PDLCs and EPCs Co-Cultured on Ta Discs: A Golden Fleece for “Compromised” Osseointegration
Abstract
:1. Introduction
2. Results
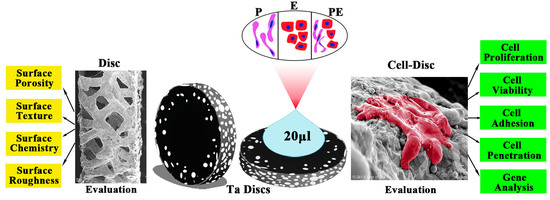
2.1. Disc Preparation and Evaluation
2.1.1. Surface Porosity and Surface Texture
2.1.2. Surface Chemistry
2.1.3. Surface Roughness
2.2. Cell Isolation and Culture
2.3. Cell-Disc Preparation and Evaluation
2.3.1. Cell Proliferation and Viability
2.3.2. Cell Adhesion and Penetration
2.3.3. RT-qPCR for the Expression of Osteogenic and Angiogenic Genes
3. Discussion
4. Materials and Methods
4.1. Disc Preparation
4.2. Disc Evaluation
4.2.1. Surface Porosity
4.2.2. Surface Texture
4.2.3. Surface Chemistry
4.2.4. Surface Roughness
4.3. Cell Isolation and Culturing
4.3.1. Rabbit PDLCs
4.3.2. Rabbit CD34+CD133+EPCs
4.4. Cell-Disc Complex (Cell-Disc) Preparation
4.5. Cell-Disc Evaluation
4.5.1. Cell Proliferation
4.5.2. Cell Viability
4.5.3. Cell Adhesion
4.5.4. Cell Penetration
4.5.5. Real-Time Quantitative Reverse-Transcription Polymerase Chain Reaction (RT-qPCR) for the Expression of Osteogenic and Angiogenic Genes
4.6. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lanza, R.; Langer, R.; Vacanti, J.P. Principles of Tissue Engineering; Academic Press: Cambridge, MA, USA, 2011. [Google Scholar]
- Chopra, H.; Hans, M.K.; Shetty, S. Stem cells-the hidden treasure: A strategic review. Dent. Res. J. 2013, 10, 421–427. [Google Scholar]
- Seo, B.-M.; Miura, M.; Gronthos, S.; Bartold, P.M.; Batouli, S.; Brahim, J.; Young, M.; Robey, P.G.; Wang, C.Y.; Shi, S. Investigation of multipotent postnatal stem cells from human periodontal ligament. Lancet 2004, 364, 149–155. [Google Scholar] [CrossRef]
- Chopra, H.; Hung, M.K.; Kwong, D.L.; Zhang, C.F.; Pow, E.H.N. Insights into endothelial progenitor cells: Origin, classification, potentials, and prospects. Stem Cells Int. 2018, 2018, 1–24. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Brånemark, P.-I.; Hansson, H.-A.; Lindström, J. Osseointegrated titanium implants: Requirements for ensuring a long-Lasting, direct bone-to-implant anchorage in man. Acta Orthop. Scand. 1981, 52, 155–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bobyn, J.D.; Stackpool, G.J.; Hacking, S.A.; Tanzer, M.; Krygier, J.J. Characteristics of bone ingrowth and interface mechanics of a new porous tantalum biomaterial. J. Bone Joint Surg. Br. 1999, 81, 907–914. [Google Scholar]
- Reach, J.S., Jr.; Dickey, I.D.; Zobitz, M.E.; Adams, J.E.; Scully, S.P.; Lewallen, D.G. Direct tendon attachment and healing to porous tantalum: An experimental animal study. J. Bone Jt. Surg. A 2007, 89, 1000–1009. [Google Scholar] [CrossRef]
- Tanzer, M.; Bobyn, J.; Krygier, J.; Karabasz, D. Histopathologic retrieval analysis of clinically failed porous tantalum osteonecrosis implants. J. Bone Jt. Surg. Am. 2008, 90, 1282–1289. [Google Scholar] [CrossRef] [PubMed]
- Hanzlik, J.A.; Day, J.S. Acknowledged Contributors: Ingrowth Retrieval Study, G., Bone ingrowth in well-fixed retrieved porous tantalum implants. J. Arthroplast. 2013, 28, 922–927. [Google Scholar] [CrossRef] [Green Version]
