Degraded Arabinogalactans and Their Binding Properties to Cancer-Associated Human Galectins
Abstract
:1. Introduction
2. Results
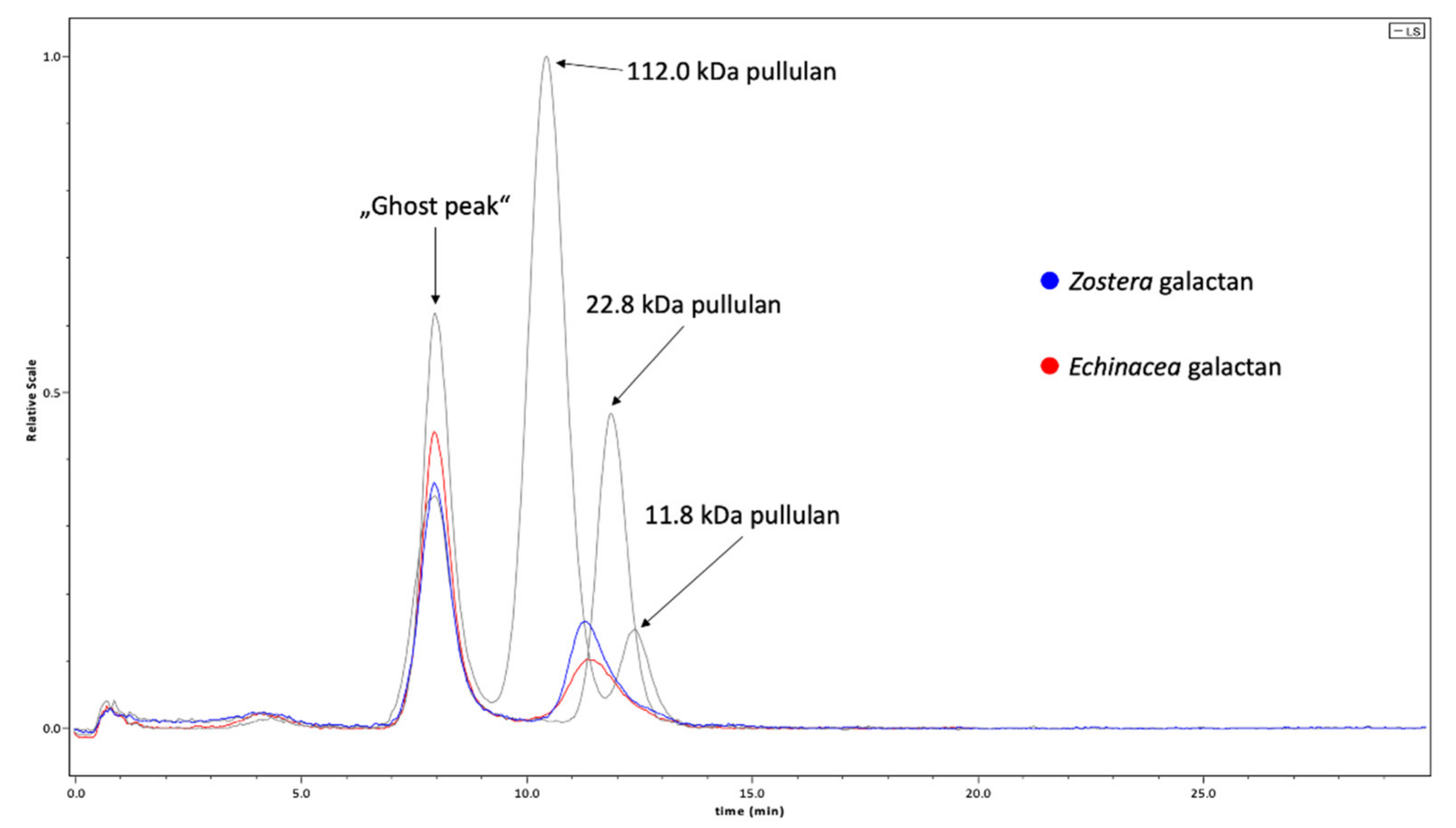
2.1. Chemical Characterization of Galactans
2.2. Cell-Free Production of Gal-1 and Gal-7
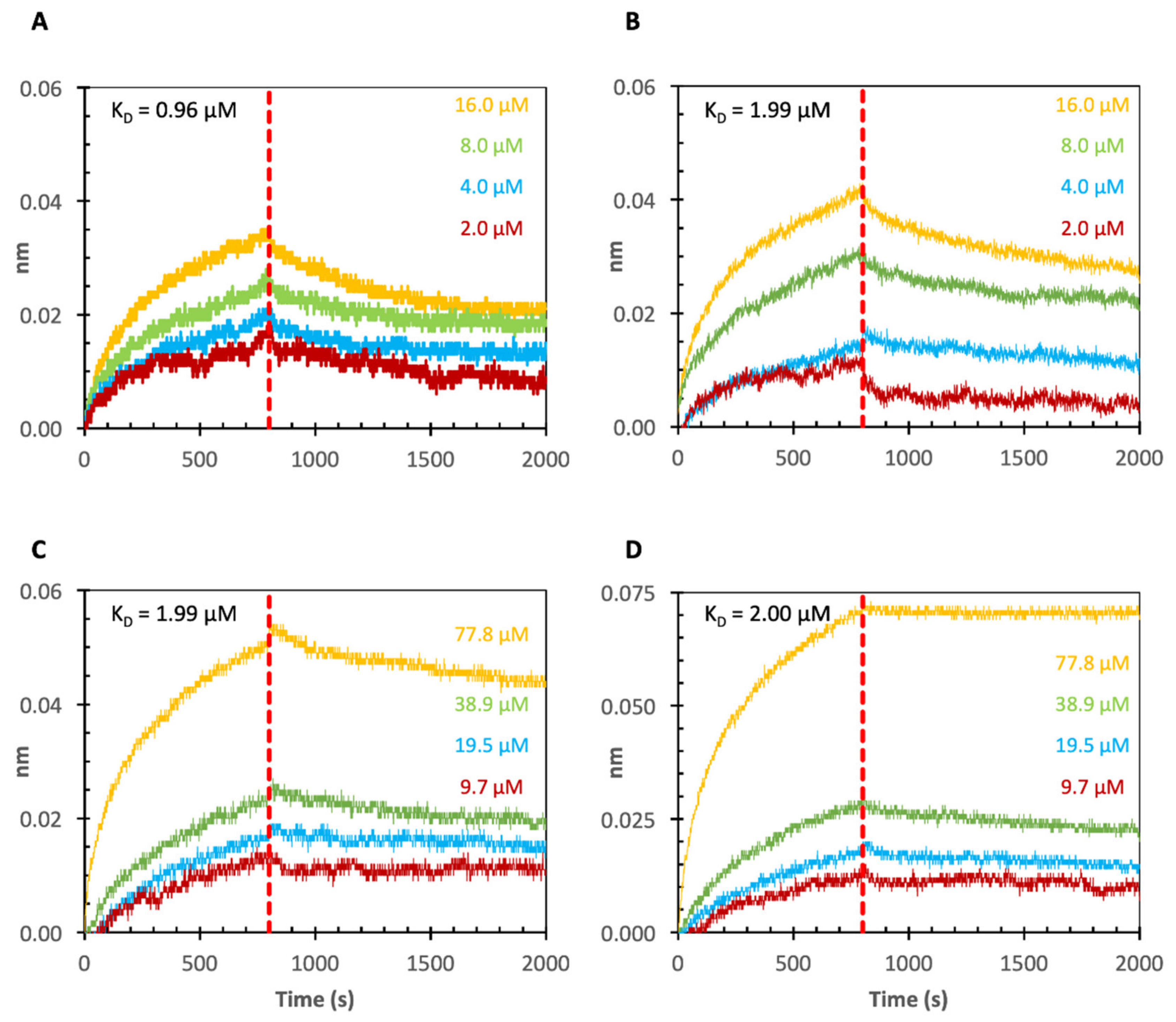
2.3. Binding of Echinacea Galactan to Gal-1 and -7 by Biolayer Interferometry (BLI)
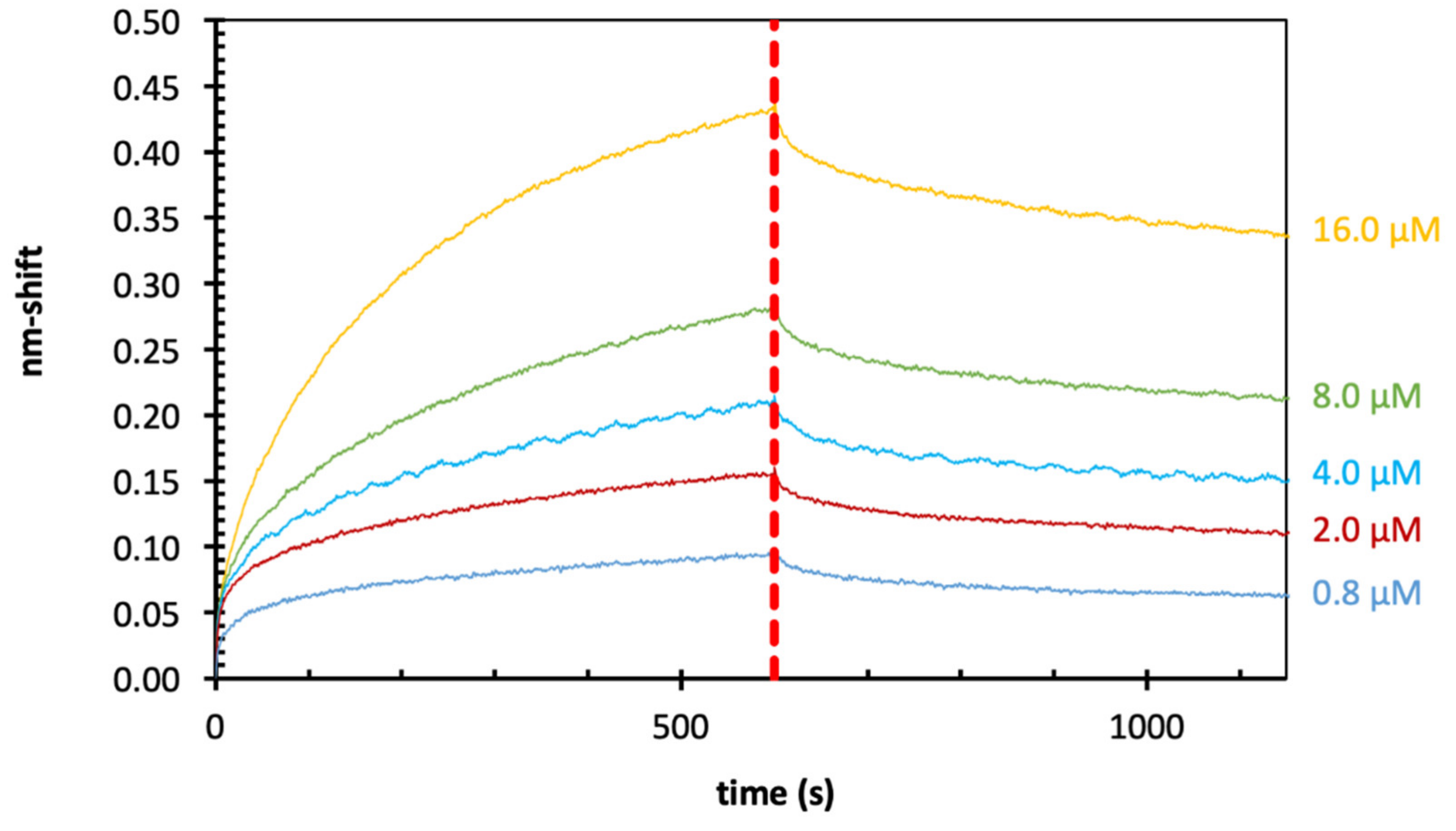
2.4. Binding of Echinacea Galactan to Gal-3 by BLI
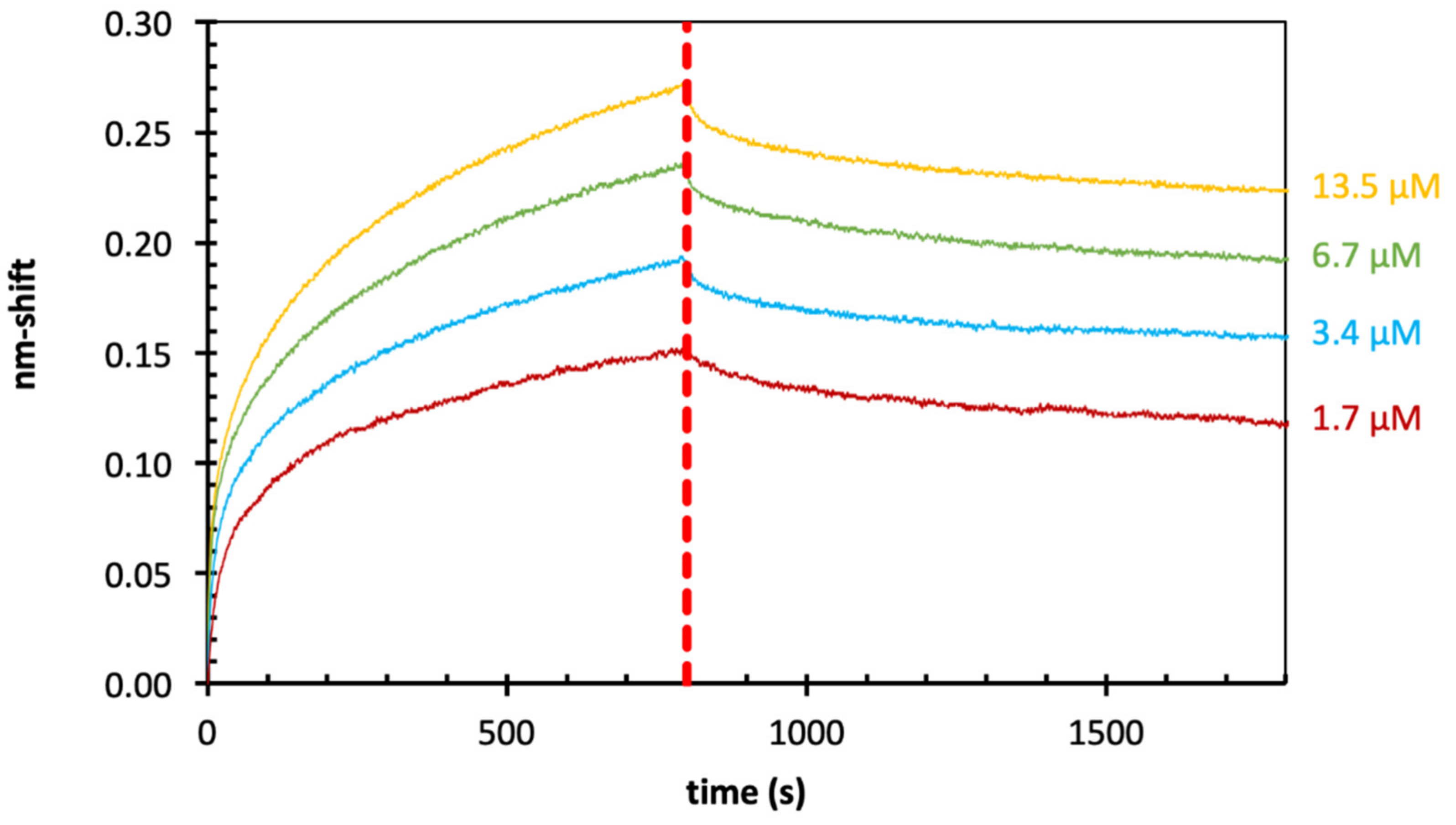
2.5. Binding of Zostera Galactan to Gal-3 by BLI
3. Discussion
3.1. Composition of Galactans
3.2. Cell-Free Production of Gal-1 and -7
3.3. Binding of Galactans to Galectins
3.3.1. Binding of Plant Saccharides to Gal-1, -3 and -7
3.3.2. Mode of Binding of Plant Saccharides to Galectins
4. Materials and Methods
4.1. Isolation of AGPs
4.2. Partial Degradation of AGPs
4.3. Determination of Neutral Monosaccharide Composition
4.4. Determination of Absolute Molecular Weight
4.5. Commercially Purchased Galectins
4.6. Cell-Free Production, Purification and Western Blotting of Gal-1 and Gal-7
4.7. Biotinylation of Galectins
4.8. BLI
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Liu, F.-T.; Rabinovich, G.A. Galectins as Modulators of Tumour Progression. Nat. Rev. Cancer 2005, 5, 29–41. [Google Scholar] [CrossRef]
- Hönig, E.; Schneider, K.; Jacob, R. Recycling of Galectin-3 in Epithelial Cells. Eur. J. Cell Biol. 2015, 94, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Thiemann, S.; Baum, L.G. Galectins and Immune Responses—Just How Do They Do Those Things They Do? Annu. Rev. Immunol. 2016, 34, 243–264. [Google Scholar] [CrossRef] [PubMed]
- Johannes, L.; Jacob, R.; Leffler, H. Galectins at a Glance. J. Cell Sci. 2018, 131, jcs208884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cummings, R.D.; Liu, F.-T.; Vasta, G.R. Galectins. In Essentials of Glycobiology; Varki, A., Cummings, R.D., Esko, J.D., Stanley, P., Hart, G.W., Aebi, M., Darvill, A.G., Kinoshita, T., Packer, N.H., Prestegard, J.H., et al., Eds.; Cold Spring Harbor Laboratory Press: Cold Spring Harbor, NY, USA, 2015. [Google Scholar]
