Structural Studies of the Lipopolysaccharide of Aeromonas veronii bv. sobria Strain K133 Which Represents New Provisional Serogroup PGO1 Prevailing among Mesophilic Aeromonads on Polish Fish Farms
Abstract
:1. Introduction
2. Results
2.1. Bacterial Cultivation, Isolation of LPS, and SDS-PAGE Study
2.2. Serological Studies of the Aeromonas veronii bv. sobria Strain K133 O-PS
2.3. Chemical and Mass Spectrometry Analyses of LPS
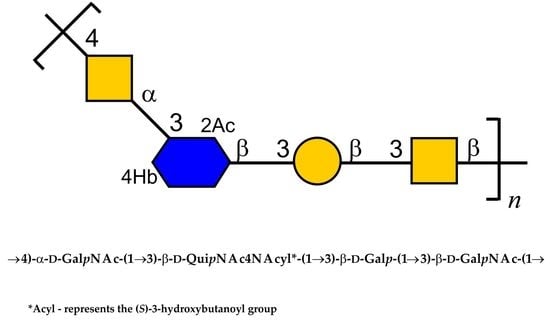
2.4. Structural Studies of O-Polysaccharide (O-PS)

3. Discussion
4. Materials and Methods
4.1. Bacterial Strain, Growth Conditions, and LPS Isolation
4.2. SDS-PAGE
4.3. Serological Studies
4.4. Degradation of LPS and Isolation of O-Polysaccharide
4.5. Chemical Analyses
4.6. NMR Spectroscopy
4.7. MALDI-TOF Mass Spectrometry (MS)
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yahia, N.; Brown, C.A.; Rapley, M.; Chung, M. Level of nutrition knowledge and its association with fat consumption among college students. BMC Public Health 2016, 16, 1047. [Google Scholar] [CrossRef] [Green Version]
- The State of World Fisheries and Aquaculture 2018-Meeting the Sustainable Development Goals. Available online: http://www.fao.org/3/i9540en/i9540en.pdf (accessed on 10 March 2021).
- Pękala-Safińska, A. Contemporary Threats of Bacterial Infections in Freshwater Fish. J. Vet. Res. 2018, 62, 261–267. [Google Scholar] [CrossRef] [Green Version]
- Schulz, P.; Terech-Majewska, E.; Siwicki, A.K.; Kazuń, B.; Demska-Zakęś, K.; Rożyński, M.; Zakęś, Z. Effect of Different Routes of Vaccination against Aeromonas salmonicida on Rearing Indicators and Survival after an Experimental Challenge of Pikeperch (Sander lucioperca) in Controlled Rearing. Vaccines 2020, 8, 476. [Google Scholar] [CrossRef]
- Forn-Cuní, G.; Fulton, K.M.; Smith, J.C.; Twine, S.M.; Mendoza-Barberà, E.; Tomás, J.M.; Merino, S. Polar Flagella Glycosylation in Aeromonas: Genomic Characterization and Involvement of a Specific Glycosyltransferase (Fgi-1) in Heterogeneous Flagella Glycosylation. Front. Microbiol. 2020, 11, 595697. [Google Scholar] [CrossRef]
- Igbinosa, I.H.; Beshiru, A.; Odjadjare, E.E.; Ateba, C.N.; Igbinosa, E.O. Pathogenic potentials of Aeromonas species isolated from aquaculture and abattoir environments. Microb. Pathog. 2017, 107, 185–192. [Google Scholar] [CrossRef]
- Talagrand-Reboul, E.; Jumas-Bilak, E.; Lamy, B. The Social Life of Aeromonas through Biofilm and Quorum Sensing Systems. Front. Microbiol. 2017, 8, 1–19. [Google Scholar] [CrossRef]
- Harnisz, M.; Korzeniewska, E. The prevalence of multidrug-resistant Aeromonas spp. in the municipal wastewater system and their dissemination in the environment. Sci. Total Environ. 2018, 626, 377–383. [Google Scholar] [CrossRef]
- Piotrowska, M.; Przygodzińska, D.; Matyjewicz, K.; Popowska, M. Occurrence and Variety of β-Lactamase Genes among Aeromonas spp. Isolated from Urban Wastewater Treatment Plant. Front. Microbiol. 2017, 8, 1–12. [Google Scholar] [CrossRef] [Green Version]
