Decreased Lymphangiogenic Activities and Genes Expression of Cord Blood Lymphatic Endothelial Progenitor Cells (VEGFR3+/Pod+/CD11b+ Cells) in Patient with Preeclampsia
Abstract
:1. Introduction
2. Results
2.1. Isolation and Characterization of Human Cord Blood-Derived VEGFR3+/Pod+/CD11b+ Cells as LEPCs and the Induction of Their Differentiation into LECs
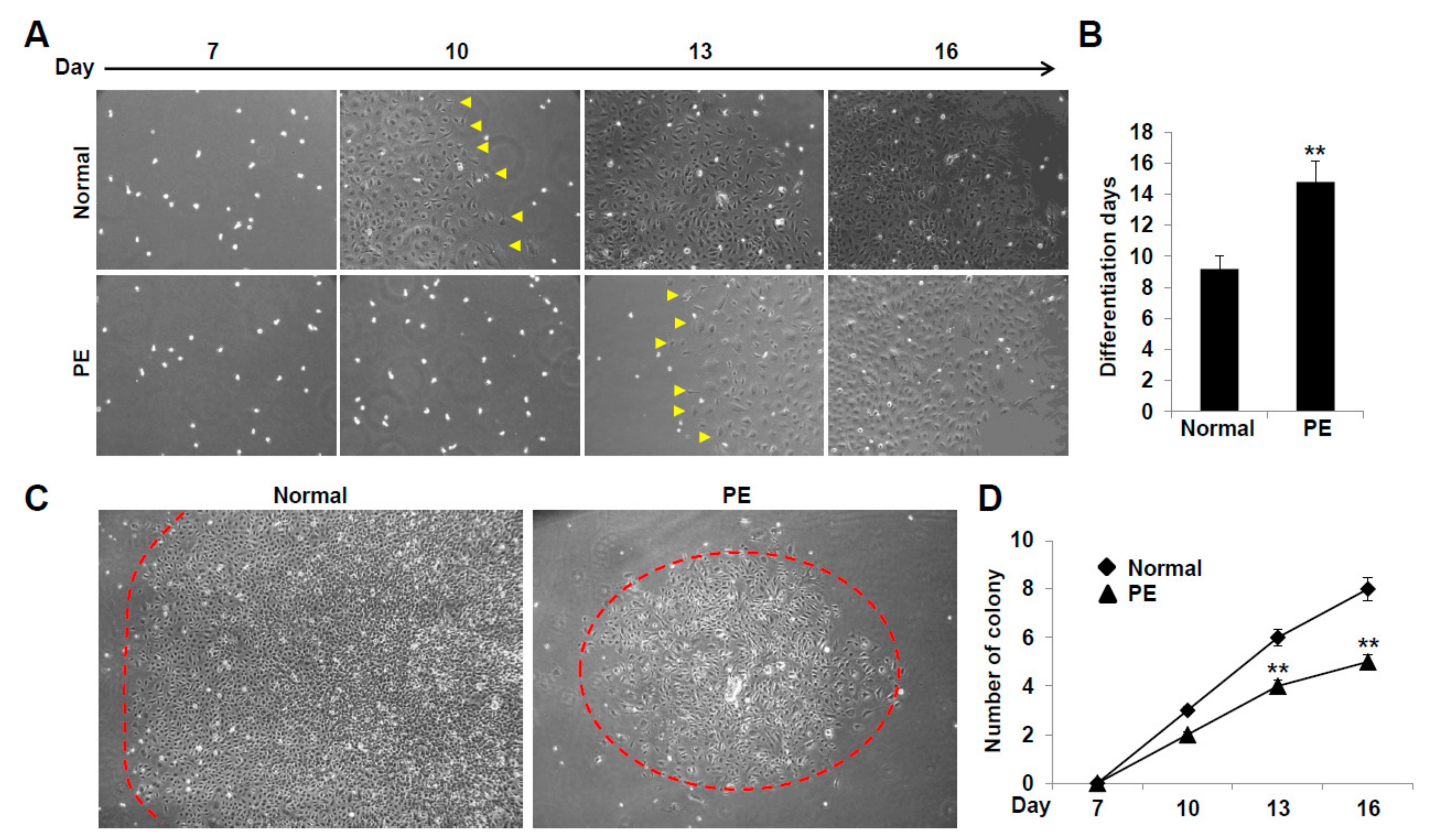
2.2. VEGFR3+/Pod+/CD11b+ LEPCs from Women with PE Show Diminished Differentiation into LECs
2.3. LECs Derived from VEGFR3+/Pod+/CD11b+ LEPCs of PE Show Decreased Lymphangiogenic Activities In Vitro
2.4. LECs Differentiated from VEGFR3+/Pod+/CD11b+ LEPCs of PE Present Reduced 3D Lymphatic Sprouting
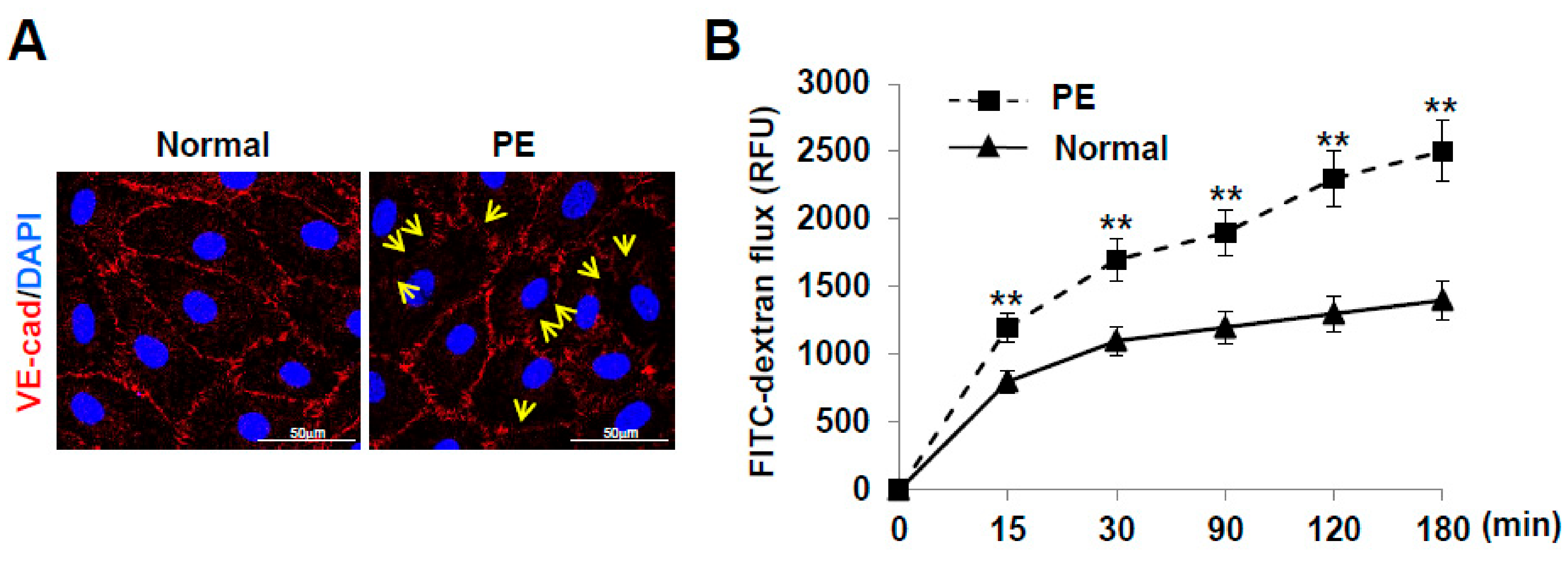
2.5. LECs Differentiated from VEGFR3+/Pod+/CD11b+ LEPCs of PE Show Increased Lymphatic Vessel Permeability
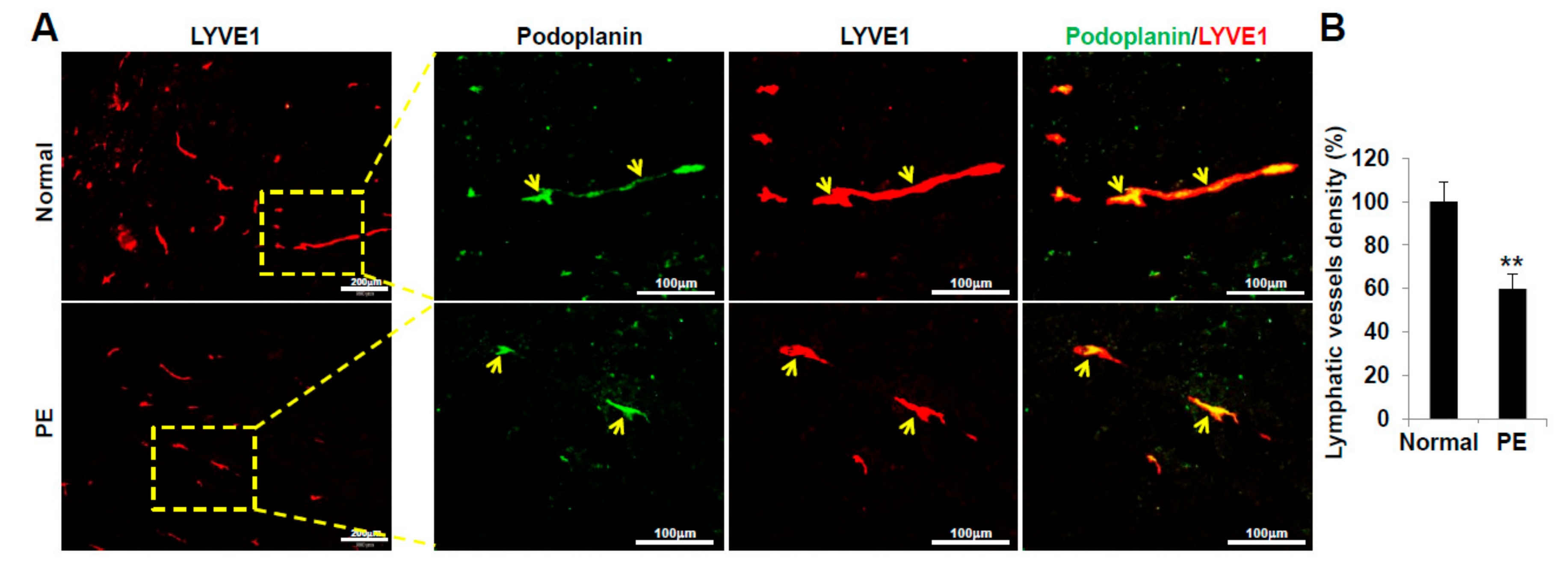
2.6. VEGFR3+/Pod+/CD11b+ LEPCs from Women with PE Show Decreased Lymphangiogenic Activity In Vivo
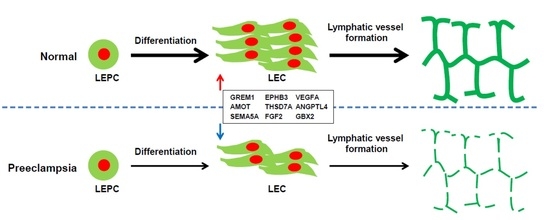
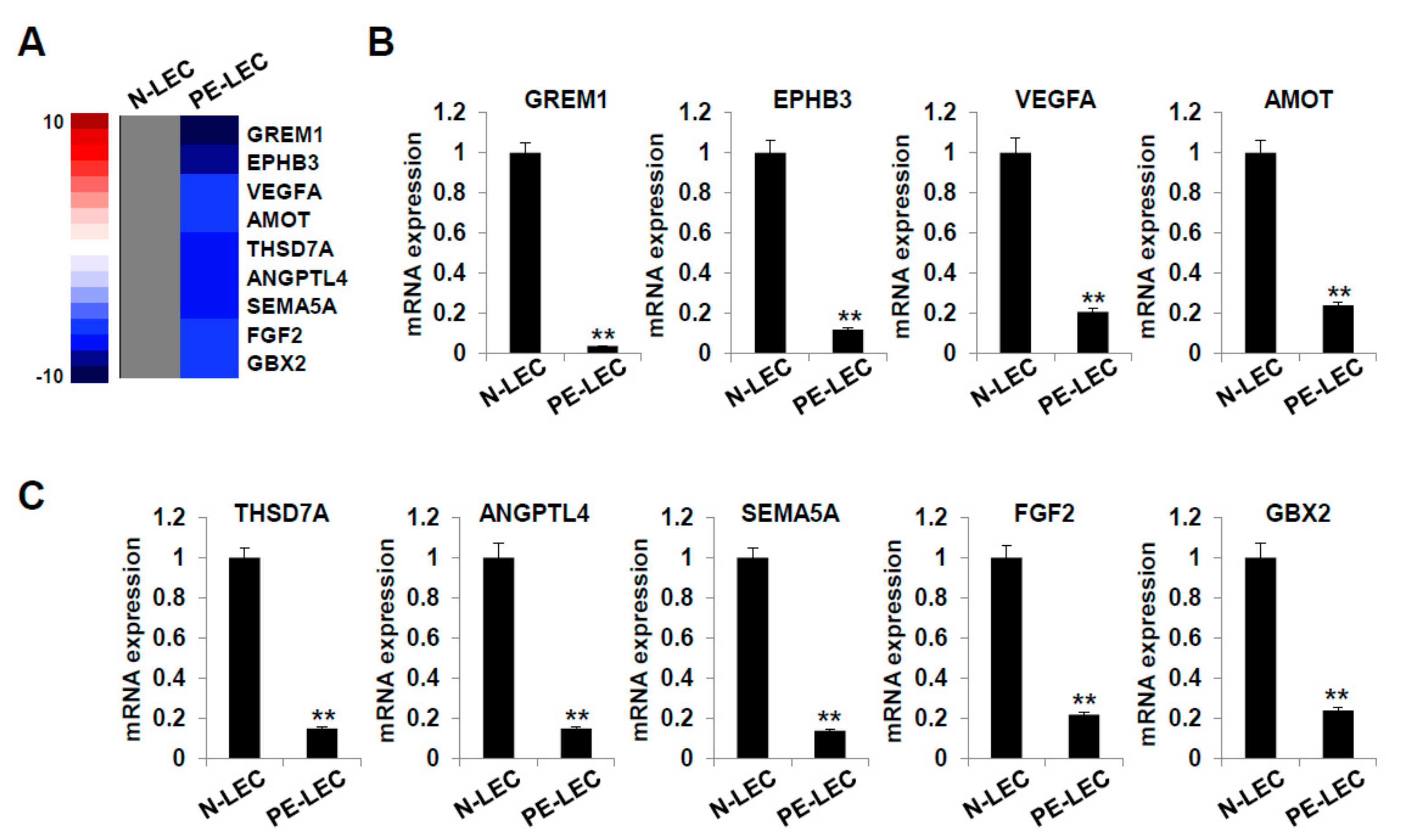
2.7. Gene Expression Analysis of LECs Derived from LEPCs of Preeclamptic Women
3. Discussion
4. Materials and Methods
4.1. Study Population and Sample Collection
4.2. Isolation and Cultivation of LEPCs (VEGFR3+/Pod+/CD11b+ Cells)
4.3. LEPC Differentiation Assay
4.4. In Vitro Tube Formation Assay
4.5. Cell Proliferation Assay
4.6. Cell Migration Assay
4.7. Wound Healing Assay
4.8. Cell Matrix Adhesion
4.9. Three-Dimensional (3D) Bead Sprouting Assay
4.10. Three-Dimensional (3D) Spheroid Sprouting Assay
4.11. Immunofluorescence Staining
4.12. Transwell Permeability Assay
4.13. LEC Differentiation Assay in Matrigel
4.14. RNA-Sequencing for Gene Expression Analysis
4.15. Real-Time Reverse-Transcription Quantitative Polymerase Chain Reaction (RT-qPCR)
4.16. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lane, M.; Zander-Fox, D.L.; Robker, R.L.; McPherson, N.O. Peri-conception parental obesity, reproductive health, and transgenerational impacts. Trends Endocrinol. Metab. 2015, 26, 84–90. [Google Scholar] [CrossRef]
- Gluckman, P.D.; Hanson, M.A.; Cooper, C.; Thornburg, K.L. Effect of in utero and early-life conditions on adult health and disease. N. Engl. J. Med. 2008, 359, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Goffin, S.M.; Derraik, J.G.B.; Groom, K.M.; Cutfield, W.S. Maternal pre-eclampsia and long-term offspring health: Is there a shadow cast? Pregnancy Hypertens. 2018, 12, 11–15. [Google Scholar] [CrossRef]
- Hakim, J.; Senterman, M.K.; Hakim, A.M. Preeclampsia is a biomarker for vascular disease in both mother and child: The need for a medical alert system. Int. J. Pediatr. 2013, 2013, 953150. [Google Scholar] [CrossRef]
- Davis, E.F.; Newton, L.; Lewandowski, A.J.; Lazdam, M.; Kelly, B.A.; Kyriakou, T.; Leeson, P. Pre-eclampsia and offspring cardiovascular health: Mechanistic insights from experimental studies. Clin. Sci. 2012, 123, 53–72. [Google Scholar] [CrossRef] [Green Version]