- Ahmad, N.; Gabius, H.-J.; André, S.; Kaltner, H.; Sabesan, S.; Roy, R.; Liu, B.; Macaluso, F.; Brewer, C.F. Galectin-3 Precipitates as a Pentamer with Synthetic Multivalent Carbohydrates and Forms Heterogeneous Cross-Linked Complexes. J. Biol. Chem. 2004, 279, 10841–10847. [Google Scholar] [CrossRef] [Green Version]
- Girotti, M.R.; Salatino, M.; Dalotto-Moreno, T.; Rabinovich, G.A. Sweetening the Hallmarks of Cancer: Galectins as Multifunctional Mediators of Tumor Progression. J. Exp. Med. 2020, 217, e20182041. [Google Scholar] [CrossRef]
- Compagno, D.; Tiraboschi, C.; Garcia, J.D.; Rondón, Y.; Corapi, E.; Velazquez, C.; Laderach, D.J. Galectins as Checkpoints of the Immune System in Cancers, Their Clinical Relevance, and Implication in Clinical Trials. Biomolecules 2020, 10, 750. [Google Scholar] [CrossRef]
- Laaf, D.; Bojarová, P.; Elling, L.; Křen, V. Galectin–Carbohydrate Interactions in Biomedicine and Biotechnology. Trends Biotechnol. 2019, 37, 402–415. [Google Scholar] [CrossRef]
- Maxwell, E.G.; Belshaw, N.J.; Waldron, K.W.; Morris, V.J. Pectin—An Emerging New Bioactive Food Polysaccharide. Trends Food Sci. Technol. 2012, 24, 64–73. [Google Scholar] [CrossRef]
- Zhang, T.; Miller, M.C.; Zheng, Y.; Zhang, Z.; Xue, H.; Zhao, D.; Su, J.; Mayo, K.H.; Zhou, Y.; Tai, G. Macromolecular Assemblies of Complex Polysaccharides with Galectin-3 and Their Synergistic Effects on Function. Biochem. J. 2017, 474, 3849–3868. [Google Scholar] [CrossRef]
- Demotte, N.; Bigirimana, R.; Wieërs, G.; Stroobant, V.; Squifflet, J.-L.; Carrasco, J.; Thielemans, K.; Baurain, J.-F.; Van Der Smissen, P.; Courtoy, P.J.; et al. A Short Treatment with Galactomannan GM-CT-01 Corrects the Functions of Freshly Isolated Human Tumor–Infiltrating Lymphocytes. Clin. Cancer Res. 2014, 20, 1823–1833. [Google Scholar] [CrossRef] [Green Version]