- El-Gohary, F.A.; Zahran, E.; Abd El-Gawad, E.A.; El-Gohary, A.H.; Abdelhamid, F.M.; El-Mleeh, A.; Elmahallawy, E.K.; Elsayed, M.M. Investigation of the Prevalence, Virulence Genes, and Antibiogram of Motile Aeromonads Isolated from Nile Tilapia Fish Farms in Egypt and Assessment of their Water Quality. Animals 2020, 10, 1432. [Google Scholar] [CrossRef]
- Janda, J.M.; Abbott, S.L. The Genus Aeromonas: Taxonomy, Pathogenicity, and Infection. Clin. Microbiol. Rev. 2010, 23, 35–73. [Google Scholar] [CrossRef] [Green Version]
- Sinha, S.; Shimada, T.; Ramamurthy, T.; Bhattacharya, S.K.; Yamasaki, S.; Takeda, Y.; Nair, G.B. Prevalence, serotype distribution, antibiotic susceptibility and genetic profiles of mesophilic Aeromonas species isolated from hospitalized diarrhoeal cases in Kolkata, India. J. Med. Microbiol. 2004, 53, 527–534. [Google Scholar] [CrossRef]
- Beaz-Hidalgo, R.; Figueras, M.J. Aeromonas spp. whole genomes and virulence factors implicated in fish disease. J. Fish Dis. 2013, 36, 371–388. [Google Scholar] [CrossRef]
- Fernández-Bravo, A.; Figueras, M.J. An Update on the Genus Aeromonas: Taxonomy, Epidemiology, and Pathogenicity. Microorganisms 2020, 8, 129. [Google Scholar] [CrossRef] [Green Version]
- Waldron, K.J.; Rutherford, J.C.; Ford, D.; Robinson, N.J. Metalloproteins and metal sensing. Nature 2009, 460, 823–830. [Google Scholar] [CrossRef]
- Rasmussen-Ivey, C.R.; Figueras, M.J.; McGarey, D.; Liles, M.R. Virulence Factors of Aeromonas hydrophila: In the Wake of Reclassification. Front. Microbiol. 2016, 7, 1–10. [Google Scholar] [CrossRef]
- Raetz, C.R.; Whitfield, C. Lipopolysaccharide endotoxins. Ann. Rev. Biochem. 2002, 71, 635–700. [Google Scholar] [CrossRef] [Green Version]
- Tomás, J.M. The main Aeromonas pathogenic factors. ISRN Microbiol. 2012, 2012, 256261. [Google Scholar] [CrossRef] [Green Version]
- Ebbensgaard, A.; Mordhorst, H.; Aarestrup, F.M.; Hansen, E.B. The Role of Outer Membrane Proteins and Lipopolysaccharides for the Sensitivity of Escherichia coli to Antimicrobial Peptides. Front. Microbiol. 2018, 9, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Anwar, M.A.; Choi, S. Gram-Negative Marine Bacteria: Structural Features of Lipopolysaccharides and Their Relevance for Economically Important Diseases. Mar. Drugs 2014, 12, 2485–2514. [Google Scholar] [CrossRef] [Green Version]
- Sakazaki, R.; Shimada, T. O-serogrouping scheme for mesophilic Aeromonas strains. Jpn. J. Med. Sci. Biol. 1984, 37, 247–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, L.V.; Gross, R.J.; Cheasty, T.; Rowe, B. Extended serogrouping scheme for motile, mesophilic Aeromonas species. J. Clin. Microbiol. 1990, 28, 980–984. [Google Scholar] [CrossRef] [Green Version]
- Cao, H.; Wang, M.; Wang, Q.; Xu, T.; Du, Y.; Li, H.; Qian, C.; Yin, Z.; Wang, L.; Wei, Y.; et al. Identifying genetic diversity of O antigens in Aeromonas hydrophila for molecular serotype detection. PLoS ONE 2018, 13, e0203445. [Google Scholar] [CrossRef] [PubMed]
