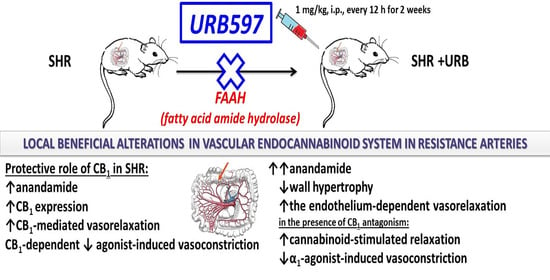
Beneficial Changes in Rat Vascular Endocannabinoid System in Primary Hypertension and under Treatment with Chronic Inhibition of Fatty Acid Amide Hydrolase by URB597
Abstract
:1. Introduction
2. Results
2.1. General
2.2. Influence of Hypertension; An Antagonist of the CB1 Receptor, AM251; and Chronic Administration of URB597 on Vasoconstrictor Responses to Phenylephrine and U46619
2.3. Influence of Hypertension and Chronic Administration of URB597 on Vasodilatory Effects of Acetylcholine and Sodium Nitroprusside
2.4. Influence of Hypertension; an Antagonist of the CB1 Receptor, AM251; and Chronic Administration of URB597 on Vasodilatory Effects of Methanandamide
2.5. Influence of Hypertension and Chronic Administration of URB597 on Vascular Remodeling and Immunohistochemical Staining of CB1 Receptors
2.6. Influence of Hypertension and Chronic Administration of URB597 on Expression of CB1 and FAAH in Isolated Mesenteric G3 Arteries and Aorta
2.7. Influence of Hypertension and Chronic Administration of URB597 on Endocannabinoid Levels in Isolated Mesenteric G3 Arteries and Aortas
3. Discussion
3.1. Vascular Changes Related to Hypertension
3.2. Vascular Changes Induced by URB597 in SHR and in WKY
3.3. Limitations
4. Material and Methods
4.1. Animals
4.2. Vessel Preparation
4.3. Concentration–Response Curves
4.4. Western Blots
4.5. Thickness of Media in Mesenteric G3 Arteries and Aortas
4.6. Immunohistochemistry
4.7. Determination of Endocannabinoids
4.8. Drugs
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-AG | 2-Arachidonoylglycerol |
| 2-AG-d8 | Octadeuterated 2-arachidonoyl glycerol |
| Ach | Acetylcholine |
| AEA | Anandamide |
| AEA-d8 | Octadeuterated anandamide |
| ANOVA | Analysis of variance |
| CRCs | Concentration-response curves |
| DMF | N,N-Dimethylformamide |
| DMSO | Dimethyl sulfoxide |
| DOCA | Deoxycorticosterone acetate |
| FAAH | Fatty acid amide hydrolase |
| H + E | Hematoxylin and eosin |
| i.p. | Intraperitoneally |
| Kca | Calcium-dependent potassium channels |
| LC–MS | Ultrahigh performance liquid chromatography-tandem mass spectrometry |
| L-NAME | NG-nitro-L-arginine methyl ester |
| MAGL | Monoacylglycerol lipase |
| MethAEA | Methanandamide |
| MRM | Multiple reaction monitoring |
| N.D. | Not determined |
| Phe | Phenylephrine |
| RIPA | Radioimmunoprecipitation assay |
| SBP | Systolic blood pressure |
| SHR | Spontaneously hypertensive rats |
| sMA | Small mesenteric G3 arteries |
| SNP | Sodium nitroprusside |
| TRPV1 | Transient receptor potential vanilloid type 1 |
| TXA2 | Thromboxane A2 |
| UNX | Uninephrectomized normotensive |
| URB597 | 3’-(Aminocarbonyl)[1,1’-biphenyl]-3-yl)-cyclohexylcarbamate |
| WKY | Wistar Kyoto rats |
References
- Malinowska, B.; Toczek, M.; Pędzińska-Betiuk, A.; Schlicker, E. Cannabinoids in arterial, pulmonary and portal hypertension—Mechanisms of action and potential therapeutic significance. Br. J. Pharmacol. 2019, 176, 1395–1411. [Google Scholar] [CrossRef]
- Toczek, M.; Malinowska, B. Enhanced endocannabinoid tone as a potential target of pharmacotherapy. Life Sci. 2018, 204, 20–45. [Google Scholar] [CrossRef] [PubMed]