- Girard, A.; Magnani, J.L. Clinical Trials and Applications of Galectin Antagonists. Trends Glycosci. Glycotechnol. 2018, 30, SE211–SE220. [Google Scholar] [CrossRef] [Green Version]
- Stegmayr, J.; Lepur, A.; Kahl-Knutson, B.; Aguilar-Moncayo, M.; Klyosov, A.A.; Field, R.A.; Oredsson, S.; Nilsson, U.J.; Leffler, H. Low or No Inhibitory Potency of the Canonical Galectin Carbohydrate-Binding Site by Pectins and Galactomannans. J. Biol. Chem. 2016, 291, 13318–13334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, M.C.; Ippel, H.; Suylen, D.; Klyosov, A.A.; Traber, P.G.; Hackeng, T.; Mayo, K.H. Binding of Polysaccharides to Human Galectin-3 at a Noncanonical Site in Its Carbohydrate Recognition Domain. Glycobiology 2016, 26, 88–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, Y.; Zeng, W.; Bacic, A.; Johnson, K. AGPs through Time and Space. Annu. Plant. Rev. Online 2018, 1, 767–804. [Google Scholar] [CrossRef]
- Classen, B.; Witthohn, K.; Blaschek, W. Characterization of an Arabinogalactan-Protein Isolated from Pressed Juice of Echinacea purpurea by Precipitation with the Beta-Glucosyl Yariv Reagent. Carbohydr. Res. 2000, 327, 497–504. [Google Scholar] [CrossRef]
- Pfeifer, L.; Shafee, T.; Johnson, K.L.; Bacic, A.; Classen, B. Arabinogalactan-Proteins of Zostera marina L. Contain Unique Glycan Structures and Provide Insight into Adaption Processes to Saline Environments. Sci. Rep. 2020, 10, 8232. [Google Scholar] [CrossRef] [PubMed]
- Thude, S.; Classen, B.; Blaschek, W.; Barz, D.; Thude, H. Binding Studies of an Arabinogalactan-Protein from Echinacea purpurea to Leucocytes. Phytomedicine 2006, 13, 425–427. [Google Scholar] [CrossRef]
