Am. Chem. Soc. 1950, 72, 3987–3991. [Google Scholar] [CrossRef]
- Di, L.; Kerns, E.H.; Fan, K.; McConnell, O.J.; Carter, G.T. High throughput artificial membrane permeability assay for blood-brain barrier. Eur. J. Med. Chem. 2003, 38, 223–232. [Google Scholar] [CrossRef]
- Carpenter, T.S.; Kirshner, D.A.; Lau, E.Y.; Wong, S.E.; Nilmeier, J.P.; Lightstone, F.C. A Method to predict blood-brain barrier permeability of drug-like compounds using molecular dynamics simulations. Biophys. J. 2014, 107, 630–641. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, J.; Rudolph, M.J.; Burshteyn, F.; Cassidy, M.S.; Gary, E.N.; Love, J.; Franklin, M.C.; Height, J.J. Structures of human acetylcholinesterase in complex with pharmacologically important ligands. J. Med. Chem. 2012, 55, 10282–10286. [Google Scholar] [CrossRef]
- Hoerr, R.; Noeldner, M. Ensaculin (KA-672 HCl): A multitransmitter approach to dementia treatment. CNS Drug Rev. 2002, 8, 143–158. [Google Scholar] [CrossRef] [PubMed]
- Lacy, A.; O’Kennedy, R. Studies on coumarins and coumarin-related compounds to determine their therapeutic role in the treatment of cancer. Curr. Pharm. Des. 2005, 10, 3797–3811. [Google Scholar] [CrossRef] [Green Version]
- Huang, R.-Z.; Hua, S.-X.; Wang, C.-Y.; Pan, Y.-M.; Qin, J.-M.; Ding, Z.-Y.; Zhang, Y.; Wang, H.-S. 4-Methylumbelliferones analogues as anticancer agents: Synthesis and in cell pharmacological studies. Anticancer. Agents Med. Chem. 2017, 17, 576–589. [Google Scholar] [CrossRef]
- Leevy, W.M.; Weber, M.E.; Gokel, M.R.; Hughes-Strange, G.B.; Daranciang, D.D.; Ferdani, R.; Gokel, G.W. Correlation of bilayer membrane cation transport and biological activity in alkyl-substituted lariat ethers. Org. Biomol. Chem. 2005, 3, 1647–1652. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Supek, F.; Ramljak, T.Š.; Marjanović, M.; Buljubašić, M.; Kragol, G.; Ilić, N.; Šmuc, T.; Zahradka, D.; Mlinarić-Majerski, K.; Kralj, M. Could LogP be a principal determinant of biological activity in 18-crown-6 ethers? Synthesis of biologically active adamantane-substituted diaza-crowns. Eur. J. Med. Chem. 2011, 46, 3444–3454. [Google Scholar] [CrossRef] [PubMed]
- Bortner, C.D.; Cidlowski, J.A. Cell shrinkage and monovalent cation fluxes: Role in apoptosis. Arch. Biochem. Biophys. 2007, 462, 176–188. [Google Scholar] [CrossRef] [Green Version]
- Bisi, A.; Cappadone, C.; Rampa, A.; Farruggia, G.; Sargenti, A.; Belluti, F.; Di Martino, R.M.C.; Malucelli, E.; Meluzzi, A.; Iotti, S.; et al. Coumarin derivatives as potential antitumor agents: Growth inhibition, apoptosis induction and multidrug resistance reverting activity. Eur. J. Med. Chem. 2017, 127, 577–585. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Mizumoto, K.; Sato, N.; Ogawa, T.; Kusumoto, M.; Niiyama, H.; Tanaka, M. Quantitative determination of apoptotic death in cultured human pancreatic cancer cells by propidium iodide and digitonin. Cancer Lett. 1999, 142, 129–137. [Google Scholar] [CrossRef]
- Firmino, S.S.; André, S.C.; Hastenreiter, Z.; Campos, V.K. In vitro assessment of the cytotoxicity of Gallium (III) complexes with Isoniazid-Derived Hydrazones: Effects on clonogenic survival of HCT-116 cells. Inorganica Chim. Acta 2019, 497, 119079. [Google Scholar] [CrossRef]
- Pettersen, E.F.; Goddard, T.D.; Huang, C.C.; Couch, G.S.; Greenblatt, D.M.; Meng, E.C.; Ferrin, T.E. UCSF Chimera—A Visualization system for exploratory research and analysis. J. Comput. Chem. 2004, 25, 1605–1612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morris, G.M.; Huey, R.; Lindstrom, W.; Sanner, M.F.; Belew, R.K.; Goodsell, D.S.; Olson, A.J. AutoDock4 and AutoDockTools4: Automated docking with selective receptor flexibility. J. Comput. Chem. 2009, 30, 2785–2791. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panek, D.; Więckowska, A.; Wichur, T.; Bajda, M.; Godyń, J.; Jończyk, J.; Mika, K.; Janockova, J.; Soukup, O.; Knez, D.; et al. Design, synthesis and biological evaluation of new phthalimide and saccharin derivatives with alicyclic amines targeting cholinesterases, beta-secretase and amyloid beta aggregation. Eur. J. Med. Chem. 2017, 125, 676–695. [Google Scholar] [CrossRef] [PubMed]