- Davis, E.F.; Lazdam, M.; Lewandowski, A.J.; Worton, S.A.; Kelly, B.; Kenworthy, Y.; Adwani, S.; Wilkinson, A.R.; McCormick, K.; Sargent, I.; et al. Cardiovascular risk factors in children and young adults born to preeclamptic pregnancies: A systematic review. Pediatrics 2012, 129, e1552–e1561. [Google Scholar] [CrossRef]
- Palmsten, K.; Buka, S.L.; Michels, K.B. Maternal pregnancy-related hypertension and risk for hypertension in offspring later in life. Obstet Gynecol 2010, 116, 858–864. [Google Scholar] [CrossRef] [Green Version]
- Kajantie, E.; Eriksson, J.G.; Osmond, C.; Thornburg, K.; Barker, D.J. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: The Helsinki birth cohort study. Stroke 2009, 40, 1176–1180. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.; Hypponen, E.; Power, C. Prenatal exposures and glucose metabolism in adulthood: Are effects mediated through birth weight and adiposity? Diabetes Care 2007, 30, 918–924. [Google Scholar] [CrossRef] [Green Version]
- Libby, G.; Murphy, D.J.; McEwan, N.F.; Greene, S.A.; Forsyth, J.S.; Chien, P.W.; Morris, A.D.; Collaboration, D.M. Pre-eclampsia and the later development of type 2 diabetes in mothers and their children: An intergenerational study from the Walker cohort. Diabetologia 2007, 50, 523–530. [Google Scholar] [CrossRef] [Green Version]
- Ogland, B.; Vatten, L.J.; Romundstad, P.R.; Nilsen, S.T.; Forman, M.R. Pubertal anthropometry in sons and daughters of women with preeclamptic or normotensive pregnancies. Arch. Dis. Child. 2009, 94, 855–859. [Google Scholar] [CrossRef]
- Vatten, L.J.; Romundstad, P.R.; Holmen, T.L.; Hsieh, C.C.; Trichopoulos, D.; Stuver, S.O. Intrauterine exposure to preeclampsia and adolescent blood pressure, body size, and age at menarche in female offspring. Obstet. Gynecol. 2003, 101, 529–533. [Google Scholar]
- Thoulass, J.C.; Robertson, L.; Denadai, L.; Black, C.; Crilly, M.; Iversen, L.; Scott, N.W.; Hannaford, P.C. Hypertensive disorders of pregnancy and adult offspring cardiometabolic outcomes: A systematic review of the literature and meta-analysis. J. Epidemiol. Community Health 2016, 70, 414–422. [Google Scholar] [CrossRef] [PubMed]
- Wu, C.S.; Nohr, E.A.; Bech, B.H.; Vestergaard, M.; Catov, J.M.; Olsen, J. Health of children born to mothers who had preeclampsia: A population-based cohort study. Am. J. Obstet. Gynecol. 2009, 201, 269.e1–269.e10. [Google Scholar] [CrossRef]
- Zugna, D.; Galassi, C.; Annesi-Maesano, I.; Baiz, N.; Barros, H.; Basterrechea, M.; Correia, S.; Duijts, L.; Esplugues, A.; Fantini, M.P.; et al. Maternal complications in pregnancy and wheezing in early childhood: A pooled analysis of 14 birth cohorts. Int. J. Epidemiol. 2015, 44, 199–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rusconi, F.; Galassi, C.; Forastiere, F.; Bellasio, M.; De Sario, M.; Ciccone, G.; Brunetti, L.; Chellini, E.; Corbo, G.; La Grutta, S.; et al. Maternal complications and procedures in pregnancy and at birth and wheezing phenotypes in children. Am. J. Respir. Crit. Care Med. 2007, 175, 16–21. [Google Scholar] [CrossRef] [PubMed]
- Herzog, E.M.; Eggink, A.J.; van der Zee, M.; Lagendijk, J.; Willemsen, S.P.; de Jonge, R.; Steegers, E.A.; Steegers-Theunissen, R.P. The impact of early- and late-onset preeclampsia on umbilical cord blood cell populations. J. Reprod. Immunol. 2016, 116, 81–85. [Google Scholar] [CrossRef] [Green Version]