- Manero-Rupérez, N.; Martínez-Bosch, N.; Barranco, L.E.; Visa, L.; Navarro, P. The Galectin Family as Molecular Targets: Hopes for Defeating Pancreatic Cancer. Cells 2020, 9, 689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schwarz, D.; Junge, F.; Durst, F.; Frölich, N.; Schneider, B.; Reckel, S.; Sobhanifar, S.; Dötsch, V.; Bernhard, F. Preparative Scale Expression of Membrane Proteins in Escherichia Coli-Based Continuous Exchange Cell-Free Systems. Nat. Protoc. 2007, 2, 2945–2957. [Google Scholar] [CrossRef] [PubMed]
- Wu, D.; Zheng, J.; Mao, G.; Hu, W.; Ye, X.; Linhardt, R.J.; Chen, S. Rethinking the Impact of RG-I Mainly from Fruits and Vegetables on Dietary Health. Crit. Rev. Food Sci. Nutr. 2020, 60, 2938–2960. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Klyosov, A.; Mayo, K.H. The α-Galactomannan Davanat Binds Galectin-1 at a Site Different from the Conventional Galectin Carbohydrate Binding Domain. Glycobiology 2009, 19, 1034–1045. [Google Scholar] [CrossRef] [Green Version]
- Scheller, H.V.; Ulvskov, P. Hemicelluloses. Annu. Rev. Plant. Biol. 2010, 61, 263–289. [Google Scholar] [CrossRef] [PubMed]
- Ramaswamy, S.; Sleiman, M.H.; Masuyer, G.; Arbez-Gindre, C.; Micha-Screttas, M.; Calogeropoulou, T.; Steele, B.R.; Acharya, K.R. Structural Basis of Multivalent Galactose-Based Dendrimer Recognition by Human Galectin-7. FEBS J. 2015, 282, 372–387. [Google Scholar] [CrossRef] [PubMed]
- Fouillit, M.; Lévi-Strauss, M.; Giudicelli, V.; Lutomski, D.; Bladier, D.; Caron, M.; Joubert-Caron, R. Affinity Purification and Characterization of Recombinant Human Galectin-1. J. Chromatogr. B. Biomed. Sci. App. 1998, 706, 167–171. [Google Scholar] [CrossRef]
- Leonidas, D.D.; Vatzaki, E.H.; Vorum, H.; Celis, J.E.; Madsen, P.; Acharya, K.R. Structural Basis for the Recognition of Carbohydrates by Human Galectin-7. Biochemistry 1998, 37, 13930–13940. [Google Scholar] [CrossRef] [PubMed]