- Zhao, G.; Raines, A.; Wieland, M.; Schwartz, Z.; Boyan, B. Requirement for both micron- and submicron scale structure for synergistic responses of osteoblasts to substrate surface energy and topography. Biomaterials 2007, 28, 2821–2829. [Google Scholar] [CrossRef] [Green Version]
- Iaculli, F.; Di Filippo, E.S.; Piattelli, A.; Mancinelli, R.; Fulle, S. Dental pulp stem cells grown on dental implant titanium surfaces: An in vitro evaluation of differentiation and micro RNA s expression. J. Biomed. Mater. Res. B Appl. Biomater. 2017, 105, 953–965. [Google Scholar] [CrossRef]
- Wessely-Szponder, J.; Szponder, T.; Bobowiec, R. Different activation of monocyte-derived macrophages by antimicrobial peptides at a titanium tibial implantation in rabbits. Res. Vet. Sci. 2017, 115, 201–210. [Google Scholar] [CrossRef]
- Ocaña, R.P.; Rabelo, G.D.; Sassi, L.M.; Rodrigues, V.P.; Alves, F.A. Implant osseointegration in irradiated bone: An experimental study. J. Periodontal Res. 2016, 52, 505–511. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, J.; Chen, M.; Liu, D. Biological activity evaluation of magnesium fluoride coated Mg-Zn-Zr alloy in vivo. Mater. Sci. Eng. C 2017, 75, 1068–1074. [Google Scholar] [CrossRef]
- Keller, J.C.; Stanford, C.M.; Wightman, J.P.; Draughn, R.A.; Zaharias, R. Characterizations of titanium implant surfaces. III. J. Biomed. Mater. Res. 1994, 28, 939–946. [Google Scholar] [CrossRef]
- Bowers, K.T.; Keller, J.C.; Randolph, B.A.; Wick, D.G.; Michaels, C.M. Optimization of surface micromorphology for enhanced osteoblast responses in vitro. Int. J. Oral Maxillofac. Implant. 1992, 7, 302–310. [Google Scholar]
- Stanford, C.; Keller, J.; Solursh, M. Bone cell expression on titanium surfaces is altered by sterilization treatments. J. Dent. Res. 1994, 73, 1061–1071. [Google Scholar] [CrossRef] [PubMed]
- Vezeau, P.; Koorbusch, G.; Draughn, R.; Keller, J. Effects of multiple sterilization on surface characteristics and in vitro biologic responses to titanium. J. Oral Maxillofac. Surg. 1996, 54, 738–746. [Google Scholar] [CrossRef]
- Neyt, J.G.; Buckwalter, J.A.; Carroll, N.C. Use of animal models in musculoskeletal research. Iowa Orthop. J. 1998, 18, 118–123. [Google Scholar] [PubMed]
- Lee, J.; Sieweke, J.H.; Rodriguez, N.A.; Schupbach, P.; Lindström, H.; Susin, C.; Wikesjö, U.M.E. Evaluation of nano-technology-modified zirconia oral implants: A study in rabbits. J. Clin. Periodontol. 2009, 36, 610–617. [Google Scholar] [CrossRef]
- Li, J.Y.; Pow, E.H.N.; Zheng, L.W.; Ma, L.; Kwong, D.L.W.; Cheung, L.K. Effects of calcium phosphate nanocrystals on osseointegration of titanium implant in irradiated bone. BioMed Res. Int. 2015, 2015, 1–6. [Google Scholar] [CrossRef]
- Lee, J.W.; Wen, H.B.; Gubbi, P.; Romanos, G.E. New bone formation and trabecular bone microarchitecture of highly porous tantalum compared to titanium implant threads: A pilot canine study. Clin. Oral Implant. Res. 2017, 29, 164–174. [Google Scholar] [CrossRef]
- Fraser, D.; Mendonca, G.; Sartori, E.; Funkenbusch, P.; Ercoli, C.; Meirelles, L. Bone response to porous tantalum implants in a gap-healing model. Clin. Oral Implant. Res. 2019, 30, 156–168. [Google Scholar] [CrossRef]