- Brown, I.A.; Diederich, L.; Good, M.E.; DeLalio, L.J.; Murphy, S.A.; Cortese-Krott, M.M.; Hall, J.L.; Le, T.H.; Isakson, B.E. Vascular Smooth Muscle Remodeling in Conductive and Resistance Arteries in Hypertension. Arter. Thromb. Vasc. Biol. 2018, 38, 1969–1985. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Karpińska, O.; Toczek, M.; Harasim, E.; Kasacka, I.; Malinowska, B. Protective role of cannabinoid CB 1 receptors and vascular effects of chronic administration of FAAH inhibitor URB597 in DOCA-salt hypertensive rats. Life Sci. 2016, 151, 288–299. [Google Scholar] [CrossRef] [PubMed]
- Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Sadowska, O.; Kozłowski, M.; Kusaczuk, M.; Kasacka, I.; Malinowska, B. Vasodilatory effects of cannabidiol in human pulmonary and rat small mesenteric arteries: Modification by hypertension and the potential pharmacological opportunities. J. Hypertens. 2020, 38, 896–911. [Google Scholar] [CrossRef] [PubMed]
- Bátkai, S.; Pacher, P.; Osei-Hyiaman, D.; Radaeva, S.; Liu, J.; Harvey-White, J.; Offertáler, L.; Mackie, K.; Rudd, M.A.; Bukoski, R.D.; et al. Endocannabinoids Acting at Cannabinoid-1 Receptors Regulate Cardiovascular Function in Hypertension. Circulation 2004, 110, 1996–2002. [Google Scholar] [CrossRef] [Green Version]
- Wheal, A.J.; Randall, M.D. Effects of hypertension on vasorelaxation to endocannabinoids in vitro. Eur. J. Pharmacol. 2009, 603, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Ho, W.-S.V.; Gardiner, S.M. Acute hypertension reveals depressor and vasodilator effects of cannabinoids in conscious rats. Br. J. Pharmacol. 2009, 156, 94–104. [Google Scholar] [CrossRef] [Green Version]
- Godlewski, G.; Alapafuja, S.O.; Batkai, S.; Nikas, S.P.; Cinar, R.; Offertáler, L.; Osei-Hyiaman, D.; Liu, J.; Mukhopadhyay, B.; Harvey-White, J.; et al. Inhibitor of Fatty Acid Amide Hydrolase Normalizes Cardiovascular Function in Hypertension without Adverse Metabolic Effects. Chem. Biol. 2010, 17, 1256–1266. [Google Scholar] [CrossRef] [Green Version]
- Bondarenko, A.I. Cannabinoids and Cardiovascular System. Adv. Exp. Med. Biol. 2019, 1162, 63–87. [Google Scholar] [CrossRef]
- Toczek, M.; Schlicker, E.; Grzęda, E.; Malinowska, B. Enhanced function of inhibitory presynaptic cannabinoid CB1 receptors on sympathetic nerves of DOCA–salt hypertensive rats. Life Sci. 2015, 138, 78–85. [Google Scholar] [CrossRef]
- Malinowska, B.; Toczek, M.; Kossakowski, R. Function of presynaptic inhibitory cannabinoid CB1 receptors on sympathetic nerves of spontaneously hypertensive rats. Proceedings of 83rd Annual Meeting of the German Society for Experimental and Clinical Pharmacology and Toxicology (DGPT) and the 19th Annual Meeting of the Association of the Clinical Pharmacology Germany (VKliPha) with Contribution of the Arbeitsgemeinschaft Für Angewandte Humanpharmakologie E. V. (AGAH). Naunyn-Schmiedeberg’s Arch. Pharmacol. 2017, 390 (Suppl. 1), S36. [Google Scholar]