- Alavandi, S.V.; Ananthan, S. Biochemical characteristics, serogroups, and virulence factors of Aeromonas species isolated from cases of diarrhoea and domestic water samples in Chennai. Indian J. Med. Microbiol. 2003, 21, 233–238. [Google Scholar] [PubMed]
- Dworaczek, K.; Drzewiecka, D.; Pękala-Safińska, A.; Turska-Szewczuk, A. Structural and Serological Studies of the O6-Related Antigen of Aeromonas veronii bv. sobria Strain K557 Isolated from Cyprinus carpio on a Polish Fish Farm, which Contains l-perosamine (4-amino-4,6-dideoxy-l-mannose), a Unique Sugar Characteristic for Aeromonas Serogroup O6. Mar. Drugs 2019, 17, 399. [Google Scholar] [CrossRef] [Green Version]
- Kozińska, A.; Pękala, A. Characteristics of disease spectrum in relation to species, serogroups, and adhesion ability of motile aeromonads in fish. Sci. World J. 2012, 2012, 949358. [Google Scholar] [CrossRef] [Green Version]
- Kozińska, A.; Pękala, A. Serotyping of Aeromonas species isolated from Polish fish farms in relation to species and virulence phenotype of the bacteria. Bull. Vet. Inst. Pulawy 2010, 54, 315–320. Available online: http://agro.icm.edu.pl/agro/element/bwmeta1.element.dl-catalog-a1889583-5c2c-4f57-9f69-92857b6ffa38 (accessed on 10 March 2021).
- Westphal, O.; Jann, K. Bacterial lipopolysaccharide. Extraction with phenol-water and further applications of the procedure. Methods Carbohydr. Chem. 1965, 5, 83–91. [Google Scholar]
- Kozińska, A. Genotypic and Serological Analysis of Domestic Mesophilic Isolates Aeromonas sp. in Terms of Pathogenicity and the Type of Disease Symptoms Caused by Them in Fish. Habilitation Thesis, The National Veterinary Institute—The National Research Institute, Pulawy, Poland, 2009. [Google Scholar]
- Dworaczek, K.; Kurzylewska, M.; Karas, M.A.; Janczarek, M.; Pekala-Safinska, A.; Turska-Szewczuk, A. A Unique Sugar l-Perosamine (4-Amino-4,6-dideoxy-l-mannose) Is a Compound Building Two O-Chain Polysaccharides in the Lipopolysaccharide of Aeromonas hydrophila Strain JCM 3968, Serogroup O6. Mar. Drugs 2019, 17, 254. [Google Scholar] [CrossRef] [Green Version]
- Knirel, Y.A.; Vinogradov, E.; Jimenez, N.; Merino, S.; Tomás, J.M. Structural studies on the R-type lipopolysaccharide of Aeromonas hydrophila. Carbohydr. Res. 2004, 339, 787–793. [Google Scholar] [CrossRef]
- Pieretti, G.; Corsaro, M.M.; Lanzetta, R.; Parrilli, M.; Nicolaus, B.; Gambacorta, A.; Lindner, B.; Holst, O. Structural Characterization of the Core Region of the Lipopolysaccharide from the Haloalkaliphilic Halomonas pantelleriensis: Identification of the Biological O-Antigen Repeating Unit. Eur. J. Organ. Chem. 2008, 2008, 721–728. [Google Scholar] [CrossRef]
- Domon, B.; Costello, C.E. A systematic nomenclature for carbohydrate fragmentations in FAB-MS/MS spectra of glycoconjugates. Glycoconjug. J. 1988, 5, 397–409. [Google Scholar] [CrossRef]