- Sugawara, J.; Mitsui-Saito, M.; Hayashi, C.; Hoshiai, T.; Senoo, M.; Chisaka, H.; Yaegashi, N.; Okamura, K. Decrease and senescence of endothelial progenitor cells in patients with preeclampsia. J. Clin. Endocrinol. Metab. 2005, 90, 5329–5332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, L.; Zhou, X.P.; Zhu, J.H.; Xie, X.D.; Zhang, H.; Wang, X.X.; Chen, J.Z.; Jian, S. Decrease and dysfunction of endothelial progenitor cells in umbilical cord blood with maternal pre-eclampsia. J. Obstet. Gynaecol. Res. 2007, 33, 465–474. [Google Scholar] [CrossRef]
- Jung, Y.J.; Park, Y.; Kim, H.S.; Lee, H.J.; Kim, Y.N.; Lee, J.; Kim, Y.H.; Maeng, Y.S.; Kwon, J.Y. Abnormal lymphatic vessel development is associated with decreased decidual regulatory T cells in severe preeclampsia. Am. J. Reprod. Immunol. 2018, 80, e12970. [Google Scholar] [CrossRef]
- Kwon, J.Y.; Maeng, Y.S.; Kwon, Y.G.; Kim, Y.H.; Kang, M.H.; Park, Y.W. Decreased endothelial progenitor cells in umbilical cord blood in severe preeclampsia. Gynecol. Obstet. Investig. 2007, 64, 103–108. [Google Scholar] [CrossRef] [PubMed]
- Vo, T.; Hardy, D.B. Molecular mechanisms underlying the fetal programming of adult disease. J. Cell. Commun. Signal. 2012, 6, 139–153. [Google Scholar] [CrossRef] [Green Version]
- Novakovic, B.; Saffery, R. The ever growing complexity of placental epigenetics—Role in adverse pregnancy outcomes and fetal programming. Placenta 2012, 33, 959–970. [Google Scholar] [CrossRef]
- Ching, T.; Ha, J.; Song, M.A.; Tiirikainen, M.; Molnar, J.; Berry, M.J.; Towner, D.; Garmire, L.X. Genome-scale hypomethylation in the cord blood DNAs associated with early onset preeclampsia. Clin. Epigenetics 2015, 7, 21. [Google Scholar] [CrossRef] [Green Version]
- Blue, E.K.; Sheehan, B.M.; Nuss, Z.V.; Boyle, F.A.; Hocutt, C.M.; Gohn, C.R.; Varberg, K.M.; McClintick, J.N.; Haneline, L.S. Epigenetic regulation of placenta-specific 8 contributes to altered function of endothelial colony-forming cells exposed to intrauterine gestational diabetes mellitus. Diabetes 2015, 64, 2664–2675. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oliver, G. Lymphatic vasculature development. Nat. Rev. Immunol. 2004, 4, 35–45. [Google Scholar] [CrossRef]
- Cueni, L.N.; Detmar, M. The lymphatic system in health and disease. Lymphat. Res. Biol. 2008, 6, 109–122. [Google Scholar] [CrossRef]
- Randolph, G.J.; Ivanov, S.; Zinselmeyer, B.H.; Scallan, J.P. The lymphatic system: Integral roles in immunity. Annu. Rev. Immunol. 2017, 35, 31–52. [Google Scholar] [CrossRef] [Green Version]
- Guerin, L.R.; Prins, J.R.; Robertson, S.A. Regulatory T-cells and immune tolerance in pregnancy: A new target for infertility treatment? Hum. Reprod. Update 2009, 15, 517–535. [Google Scholar] [CrossRef] [PubMed]
- Humbert, M.; Hugues, S.; Dubrot, J. Shaping of oeripheral T cell responses by lymphatic endothelial cells. Front. Immunol. 2016, 7, 684. [Google Scholar] [PubMed] [Green Version]