- López-Lucendo, M.F.; Solís, D.; André, S.; Hirabayashi, J.; Kasai, K.; Kaltner, H.; Gabius, H.-J.; Romero, A. Growth-Regulatory Human Galectin-1: Crystallographic Characterisation of the Structural Changes Induced by Single-Site Mutations and Their Impact on the Thermodynamics of Ligand Binding. J. Mol. Biol. 2004, 343, 957–970. [Google Scholar] [CrossRef]
- Tracey, B.M.; Feizi, T.; Abbott, W.M.; Carruthers, R.A.; Green, B.N.; Lawson, A.M. Subunit Molecular Mass Assignment of 14,654 Da to the Soluble Beta-Galactoside-Binding Lectin from Bovine Heart Muscle and Demonstration of Intramolecular Disulfide Bonding Associated with Oxidative Inactivation. J. Biol. Chem. 1992, 267, 10342–10347. [Google Scholar] [CrossRef]
- Hirabayashi, J.; Kasai, K. Effect of Amino Acid Substitution by Sited-Directed Mutagenesis on the Carbohydrate Recognition and Stability of Human 14-KDa Beta-Galactoside-Binding Lectin. J. Biol. Chem. 1991, 266, 23648–23653. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, P.; Qin, Y.; Cong, Q.; Shao, C.; Du, Z.; Ni, X.; Li, P.; Ding, K. RN1, A Novel Galectin-3 Inhibitor, Inhibits Pancreatic Cancer Cell Growth In Vitro and In Vivo via Blocking Galectin-3 Associated Signaling Pathways. Oncogene 2017, 36, 1297–1308. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Zheng, Y.; Zhao, D.; Yan, J.; Sun, C.; Zhou, Y.; Tai, G. Multiple Approaches to Assess Pectin Binding to Galectin-3. Int. J. Biol. Macromol. 2016, 91, 994–1001. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, T.-J.; Lin, H.-Y.; Tu, Z.; Huang, B.-S.; Wu, S.-C.; Lin, C.-H. Structural Basis Underlying the Binding Preference of Human Galectins-1, -3 and -7 for Galβ1-3/4GlcNAc. PLoS ONE 2015, 10, e0125946. [Google Scholar] [CrossRef] [PubMed]
- Su, J.; Wang, Y.; Si, Y.; Gao, J.; Song, C.; Cui, L.; Wu, R.; Tai, G.; Zhou, Y. Galectin-13, a Different Prototype Galectin, Does Not Bind β-Galacto-Sides and Forms Dimers via Intermolecular Disulfide Bridges between Cys-136 and Cys-138. Sci. Rep. 2018, 8, 980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cui, L.; Wang, J.; Huang, R.; Tan, Y.; Zhang, F.; Zhou, Y.; Sun, L. Analysis of Pectin from Panax ginseng Flower Buds and Their Binding Activities to Galectin-3. Int. J. Biol. Macromol. 2019, 128, 459–467. [Google Scholar] [CrossRef] [PubMed]