- Leontein, K.; Lindberg, B.; Lōnngren, J. Assignment of absolute configuration of sugars by g.l.c. of their acetylated glycosides formed from chiral alcohols. Carbohydr. Res. 1978, 62, 359–362. [Google Scholar] [CrossRef]
- Kenne, L.; Lindberg, B.; Rahman, M.M.; Mosihuzzaman, M. Structural studies of the Vibrio mimicus W-26768 O-antigen polysaccharide. Carbohydr. Res. 1993, 243, 131–138. [Google Scholar] [CrossRef]
- Bock, K.; Pedersen, C. Carbon-13 Nuclear Magnetic Resonance Spectroscopy of Monosaccharides. In Advances in Carbohydrate Chemistry and Biochemistry; Tipson, R.S., Horton, D., Eds.; Academic Press: Cambridge, MA, USA, 1983; Volume 41, pp. 27–66. [Google Scholar] [CrossRef]
- Lipkind, G.M.; Shashkov, A.S.; Knirel, Y.A.; Vinogradov, E.V.; Kochetkov, N.K. A computer-assisted structural analysis of regular polysaccharides on the basis of 13C-n.m.r. data. Carbohydr. Res. 1988, 175, 59–75. [Google Scholar] [CrossRef]
- Kocharova, N.A.; Perepelov, A.V.; Zatonsky, G.V.; Shashkov, A.S.; Knirel, Y.A.; Jansson, P.E.; Weintraub, A. Structural studies of the O-specific polysaccharide of Vibrio cholerae O8 using solvolysis with triflic acid. Carbohydr. Res. 2001, 330, 83–92. [Google Scholar] [CrossRef]
- Jansson, P.E.; Kenne, L.; Widmalm, G. Computer-assisted structural analysis of polysaccharides with an extended version of CASPER using 1H- and 13C-n.m.r. data. Carbohydr. Res. 1989, 188, 169–191. [Google Scholar] [CrossRef]
- Hanniffy, O.M.; Shashkov, A.S.; Senchenkova, S.N.; Tomshich, S.V.; Komandrova, N.A.; Romanenko, L.A.; Knirel, Y.A.; Savage, A.V. Structure of an acidic O-specific polysaccharide of Pseudoalteromonas haloplanktis type strain ATCC 14393 containing 2-acetamido-2-deoxy-D- and -L-galacturonic acids and 3-(N-acetyl-D-alanyl)amino-3,6-dideoxy-D-glucose. Carbohydr. Res. 1999, 321, 132–138. [Google Scholar] [CrossRef]
- Shashkov, A.S.; Lipkind, G.M.; Knirel, Y.A.; Kochetkov, N.K. Stereochemical factors determining the effects of glycosylation on the 13C chemical shifts in carbohydrates. Magnet. Reson. Chem. 1988, 26, 735–747. [Google Scholar] [CrossRef]
- Perepelov, A.V.; Babicka, D.; Senchenkova, S.N.; Shashkov, A.S.; Moll, H.; Rozalski, A.; Zähringer, U.; Knirel, Y.A. Structure of the O-specific polysaccharide of Proteus vulgaris O4 containing a new component of bacterial polysaccharides, 4,6-dideoxy-4-{N-[(R)-3-hydroxybutyryl]-l-alanyl}amino-d-glucose. Carbohydr. Res. 2001, 331, 195–202. [Google Scholar] [CrossRef]
- Toukach, P.V.; Egorova, K.S. Carbohydrate structure database merged from bacterial, archaeal, plant and fungal parts. Nucleic Acids Res. 2016, 44, D1229–D1236. [Google Scholar] [CrossRef]
- Lirski, A.; Myszkowski, L. Polish aquaculture in 2016 based on the analysis of questionnaire RRW-22. Part 1. Komun. Ryb. 2017, 6, 20–27. Available online: https://www.infish.com.pl/article/polska-akwakultura-w-2016-roku-na-podstawie-analizy-kwestionariuszy-rrw-22-cz%C4%99%C5%9B%C4%87-1 (accessed on 10 March 2021).