- Guo, Z.; Liu, Y.-X.; Yuan, F.; Ma, H.-J.; Maslov, L.; Zhang, Y. Enhanced vasorelaxation effect of endogenous anandamide on thoracic aorta in renal vascular hypertension rats. Clin. Exp. Pharmacol. Physiol. 2015, 42, 950–955. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Nádasy, G.L.; Turu, G.; Soltész-Katona, E.; Tóth, Z.E.; Balla, A.; Catt, K.J.; Hunyady, L. Angiotensin II Induces Vascular Endocannabinoid Release, Which Attenuates Its Vasoconstrictor Effect via CB1 Cannabinoid Receptors. J. Biol. Chem. 2012, 287, 31540–31550. [Google Scholar] [CrossRef] [Green Version]
- Szekeres, M.; Nádasy, G.L.; Turu, G.; Soltész-Katona, E.; Benyó, Z.; Offermanns, S.; Ruisanchez, É.; Szabó, E.; Takáts, Z.; Bátkai, S.; et al. Endocannabinoid-mediated modulation of Gq/11 protein-coupled receptor signaling-induced vasoconstriction and hypertension. Mol. Cell. Endocrinol. 2015, 403, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Szekeres, M.; Nádasy, G.L.; Soltész-Katona, E.; Hunyady, L. Control of myogenic tone and agonist induced contraction of intramural coronary resistance arterioles by cannabinoid type 1 receptors and endocannabinoids. Prostaglandins Other Lipid Mediat. 2018, 134, 77–83. [Google Scholar] [CrossRef]
- Hillard, C.J.; Ho, W.-S.; Thompson, J.; Gauthier, K.M.; Wheelock, C.E.; Huang, H.; Hammock, B.D. Inhibition of 2-arachidonoylglycerol catabolism modulates vasoconstriction of rat middle cerebral artery by the thromboxane mimetic, U-46619. Br. J. Pharmacol. 2007, 152, 691–698. [Google Scholar] [CrossRef] [Green Version]
- Karpińska, O.; Baranowska-Kuczko, M.; Kloza, M.; Ambroz˙ewicz, E.; Kozłowski, T.; Kasacka, I.; Malinowska, B.; Kozłowska, H. Activation of CB1receptors by 2-arachidonoylglycerol attenuates vasoconstriction induced by U46619 and angiotensin II in human and rat pulmonary arteries. Am. J. Physiol. Integr. Comp. Physiol. 2017, 312, R883–R893. [Google Scholar] [CrossRef] [Green Version]
- Karpińska, O.; Baranowska-Kuczko, M.; Kloza, M.; Kozłowska, H. Endocannabinoids modulate Gq/11 protein-coupled receptor agonist-induced vasoconstriction via a negative feedback mechanism. J. Pharm. Pharmacol. 2018, 70, 214–222. [Google Scholar] [CrossRef] [Green Version]
- Pędzińska-Betiuk, A.; Weresa, J.; Toczek, M.; Baranowska-Kuczko, M.; Kasacka, I.; Harasim-Symbor, E.; Malinowska, B. Chronic inhibition of fatty acid amide hydrolase by URB597 produces differential effects on cardiac performance in normotensive and hypertensive rats. Br. J. Pharmacol. 2017, 174, 2114–2129. [Google Scholar] [CrossRef] [Green Version]
- Pędzińska-Betiuk, A.; Weresa, J.; Schlicker, E.; Harasim-Symbor, E.; Toczek, M.; Kasacka, I.; Gajo, B.; Malinowska, B. Chronic cannabidiol treatment reduces the carbachol-induced coronary constriction and left ventricular cardiomyocyte width of the isolated hypertensive rat heart. Toxicol. Appl. Pharmacol. 2021, 411, 115368. [Google Scholar] [CrossRef] [PubMed]
- Lerman, L.O.; Kurtz, T.W.; Touyz, R.M.; Ellison, D.H.; Chade, A.R.; Crowley, S.D.; Mattson, D.L.; Mullins, J.J.; Osborn, J.; Eirin, A.; et al. Animal Models of Hypertension: A Scientific Statement From the American Heart Association. Hypertension 2019, 73, e87–e120. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Biernacki, M.; Malinowska, B.; Timoszuk, M.; Toczek, M.; Jastrząb, A.; Remiszewski, P.; Skrzydlewska, E. Hypertension and chronic inhibition of endocannabinoid degradation modify the endocannabinoid system and redox balance in rat heart and plasma. Prostaglandins Other Lipid Mediat. 2018, 138, 54–63. [Google Scholar] [CrossRef]