- Gao, X.; Zhi, Y.; Sun, L.; Peng, X.; Zhang, T.; Xue, H.; Tai, G.; Zhou, Y. The Inhibitory Effects of a Rhamnogalacturonan Ι (RG-I) Domain from Ginseng Pectin on Galectin-3 and Its Structure-Activity Relationship. J. Biol. Chem. 2013, 288, 33953–33965. [Google Scholar] [CrossRef] [Green Version]
- Gunning, A.P.; Bongaerts, R.J.M.; Morris, V.J. Recognition of Galactan Components of Pectin by Galectin-3. FASEB J. 2009, 23, 415–424. [Google Scholar] [CrossRef]
- Gao, X.; Zhi, Y.; Zhang, T.; Xue, H.; Wang, X.; Foday, A.D.; Tai, G.; Zhou, Y. Analysis of the Neutral Polysaccharide Fraction of MCP and Its Inhibitory Activity on Galectin-3. Glycoconj. J. 2012, 29, 159–165. [Google Scholar] [CrossRef]
- Pelletier, I.; Hashidate, T.; Urashima, T.; Nishi, N.; Nakamura, T.; Futai, M.; Arata, Y.; Kasai, K.-C.; Hirashima, M.; Hirabayashi, J.; et al. Specific Recognition of Leishmania major Poly-Beta-Galactosyl Epitopes by Galectin-9: Possible Implication of Galectin-9 in Interaction between L. major and Host Cells. J. Biol. Chem. 2003, 278, 22223–22230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schöll-Naderer, M.; Helm, O.; Spencker, J.; Pfeifer, L.; Rätsch, T.; Sebens, S.; Classen, B. Plant-Derived Saccharides and Their Inhibitory Potential on Metastasis Associated Cellular Processes of Pancreatic Ductal Adenocarcinoma Cells. Carbohydr. Res. 2020, 490, 107903. [Google Scholar] [CrossRef] [PubMed]
- Chan, Y.-C.; Lin, H.-Y.; Tu, Z.; Kuo, Y.-H.; Hsu, S.-T.; Lin, C.-H. Dissecting the Structure–Activity Relationship of Galectin–Ligand Interactions. Int. J. Mol. Sci. 2018, 19, 392. [Google Scholar] [CrossRef] [Green Version]