- Rouzaut, A.; Garasa, S.; Teijeira, A.; Gonzalez, I.; Martinez-Forero, I.; Suarez, N.; Larrea, E.; Alfaro, C.; Palazon, A.; Dubrot, J.; et al. Dendritic cells adhere to and transmigrate across lymphatic endothelium in response to IFN-alpha. Eur. J. Immunol. 2010, 40, 3054–3063. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.D. Lymphatics: At the interface of immunity, tolerance, and tumor metastasis. Microcirculation 2011, 18, 517–531. [Google Scholar] [CrossRef]
- McNamee, E.N.; Rivera-Nieves, J. Defective lymphatics in Crohn’s Disease: Tertiary lymphoid follicles plug the gap. Gastroenterology 2017, 152, 908–910. [Google Scholar] [CrossRef] [PubMed]
- Yin, N.; Zhang, N.; Lal, G.; Xu, J.; Yan, M.; Ding, Y.; Bromberg, J.S. Lymphangiogenesis is required for pancreatic islet inflammation and diabetes. PLoS ONE 2011, 6, e28023. [Google Scholar] [CrossRef] [PubMed]
- Abouelkheir, G.R.; Upchurch, B.D.; Rutkowski, J.M. Lymphangiogenesis: Fuel, smoke, or extinguisher of inflammation’s fire? Exp. Biol. Med. 2017, 242, 884–895. [Google Scholar] [CrossRef]
- Ruddle, N.H. Lymphatic vessels and tertiary lymphoid organs. J. Clin. Investig. 2014, 124, 953–959. [Google Scholar] [CrossRef] [Green Version]
- Escobedo, N.; Oliver, G. The lymphatic vasculature: Its role in adipose metabolism and obesity. Cell Metab. 2017, 26, 598–609. [Google Scholar] [CrossRef] [Green Version]
- Haemmerle, M.; Keller, T.; Egger, G.; Schachner, H.; Steiner, C.W.; Stokic, D.; Neumayer, C.; Brown, M.K.; Kerjaschki, D.; Hantusch, B. Enhanced lymph vessel density, remodeling, and inflammation are reflected by gene expression signatures in dermal lymphatic endothelial cells in type 2 diabetes. Diabetes 2013, 62, 2509–2529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coma, S.; Allard-Ratick, M.; Akino, T.; van Meeteren, L.A.; Mammoto, A.; Klagsbrun, M. GATA2 and Lmo2 control angiogenesis and lymphangiogenesis via direct transcriptional regulation of neuropilin-2. Angiogenesis 2013, 16, 939–952. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jankowska-Konsur, A.; Kobierzycki, C.; Dziegiel, P. Angiogenesis and lymphangiogenesis in primary cutaneous T-cell lymphomas. Postepy Hig. Med. Dosw. 2015, 69, 1205–1214. [Google Scholar] [CrossRef]
- Hey-Cunningham, A.J.; Peters, K.M.; Zevallos, H.B.; Berbic, M.; Markham, R.; Fraser, I.S. Angiogenesis, lymphangiogenesis and neurogenesis in endometriosis. Front. Biosci. 2013, 5, 1033–1056. [Google Scholar] [CrossRef]
- Stojanovska, V.; Scherjon, S.A.; Plosch, T. Preeclampsia As modulator of offspring health. Biol. Reprod. 2016, 94, 53. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Hafner, H.; Varghese, M.; Griffin, C.; Clemente, J.; Islam, M.; Carlson, Z.; Zhu, A.; Hak, L.; Abrishami, S.; et al. Programming effects of maternal and gestational obesity on offspring metabolism and metabolic inflammation. Sci. Rep. 2019, 9, 16027. [Google Scholar] [CrossRef]
- Mudyanadzo, T.A. Endothelial progenitor cells and cardiovascular correlates. Cureus 2018, 10, e3342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Algaba-Chueca, F.; Maymo-Masip, E.; Ejarque, M.; Ballesteros, M.; Llaurado, G.; Lopez, C.; Guarque, A.; Serena, C.; Martinez-Guasch, L.; Gutierrez, C.; et al. Gestational diabetes impacts fetal precursor cell responses with potential consequences for offspring. Stem Cells Transl. Med. 2020, 9, 351–363. [Google Scholar] [CrossRef] [Green Version]