- Jimenez, N.; Canals, R.; Lacasta, A.; Kondakova, A.N.; Lindner, B.; Knirel, Y.A.; Merino, S.; Regué, M.; Tomás, J.M. Molecular analysis of three Aeromonas hydrophila AH-3 (serotype O34) lipopolysaccharide core biosynthesis gene clusters. J. Bacteriol. 2008, 190, 3176–3184. [Google Scholar] [CrossRef] [Green Version]
- Turska-Szewczuk, A.; Lindner, B.; Komaniecka, I.; Kozinska, A.; Pekala, A.; Choma, A.; Holst, O. Structural and immunochemical studies of the lipopolysaccharide from the fish pathogen, Aeromonas bestiarum strain K296, serotype O18. Mar. Drugs 2013, 11, 1235–1255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, Z.; Li, J.; Vinogradov, E.; Altman, E. Structural studies of the core region of Aeromonas salmonicida subsp. salmonicida lipopolysaccharide. Carbohydr. Res. 2006, 341, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Shashkov, A.S.; Paramonov, N.A.; Veremeychenko, S.P.; Grosskurth, H.; Zdorovenko, G.M.; Knirel, Y.A.; Kochetkov, N.K. Somatic antigens of pseudomonads: Structure of the O-specific polysaccharide of Pseudomonas fluorescens biovar B, strain IMV 247. Carbohydr. Res. 1998, 306, 297–303. [Google Scholar] [CrossRef]
- Pantophlet, R.; Haseley, S.R.; Vinogradov, E.V.; Brade, L.; Holst, O.; Brade, H. Chemical and antigenic structure of the O-polysaccharide of the lipopolysaccharides from two Acinetobacter haemolyticus strains differing only in the anomeric configuration of one glycosyl residue in their O-antigens. Eur. J. Biochem. 1999, 263, 587–595. [Google Scholar] [CrossRef]
- Hermansson, K.; Jansson, P.E.; Holme, T.; Gustavsson, B. Structural studies of the Vibrio cholerae O:5 O-antigen polysaccharide. Carbohydr. Res. 1993, 248, 199–211. [Google Scholar] [CrossRef]
- Perepelov, A.V.; Guo, X.; Filatov, A.V.; Shashkov, A.S.; Senchenkova, S.N.; Li, B. Structure elucidation and gene cluster annotation of the O-antigen of Vibrio cholerae O100 containing two rarely occurred amino sugar derivatives. Carbohydr. Res. 2019, 472, 98–102. [Google Scholar] [CrossRef]
- Kilcoyne, M.; Shashkov, A.S.; Perepelov, A.V.; Nazarenko, E.L.; Gorshkova, R.P.; Ivanova, E.P.; Widmalm, G.; Savage, A.V. Structure of the O-specific polysaccharide from Shewanella japonica type strain KMM 3299T containing the rare amino sugar Fuc4NAc. Carbohydr. Res. 2005, 340, 1557–1561. [Google Scholar] [CrossRef]
- Pieretti, G.; Corsaro, M.M.; Lanzetta, R.; Parrilli, M.; Vilches, S.; Merino, S.; Tomás, J.M. Structure of the Core Region from the Lipopolysaccharide of Plesiomonas shigelloides Strain 302-73 (Serotype O1). Eur. J. Organ. Chem. 2009, 2009, 1365–1371. [Google Scholar] [CrossRef]
- Zdorovenko, E.L.; Varbanets, L.D.; Shashkov, A.S.; Kiprianova, E.A.; Knirel, Y.A. Structure of the O-polysaccharide of the lipopolysaccharide of Pseudomonas chlororaphis subsp. aureofaciens UCM B-306. Carbohydr. Res. 2015, 410, 47–50. [Google Scholar] [CrossRef]