- Miller, M.C.; Zheng, Y.; Suylen, D.; Ippel, H.; Cañada, F.J.; Berbís, M.A.; Jiménez-Barbero, J.; Tai, G.; Gabius, H.; Mayo, K.H. Targeting the CRD F-face of Human Galectin-3 and Allosterically Modulating Glycan Binding by Angiostatic PTX008 and a Structurally Optimized Derivative. ChemMedChem 2021, 16, 713–723. [Google Scholar] [CrossRef] [PubMed]
- Miller, M.C.; Zheng, Y.; Yan, J.; Zhou, Y.; Tai, G.; Mayo, K.H. Novel Polysaccharide Binding to the N-Terminal Tail of Galectin-3 Is Likely Modulated by Proline Isomerization. Glycobiology 2017, 27, 1038–1051. [Google Scholar] [CrossRef]
- Elola, M.T.; Ferragut, F.; Méndez-Huergo, S.P.; Croci, D.O.; Bracalente, C.; Rabinovich, G.A. Galectins: Multitask Signaling Molecules Linking Fibroblast, Endothelial and Immune Cell Programs in the Tumor Microenvironment. Cell. Immunol. 2018, 333, 34–45. [Google Scholar] [CrossRef] [Green Version]
- Blakeney, A.B.; Harris, P.J.; Henry, R.J.; Stone, B.A. A Simple and Rapid Preparation of Alditol Acetates for Monosaccharide Analysis. Carbohydr. Res. 1983, 113, 291–299. [Google Scholar] [CrossRef]
- Helmstetter, F.; Arnold, P.; Höger, B.; Petersen, L.M.; Beitz, E. Formate–Nitrite Transporters Carrying Nonprotonatable Amide Amino Acids Instead of a Central Histidine Maintain PH-Dependent Transport. J. Biol. Chem. 2019, 294, 623–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]


| Monosaccharide | Echinacea Galactan | Zostera Galactan |
|---|---|---|
| Galactose | 80.7 | 86.4 |
| Arabinose | 16.1 | 6.8 |
| Others | 3.2 | 6.8 |
| Sample | Binding Partner | Average KD-Value |
|---|---|---|
| Echinacea galactan | Gal-1 | 0.96 µM |
| Echinacea galactan | Gal-1 Cell-free produced | 1.99 µM |
| Echinacea galactan | Gal-7 | 1.99 µM |
| Echinacea galactan | Gal-7 Cell-free produced | 2.00 µM |
| Sample | Binding Partner | Sensor | Average KD-Value |
|---|---|---|---|
| Echinacea galactan | Gal-3 | Super- streptavidin | 0.36 µM |
| Echinacea galactan | Gal-3 | Streptavidin | 0.70 µM |