- Vivien, C.J.; Pichol-Thievend, C.; Sim, C.B.; Smith, J.B.; Bower, N.I.; Hogan, B.M.; Hudson, J.E.; Francois, M.; Porrello, E.R. Vegfc/d-dependent regulation of the lymphatic vasculature during cardiac regeneration is influenced by injury context. NPJ Regen. Med. 2019, 4, 18. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shimizu, Y.; Polavarapu, R.; Eskla, K.L.; Pantner, Y.; Nicholson, C.K.; Ishii, M.; Brunnhoelzl, D.; Mauria, R.; Husain, A.; Naqvi, N.; et al. Impact of lymphangiogenesis on cardiac remodeling after ischemia and reperfusion injury. J. Am. Heart Assoc. 2018, 7, e009565. [Google Scholar] [CrossRef] [Green Version]
- Escobedo, N.; Proulx, S.T.; Karaman, S.; Dillard, M.E.; Johnson, N.; Detmar, M.; Oliver, G. Restoration of lymphatic function rescues obesity in Prox1-haploinsufficient mice. JCI Insight 2016, 1, e85096. [Google Scholar] [CrossRef]
- Harvey, N.L.; Srinivasan, R.S.; Dillard, M.E.; Johnson, N.C.; Witte, M.H.; Boyd, K.; Sleeman, M.W.; Oliver, G. Lymphatic vascular defects promoted by Prox1 haploinsufficiency cause adult-onset obesity. Nat. Genet. 2005, 37, 1072–1081. [Google Scholar] [CrossRef]
- Meltzer, A.; Van de Water, J. The Role of the Immune System in Autism Spectrum Disorder. Neuropsychopharmacology 2017, 42, 284–298. [Google Scholar] [CrossRef] [Green Version]
- Wakefield, A.J.; Ashwood, P.; Limb, K.; Anthony, A. The significance of ileo-colonic lymphoid nodular hyperplasia in children with autistic spectrum disorder. Eur. J. Gastroenterol.Hepatol. 2005, 17, 827–836. [Google Scholar] [CrossRef] [PubMed]
- Shibuya, M.; Claesson-Welsh, L. Signal transduction by VEGF receptors in regulation of angiogenesis and lymphangiogenesis. Exp. Cell Res. 2006, 312, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Mitola, S.; Ravelli, C.; Moroni, E.; Salvi, V.; Leali, D.; Ballmer-Hofer, K.; Zammataro, L.; Presta, M. Gremlin is a novel agonist of the major proangiogenic receptor VEGFR2. Blood 2010, 116, 3677–3680. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Li, Y.; Hou, R.; Shu, Z. Knockdown GREM1 suppresses cell growth, angiogenesis, and epithelial-mesenchymal transition in colon cancer. J. Cell Biochem. 2019, 120, 5583–5596. [Google Scholar] [CrossRef] [PubMed]
- Adams, R.H.; Wilkinson, G.A.; Weiss, C.; Diella, F.; Gale, N.W.; Deutsch, U.; Risau, W.; Klein, R. Roles of ephrinB ligands and EphB receptors in cardiovascular development: Demarcation of arterial/venous domains, vascular morphogenesis, and sprouting angiogenesis. Genes Dev. 1999, 13, 295–306. [Google Scholar] [CrossRef]
- Darling, T.K.; Lamb, T.J. Emerging Roles for Eph Receptors and Ephrin Ligands in immunity. Front. Immunol. 2019, 10, 1473. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.; Zhu, H.; Guo, Q.; Qian, T.; Zhang, P.; Li, S.; Xue, C.; Gu, X. Overlapping Mechanisms of Peripheral Nerve Regeneration and Angiogenesis Following Sciatic Nerve Transection. Front. Cell Neurosci. 2017, 11, 323. [Google Scholar] [CrossRef]