- Kokoulin, M.S.; Komandrova, N.A.; Kalinovskiy, A.I.; Tomshich, S.V.; Romanenko, L.A.; Vaskovsky, V.V. Structure of the O-specific polysaccharide from the deep-sea marine bacterium Idiomarina abyssalis KMM 227(T) containing a 2-O-sulfate-3-N-(4-hydroxybutanoyl)-3,6-dideoxy-d-glucose. Carbohydr. Res. 2015, 413, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Kondakova, A.N.; Novototskaya-Vlasova, K.A.; Shashkov, A.S.; Drutskaya, M.S.; Senchenkova, S.N.; Shcherbakova, V.A.; Gilichinsky, D.A.; Nedospasov, S.A.; Knirel, Y.A. Structure of an acidic polysaccharide isolated from Psychrobacter maritimus 3pS containing a bacillosamine derivative. Carbohydr. Res. 2012, 359, 7–10. [Google Scholar] [CrossRef] [PubMed]
- Ovchinnikova, O.G.; Arbatsky, N.P.; Chizhov, A.O.; Kocharova, N.A.; Shashkov, A.S.; Rozalski, A.; Knirel, Y.A. Structure of a polysaccharide from Providencia rustigianii O11 containing a novel amide of 2-acetamido-2-deoxygalacturonic acid with L-glutamyl-L-alanine. Carbohydr. Res. 2012, 349, 95–102. [Google Scholar] [CrossRef]
- Fregolino, E.; Gargiulo, V.; Lanzetta, R.; Parrilli, M.; Holst, O.; Castro, C.D. Identification and structural determination of the capsular polysaccharides from two Acinetobacter baumannii clinical isolates, MG1 and SMAL. Carbohydr. Res. 2011, 346, 973–977. [Google Scholar] [CrossRef] [Green Version]
- Sadovskaya, I.; Brisson, J.R.; Altman, E.; Mutharia, L.M. Structural studies of the lipopolysaccharide O-antigen and capsular polysaccharide of Vibrio anguillarum serotype O:2. Carbohydr. Res. 1996, 283, 111–127. [Google Scholar] [CrossRef]
- Chowdhury, T.A.; Jansson, P.E.; Lindberg, B.; Lindberg, J.; Gustafsson, B.; Holme, T. Structural studies of the Vibrio cholerae O:3 O-antigen polysaccharide. Carbohydr. Res. 1991, 215, 303–314. [Google Scholar] [CrossRef]
- Tsai, C.M.; Frasch, C.E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal. Biochem. 1982, 119, 115–119. [Google Scholar] [CrossRef]
- Sidorczyk, Z.; Zych, K.; Toukach, F.V.; Arbatsky, N.P.; Zablotni, A.; Shashkov, A.S.; Knirel, Y.A. Structure of the O-polysaccharide and classification of Proteus mirabilis strain G1 in Proteus serogroup O3. Eur. J. Biochem. 2002, 269, 1406–1412. [Google Scholar] [CrossRef]
- Drzewiecka, D.; Arbatsky, N.P.; Shashkov, A.S.; Staczek, P.; Knirel, Y.A.; Sidorczyk, Z. Structure and serological properties of the O-antigen of two clinical Proteus mirabilis strains classified into a new Proteus O77 serogroup. FEMS Immunol. Med. Microbiol. 2008, 54, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Ciucanu, I.; Kerek, F. A simple and rapid method for the permethylation of carbohydrates. Carbohydr. Res. 1984, 131, 209–217. [Google Scholar] [CrossRef]
- Komaniecka, I.; Choma, A.; Lindner, B.; Holst, O. The structure of a novel neutral lipid A from the lipopolysaccharide of Bradyrhizobium elkanii containing three mannose units in the backbone. Chemistry 2010, 16, 2922–2929. [Google Scholar] [CrossRef] [PubMed]
- Silipo, A.; Molinaro, A.; Sturiale, L.; Dow, J.M.; Erbs, G.; Lanzetta, R.; Newman, M.A.; Parrilli, M. The elicitation of plant innate immunity by lipooligosaccharide of Xanthomonas campestris. J. Biol. Chem. 2005, 280, 33660–33668. [Google Scholar] [CrossRef] [PubMed] [Green Version]