| Sample | Binding Partner | Sensor | Average KD-Value |
|---|---|---|---|
| Zostera galactan | Gal-3 | Streptavidin | 0.08 µM |
| Zostera galactan | Gal-3 | Streptavidin | 0.28 µM |
| Zostera galactan | Gal-3 | Streptavidin | 0.17 µM |
| Saccharide | KD-Values | Sensor-Type | Reference | |
|---|---|---|---|---|
| Disaccharides | Galβ1-3GlcNAc | 0.23 µM | Super-streptavidin | [33] |
| Galβ1-4GlcNAc | 0.28 µM | |||
| Pectin-derived | 1,4-β-D-galactan | 0.60 µM | Streptavidin | [32] |
| RGI-4 | 0.10 µM | |||
| MCP | 15 µM | |||
| RG | 0.05 µM | Streptavidin | [11] | |
| HG1 | 46 µM | |||
| HG2 | 138 µM | |||
| RGI | 0.07 nM | Ni-NTA | [34] | |
| RG-I (WGFPA-1a) | 4.90 µM | Ni-NTA | [35] | |
| RG-I (WGFPA-1b) | 0.24 µM | |||
| RG-I (WGFPA-1c) | 0.71 µM | |||
| AGP-derived | Galactan from Echinacea | 0.70 µM | Streptavidin | This work |
| Galactan from Echinacea | 0.36 µM | Super-streptavidin | ||
| Galactan from Zostera | 0.08–0.28 µM | Streptavidin | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeifer, L.; Baumann, A.; Petersen, L.M.; Höger, B.; Beitz, E.; Classen, B. Degraded Arabinogalactans and Their Binding Properties to Cancer-Associated Human Galectins. Int. J. Mol. Sci. 2021, 22, 4058. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22084058
Pfeifer L, Baumann A, Petersen LM, Höger B, Beitz E, Classen B. Degraded Arabinogalactans and Their Binding Properties to Cancer-Associated Human Galectins. International Journal of Molecular Sciences. 2021; 22(8):4058. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22084058
Chicago/Turabian StylePfeifer, Lukas, Alexander Baumann, Lea Madlen Petersen, Bastian Höger, Eric Beitz, and Birgit Classen. 2021. "Degraded Arabinogalactans and Their Binding Properties to Cancer-Associated Human Galectins" International Journal of Molecular Sciences 22, no. 8: 4058. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22084058