- Wei, L.; Sun, C.; Zhang, Y.; Han, N.; Sun, S. miR-503–5p inhibits colon cancer tumorigenesis, angiogenesis, and lymphangiogenesis by directly downregulating VEGF-A. Gene Ther. 2020, 1–13. [Google Scholar] [CrossRef]
- Shrestha, B.; Hashiguchi, T.; Ito, T.; Miura, N.; Takenouchi, K.; Oyama, Y.; Kawahara, K.; Tancharoen, S.; Ki, I.Y.; Arimura, N.; et al. B cell-derived vascular endothelial growth factor A promotes lymphangiogenesis and high endothelial venule expansion in lymph nodes. J. Immunol. 2010, 184, 4819–4826. [Google Scholar] [CrossRef]
- Murakami, M.; Zheng, Y.; Hirashima, M.; Suda, T.; Morita, Y.; Ooehara, J.; Ema, H.; Fong, G.H.; Shibuya, M. VEGFR1 tyrosine kinase signaling promotes lymphangiogenesis as well as angiogenesis indirectly via macrophage recruitment. Arterioscler. Thromb. Vasc. Biol. 2008, 28, 658–664. [Google Scholar] [CrossRef] [Green Version]
- Aase, K.; Ernkvist, M.; Ebarasi, L.; Jakobsson, L.; Majumdar, A.; Yi, C.; Birot, O.; Ming, Y.; Kvanta, A.; Edholm, D.; et al. Angiomotin regulates endothelial cell migration during embryonic angiogenesis. Genes Dev. 2007, 21, 2055–2068. [Google Scholar] [CrossRef] [Green Version]
- Troyanovsky, B.; Levchenko, T.; Mansson, G.; Matvijenko, O.; Holmgren, L. Angiomotin: An angiostatin binding protein that regulates endothelial cell migration and tube formation. J. Cell Biol. 2001, 152, 1247–1254. [Google Scholar] [CrossRef]
- Levchenko, T.; Veitonmaki, N.; Lundkvist, A.; Gerhardt, H.; Ming, Y.; Berggren, K.; Kvanta, A.; Carlsson, R.; Holmgren, L. Therapeutic antibodies targeting angiomotin inhibit angiogenesis in vivo. FASEB J. 2008, 22, 880–889. [Google Scholar] [CrossRef] [PubMed]
- Holmgren, L.; Ambrosino, E.; Birot, O.; Tullus, C.; Veitonmaki, N.; Levchenko, T.; Carlson, L.M.; Musiani, P.; Iezzi, M.; Curcio, C.; et al. A DNA vaccine targeting angiomotin inhibits angiogenesis and suppresses tumor growth. Proc. Natl. Acad. Sci. USA 2006, 103, 9208–9213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.H.; Su, P.T.; Du, X.Y.; Kuo, M.W.; Lin, C.Y.; Yang, C.C.; Chan, H.S.; Chang, S.J.; Kuo, C.; Seo, K.; et al. Thrombospondin type I domain containing 7A (THSD7A) mediates endothelial cell migration and tube formation. J. Cell Physiol. 2010, 222, 685–694. [Google Scholar] [CrossRef] [PubMed]
- Kuo, M.W.; Wang, C.H.; Wu, H.C.; Chang, S.J.; Chuang, Y.J. Soluble THSD7A is an N-glycoprotein that promotes endothelial cell migration and tube formation in angiogenesis. PLoS ONE 2011, 6, e29000. [Google Scholar] [CrossRef]
- Wang, C.H.; Chen, I.H.; Kuo, M.W.; Su, P.T.; Lai, Z.Y.; Wang, C.H.; Huang, W.C.; Hoffman, J.; Kuo, C.J.; You, M.S.; et al. Zebrafish Thsd7a is a neural protein required for angiogenic patterning during development. Dev. Dyn. 2011, 240, 1412–1421. [Google Scholar] [CrossRef]
- Liu, L.Y.; Lin, M.H.; Lai, Z.Y.; Jiang, J.P.; Huang, Y.C.; Jao, L.E.; Chuang, Y.J. Motor neuron-derived Thsd7a is essential for zebrafish vascular development via the Notch-dll4 signaling pathway. J. Biomed. Sci. 2016, 23, 59. [Google Scholar] [CrossRef] [Green Version]