| Type of PGO1 Antiserum | PGO1 Cells | K133 Cells | K133 LPS |
|---|---|---|---|
| intact | 512,000 | 128,000 | 64,000 |
| adsorbed on K133 cells | 64,000 | <1000 | <1000 |
| Observed Mass [M − H]− | Calculated Mass [M − H]− | Monoisotopic Mass [M] | Composition |
|---|---|---|---|
| 1768.196 | 1768.181 | 1769.188 | HexN2P2[14:0(3-OH)]4(12:0)2 |
| 1796.230 | 1796.139 | 1797.146 | HexN2P2[14:0(3-OH)]3[i15:0(3-OH)](12:0)2 |
| 1824.261 | 1824.243 | 1825.250 | HexN2P2[14:0(3-OH)]4(14:0)2 |
| 1903.608 | 1903.598 | 1904.605 | [HexNAc1HexN1Hex2Hep5KdoanhP]-COO |
| 1947.605 | 1947.588 | 1948.595 | HexNAc1HexN1Hex2Hep5KdoanhP |
| 2027.614 | 2027.554 | 2028.561 | HexNAc1HexN1Hex2Hep5KdoanhP2 |
| 1705.536 | 1705.568 | 1706.576 | HexNAc1HexN1Hex1Hep5Kdoanh |
| 1867.586 | 1867.621 | 1868.629 | HexNAc1HexN1Hex2Hep5Kdoanh |
| 2708.903 | 2708.953 | 2709.961 | 6dHexNAcNAcyl1HexNAc3HexN1Hex3Hep5Kdoanh |
| Residue | Chemical Shifts (δ, ppm) | |||||||
|---|---|---|---|---|---|---|---|---|
| H-1 C-1 | H-2 C-2 | H-3 C-3 | H-4 C-4 | H-5 C-5 | H-6 C-6 | NAc | ||
| →4)-α-d-GalpNAc-(1→ | A | 5.16 98.2 | 4.12 50.6 | 3.89 68.8 | 4.21 76.3 | 3.87 71.4 | 3.73; 3.87 61.3 | 2.05 23.7; 175.5 |
| →3)-β-d-GalpNAc-(1→ | B | 4.74 103.3 | 4.05 52.8 | 3.93 80.8 | 4.17 69.5 | 3.71 75.9 | 3.76; 3.81 62.3 | 2.07 23.7; 176.4 |
| →3)-β-d-QuipNAc4NAcyl-(1→ | C | 4.74 103.3 | 3.87 56.2 | 3.87 75.9 | 3.87 58.0 | 3.54 72.3 | 1.21 17.7 | 2.03 23.7; 176.4 |
| →3)-β-d-Galp-(1→ | D | 4.46 106.1 | 3.61 71.2 | 3.70 82.8 | 4.10 69.6 | 3.65 75.9 | 3.76; 3.81 62.3 | |
| (S)-3-hydroxybutanoyl | Hb | 175.0 | 2.35 46.2 | 4.20 66.0 | 1.25 23.7 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dworaczek, K.; Kurzylewska, M.; Laban, M.; Drzewiecka, D.; Pękala-Safińska, A.; Turska-Szewczuk, A. Structural Studies of the Lipopolysaccharide of Aeromonas veronii bv. sobria Strain K133 Which Represents New Provisional Serogroup PGO1 Prevailing among Mesophilic Aeromonads on Polish Fish Farms. Int. J. Mol. Sci. 2021, 22, 4272. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22084272
Dworaczek K, Kurzylewska M, Laban M, Drzewiecka D, Pękala-Safińska A, Turska-Szewczuk A. Structural Studies of the Lipopolysaccharide of Aeromonas veronii bv. sobria Strain K133 Which Represents New Provisional Serogroup PGO1 Prevailing among Mesophilic Aeromonads on Polish Fish Farms. International Journal of Molecular Sciences. 2021; 22(8):4272. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22084272
Chicago/Turabian StyleDworaczek, Katarzyna, Maria Kurzylewska, Magdalena Laban, Dominika Drzewiecka, Agnieszka Pękala-Safińska, and Anna Turska-Szewczuk. 2021. "Structural Studies of the Lipopolysaccharide of Aeromonas veronii bv. sobria Strain K133 Which Represents New Provisional Serogroup PGO1 Prevailing among Mesophilic Aeromonads on Polish Fish Farms" International Journal of Molecular Sciences 22, no. 8: 4272. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22084272