- Chopra, H.; Liao, C.; Zhang, C.F.; Pow, E.H.N. Lapine periodontal ligament stem cells for musculoskeletal research in preclinical animal trials. J. Transl. Med. 2018, 16, 1–14. [Google Scholar] [CrossRef]
- Chopra, H.; Han, Y.; Zhang, C.; Pow, E.H.N. CD133+CD34+ cells can give rise to EPCs: A comparative rabbit and human study. Blood Cells, Mol. Dis. 2021, 86, 102487. [Google Scholar] [CrossRef] [PubMed]
- Winning, L.; El Karim, I.A.; Lundy, F.T. A comparative analysis of the osteogenic potential of dental mesenchymal stem cells. Stem Cells Dev. 2019, 28, 1050–1058. [Google Scholar] [CrossRef] [PubMed]
- Pandula, P.K.C.P.; Samaranayake, L.P.; Jin, L.J.; Zhang, C.F. Human umbilical vein endothelial cells synergize osteo/odontogenic differentiation of periodontal ligament stem cells in 3D cell sheets. J. Periodontal Res. 2013, 49, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Rajaraman, R.; Rounds, D.; Yen, S.; Rembaum, A. A scanning electron microscope study of cell adhesion and spreading in vitro. Exp. Cell Res. 1974, 88, 327–339. [Google Scholar] [CrossRef]
- Klein, M.O.; Bijelic, A.; Ziebart, T.; Koch, F.; Kämmerer, P.W.; Wieland, M.; Konerding, M.A.; Al-Nawas, B. Submicron Scale-Structured Hydrophilic Titanium Surfaces Promote Early Osteogenic Gene Response for Cell Adhesion and Cell Differentiation. Clin. Implant. Dent. Relat. Res. 2011, 15, 166–175. [Google Scholar] [CrossRef] [PubMed]
- An, N.; Schedle, A.; Wieland, M.; Andrukhov, O.; Matejka, M.; Rausch-Fan, X. Proliferation, behavior, and cytokine gene expression of human umbilical vascular endothelial cells in response to different titanium surfaces. J. Biomed. Mater. Res. Part A 2009, 93, 364–372. [Google Scholar] [CrossRef] [PubMed]
- Ziebart, T.; Schnell, A.; Walter, C.; Kämmerer, P.W.; Pabst, A.; Lehmann, K.M.; Ziebart, J.; Klein, M.O.; Al-Nawas, B. Interactions between endothelial progenitor cells (EPC) and titanium implant surfaces. Clin. Oral Investig. 2012, 17, 301–309. [Google Scholar] [CrossRef]
- Heo, Y.-Y.; Um, S.; Kim, S.-K.; Park, J.-M.; Seo, B. Responses of periodontal ligament stem cells on various titanium surfaces. Oral Dis. 2010, 17, 320–327. [Google Scholar] [CrossRef]
- Zou, X.; Li, H.; Baatrup, A.; Lind, M.; Bünger, C. Engineering of bone tissue with porcine bone marrow stem cells in three-dimensional trabecular metal: In vitro and in vivo studies. APMIS Suppl. 2003, 109, 127–132. [Google Scholar]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chopra, H.; Han, Y.; Zhang, C.F.; Pow, E.H.N. PDLCs and EPCs Co-Cultured on Ta Discs: A Golden Fleece for “Compromised” Osseointegration. Int. J. Mol. Sci. 2021, 22, 4486. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22094486
Chopra H, Han Y, Zhang CF, Pow EHN. PDLCs and EPCs Co-Cultured on Ta Discs: A Golden Fleece for “Compromised” Osseointegration. International Journal of Molecular Sciences. 2021; 22(9):4486. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22094486
Chicago/Turabian StyleChopra, Hitesh, Yuanyuan Han, Cheng F. Zhang, and Edmond H. N. Pow. 2021. "PDLCs and EPCs Co-Cultured on Ta Discs: A Golden Fleece for “Compromised” Osseointegration" International Journal of Molecular Sciences 22, no. 9: 4486. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22094486