- Bäckhed, F.; Crawford, P.A.; O’Donnell, D.; Gordon, J.I. Postnatal lymphatic partitioning from the blood vasculature in the small intestine requires fasting-induced adipose factor. Proc. Natl. Acad. Sci. USA 2007, 104, 606–611. [Google Scholar] [CrossRef] [Green Version]
- Qiu, Z.; Yang, J.; Deng, G.; Li, D.; Zhang, S. Angiopoietin-like 4 promotes angiogenesis and neurogenesis in a mouse model of acute ischemic stroke. Brain Res. Bull. 2021, 168, 156–164. [Google Scholar] [CrossRef]
- Sadanandam, A.; Rosenbaugh, E.G.; Singh, S.; Varney, M.; Singh, R.K. Semaphorin 5A promotes angiogenesis by increasing endothelial cell proliferation, migration, and decreasing apoptosis. Microvasc. Res. 2010, 79, 1–9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fiore, R.; Rahim, B.; Christoffels, V.M.; Moorman, A.F.; Puschel, A.W. Inactivation of the Sema5a gene results in embryonic lethality and defective remodeling of the cranial vascular system. Mol. Cell Biol. 2005, 25, 2310–2319. [Google Scholar] [CrossRef] [Green Version]
- Tan, Y. Basic fibroblast growth factor-mediated lymphangiogenesis of lymphatic endothelial cells isolated from dog thoracic ducts: Effects of heparin. Jpn. J. Physiol 1998, 48, 133–141. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Eriksson, A.; Kubo, H.; Alitalo, K.; Cao, Y.; Thyberg, J. Comparative evaluation of FGF-2-, VEGF-A-, and VEGF-C-induced angiogenesis, lymphangiogenesis, vascular fenestrations, and permeability. Circ. Res. 2004, 94, 664–670. [Google Scholar] [CrossRef] [Green Version]
- Hajrasouliha, A.R.; Sadrai, Z.; Chauhan, S.K.; Dana, R. b-FGF induces corneal blood and lymphatic vessel growth in a spatially distinct pattern. Cornea 2012, 31, 804–809. [Google Scholar] [CrossRef] [Green Version]
- Cao, R.; Ji, H.; Feng, N.; Zhang, Y.; Yang, X.; Andersson, P.; Sun, Y.; Tritsaris, K.; Hansen, A.J.; Dissing, S.; et al. Collaborative interplay between FGF-2 and VEGF-C promotes lymphangiogenesis and metastasis. Proc. Natl. Acad. Sci. USA 2012, 109, 15894–15899. [Google Scholar] [CrossRef] [Green Version]
- Tzeng, H.E.; Chang, A.C.; Tsai, C.H.; Wang, S.W.; Tang, C.H. Basic fibroblast growth factor promotes VEGF-C-dependent lymphangiogenesis via inhibition of miR-381 in human chondrosarcoma cells. Oncotarget 2016, 7, 38566–38578. [Google Scholar] [CrossRef] [Green Version]
- Fang, Y.; Yuan, Y.; Zhang, L.L.; Lu, J.W.; Feng, J.F.; Hu, S.N. Downregulated GBX2 gene suppresses proliferation, invasion and angiogenesis of breast cancer cells through inhibiting the Wnt/beta-catenin signaling pathway. Cancer Biomark 2018, 23, 405–418. [Google Scholar] [CrossRef] [PubMed]
- ACOG Committee on Obstetric Practice. Practice bulletin #33: Diagnosis and management of preeclampsia and eclampsia. Obstet. Gynecol. 2002, 99, 159–167. [Google Scholar]
- American College of Obstreticians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet. Gynecol. 2013, 122, 1122–1131. [Google Scholar]