- Hepnarova, V.; Korabecny, J.; Matouskova, L.; Jost, P.; Muckova, L.; Hrabinova, M.; Vykoukalova, N.; Kerhartova, M.; Kucera, T.; Dolezal, R.; et al. The concept of hybrid molecules of tacrine and benzyl quinolone carboxylic acid (BQCA) as multifunctional agents for Alzheimer’s disease. Eur. J. Med. Chem. 2018, 150, 292–306. [Google Scholar] [CrossRef] [PubMed]
- Svobodova, B.; Mezeiova, E.; Hepnarova, V.; Hrabinova, M.; Muckova, L.; Kobrlova, T.; Jun, D.; Soukup, O.; Jimeno, M.L.; Marco-Contelles, J.; et al. Exploring structure-activity relationship in tacrine-squaramide derivatives as potent cholinesterase inhibitors. Biomolecules 2019, 9, 379. [Google Scholar] [CrossRef] [Green Version]
- O’Boyle, N.M.; Banck, M.; James, C.A.; Morley, C.; Vandermeersch, T.; Hutchison, G.R. Open Babel: An open chemical toolbox. J. Cheminform. 2011, 3, 33. [Google Scholar] [CrossRef] [Green Version]
- Trott, O.; Olson, A.J. AutoDock Vina: Improving the speed and accuracy of docking with a new scoring function, efficient optimization, and multithreading. J. Comput. Chem. 2010, 31, 455–461. [Google Scholar] [CrossRef] [Green Version]







| Compound No. | n | IC50 ± S.E.M. a (µM) hAChE | IC50 ± S.E.M. a (µM) hBChE | Selectivity for hAChE b | Pe ± S.E.M. (10−6 cm s−1) c | CNS Predicted Availability d |
|---|---|---|---|---|---|---|
| 12a | 3 | >500 | >500 | - | 1.53 ± 0.64 | CNS− |
| 12b | 4 | >500 | >500 | - | 0.3 4± 0.10 | CNS− |
| 12c | 6 | 88.4 ± 12.6 | >500 | - | 2.96 ± 0.73 | CNS+/− |
| 12d | 7 | 6.30 ± 0.40 | 49 | 14.60 | 2.11 ± 1.19 | CNS+/− |
| 12e | 8 | >500 | >500 | - | 13.4 ± 3.56 | CNS+ |
| 7-MEOTA | 15 ± 2.9 | 21 ± 3.4 | 1.4 | - | - | |
| Tacrine | 0.500 ± 0.100 | 0.023 ± 0.004 | 0.046 | 6.0 ± 0.6 | CNS+ | |
| Donepezil | - | - | - | 21.9 ± 2.1 | CNS+ | |
| Rivastigmine | - | - | - | 20.0 ± 2.1 | CNS+ | |
| Ibuprofen | - | - | - | 18.0 ± 4.3 | CNS+ | |
| Chlorothiazide | - | - | - | 1.1 ± 0.5 | CNS− | |
| Furosemide | - | - | - | 0.2 ± 0.07 | CNS− | |
| Ranitidine | - | - | - | 0.04 ± 0.02 | CNS− | |
| Sulfasalazine | - | - | - | 0.09 ± 0.05 | CNS− |
| Compound No. | n | IC50 (µM) a | LogP b | LogD c | |||
|---|---|---|---|---|---|---|---|
| A549 | CCD-18Co | ||||||
| 24 h | 48 h | 24 h | 48 h | ||||
| 12a | 3 | >100 | >100 | - | - | 1.27 | 0.67 |
| 12b | 4 | >100 | >100 | - | - | 1.79 | 1.01 |
| 12c | 6 | >100 | >100 | - | - | 2.68 | 1.79 |
| 12d | 7 | 94 | 58 | >100 | >100 | 3.12 | 2.24 |
| 12e | 8 | 49 | 35 | >100 | >100 | 3.57 | 2.68 |
| DMSO (1%) | - | - | 96% | 98% | - | - | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hudáčová, M.; Hamuľaková, S.; Konkoľová, E.; Jendželovský, R.; Vargová, J.; Ševc, J.; Fedoročko, P.; Soukup, O.; Janočková, J.; Ihnatova, V.; et al. Synthesis of New Biscoumarin Derivatives, In Vitro Cholinesterase Inhibition, Molecular Modelling and Antiproliferative Effect in A549 Human Lung Carcinoma Cells. Int. J. Mol. Sci. 2021, 22, 3830. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22083830
Hudáčová M, Hamuľaková S, Konkoľová E, Jendželovský R, Vargová J, Ševc J, Fedoročko P, Soukup O, Janočková J, Ihnatova V, et al. Synthesis of New Biscoumarin Derivatives, In Vitro Cholinesterase Inhibition, Molecular Modelling and Antiproliferative Effect in A549 Human Lung Carcinoma Cells. International Journal of Molecular Sciences. 2021; 22(8):3830. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22083830
Chicago/Turabian StyleHudáčová, Monika, Slávka Hamuľaková, Eva Konkoľová, Rastislav Jendželovský, Jana Vargová, Juraj Ševc, Peter Fedoročko, Ondrej Soukup, Jana Janočková, Veronika Ihnatova, and et al. 2021. "Synthesis of New Biscoumarin Derivatives, In Vitro Cholinesterase Inhibition, Molecular Modelling and Antiproliferative Effect in A549 Human Lung Carcinoma Cells" International Journal of Molecular Sciences 22, no. 8: 3830. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22083830