- Min, J.K.; Cho, Y.L.; Choi, J.H.; Kim, Y.; Kim, J.H.; Yu, Y.S.; Rho, J.; Mochizuki, N.; Kim, Y.M.; Oh, G.T.; et al. Receptor activator of nuclear factor (NF)-kappaB ligand (RANKL) increases vascular permeability: Impaired permeability and angiogenesis in eNOS-deficient mice. Blood 2007, 109, 1495–1502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maeng, Y.S.; Choi, Y.J.; Kim, E.K. TGFBIp regulates differentiation of EPC (CD133(+) C-kit(+) Lin(-) cells) to EC through activation of the Notch signaling pathway. Stem Cells 2015, 33, 2052–2062. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quinlan, A.R.; Hall, I.M. BEDTools: A flexible suite of utilities for comparing genomic features. Bioinformatics 2010, 26, 841–842. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- R Foundation for Statistical Computing. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef] [PubMed] [Green Version]





| Normal Pregnancy (n = 10) | Preeclampsia (n = 10) | p Value | |
|---|---|---|---|
| Maternal age (year) | 33.4 ± 5.1 | 32.8 ± 3.4 | 0.658 |
| Gestational age at delivery (week) | 39.1 ± 1.1 | 35.9 ± 2.2 | 0.583 |
| Gravida | 2.1 ± 1.4 | 1.8 ± 1.2 | 0.560 |
| Blood pressure (mmHg) | |||
| Systolic blood pressure | 114.6 ± 10.5 | 151.4 ± 15.1 | <0.001 |
| Diastolic blood pressure | 75.1 ± 7.5 | 94.6 ± 10.3 | <0.001 |
| Pre-pregnancy BMI (kg/m2) | 23.6 ± 4.9 | 22.2 ± 3.7 | 0.375 |
| Birthweight (g) | 3435 ± 457.0 | 2199.8 ± 654.8 | <0.001 |
| Small gestational age newborn (n) | 1 | 10 | 0.023 |
| GREM1 | Sense-gccagtgcaactctttctac | EPHB3 | Sense-tctcccagattgtcaatacc |
| Antisense-tcttggtaggtggctgtagt | Antisense-ctgtcgtgaaggttgtgtaa | ||
| VEGFA | Sense-actgaggagtccaacatcac | AMOT | Sense-ggaccacatcgtttgtctat |
| Antisense-tctgcattcacatttgttgt | Antisense-aggatctgaatgggagtttt | ||
| THSD7A | Sense-cttgtaacccaccgtgtagt | ANGPTL4 | Sense-gctggacagtaattcagagg |
| Antisense-caagggtgctgttagaagac | Antisense-gtgatgctatgcaccttctc | ||
| SEMA5A | Sense-tcaccctgctcgtctatact | FGF2 | Sense-ggcttctaaatgtgttacgg |
| Antisense-gtggttggttatgctggtat | Antisense-ttatactgcccagttcgttt | ||
| GBX2 | Sense-tagagaaggagttccactgc | GAPDH | Sense-atggggaaggtgaaggtcg |
| Antisense-ttctggaaccagattttcac | Antisense-ggggtcattgatggcaacaata |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, H.; Kwon, J.-Y.; Song, J.; Maeng, Y.-S. Decreased Lymphangiogenic Activities and Genes Expression of Cord Blood Lymphatic Endothelial Progenitor Cells (VEGFR3+/Pod+/CD11b+ Cells) in Patient with Preeclampsia. Int. J. Mol. Sci. 2021, 22, 4237. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22084237
Kwon H, Kwon J-Y, Song J, Maeng Y-S. Decreased Lymphangiogenic Activities and Genes Expression of Cord Blood Lymphatic Endothelial Progenitor Cells (VEGFR3+/Pod+/CD11b+ Cells) in Patient with Preeclampsia. International Journal of Molecular Sciences. 2021; 22(8):4237. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22084237
Chicago/Turabian StyleKwon, Hayan, Ja-Young Kwon, Jeeun Song, and Yong-Sun Maeng. 2021. "Decreased Lymphangiogenic Activities and Genes Expression of Cord Blood Lymphatic Endothelial Progenitor Cells (VEGFR3+/Pod+/CD11b+ Cells) in Patient with Preeclampsia" International Journal of Molecular Sciences 22, no. 8: 4237. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22084237