- Ho, W.-S. Modulation by 17β-estradiol of anandamide vasorelaxation in normotensive and hypertensive rats: A role for TRPV1 but not fatty acid amide hydrolase. Eur. J. Pharmacol. 2013, 701, 49–56. [Google Scholar] [CrossRef]
- Ho, W.-S.V.; Randall, M.D. Endothelium-dependent metabolism by endocannabinoid hydrolases and cyclooxygenases limits vasorelaxation to anandamide and 2-arachidonoylglycerol. Br. J. Pharmacol. 2007, 150, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Herradón, E.; Martín, M.I.; López-Miranda, V. Characterization of the vasorelaxant mechanisms of the endocannabinoid anandamide in rat aorta. Br. J. Pharmacol. 2007, 152, 699–708. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pertwee, R.G.; Howlett, A.C.; Abood, M.E.; Alexander, S.P.H.; Di Marzo, V.; Elphick, M.R.; Greasley, P.J.; Hansen, H.S.; Kunos, G.; Mackie, K.; et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid Receptors and Their Ligands: Beyond CB1 and CB2. Pharmacol. Rev. 2010, 62, 588–631. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- López-Miranda, V.; Dannert, M.T.; Herradón, E.; Alsasua, A.; Martin, M.I. Cytochrome P450 Pathway Contributes to Methanandamide-induced Vasorelaxation in Rat Aorta. Cardiovasc. Drugs Ther. 2010, 24, 379–389. [Google Scholar] [CrossRef]
- Laurent, S.; Boutouyrie, P. The Structural Factor of Hypertension. Circ. Res. 2015, 116, 1007–1021. [Google Scholar] [CrossRef]
- Bernatova, I. Endothelial Dysfunction in Experimental Models of Arterial Hypertension: Cause or Consequence? BioMed Res. Int. 2014, 2014, 1–14. [Google Scholar] [CrossRef]
- Park, J.B.; Charbonneau, F.; Schiffrin, E.L. Correlation of endothelial function in large and small arteries in human essential hypertension. J. Hypertens. 2001, 19, 415–420. [Google Scholar] [CrossRef]
- Remiszewski, P.; Jarocka-Karpowicz, I.; Biernacki, M.; Jastrząb, A.; Schlicker, E.; Toczek, M.; Harasim-Symbor, E.; Pędzińska-Betiuk, A.; Malinowska, B. Chronic Cannabidiol Administration Fails to Diminish Blood Pressure in Rats with Primary and Secondary Hypertension Despite Its Effects on Cardiac and Plasma Endocannabinoid System, Oxidative Stress and Lipid Metabolism. Int. J. Mol. Sci. 2020, 21, 1295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sadowska, O.; Baranowska-Kuczko, M.; Gromotowicz-Popławska, A.; Biernacki, M.; Kicman, A.; Malinowska, B.; Kasacka, I.; Krzyżewska, A.; Kozłowska, H. Cannabidiol Ameliorates Monocrotaline-Induced Pulmonary Hypertension in Rats. Int. J. Mol. Sci. 2020, 21, 7077. [Google Scholar] [CrossRef] [PubMed]
- Jehle, J.; Goerich, H.; Bindila, L.; Lutz, B.; Nickenig, G.; Tiyerili, V. Endocannabinoid 2-arachidonoylglycerol is elevated in the coronary circulation during acute coronary syndrome. PLoS ONE 2019, 14, e0227142. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jehle, J.; Schöne, B.; Bagheri, S.; Avraamidou, E.; Danisch, M.; Frank, I.; Pfeifer, P.; Bindila, L.; Lutz, B.; Lütjohann, D.; et al. Elevated levels of 2-arachidonoylglycerol promote atherogenesis in ApoE−/− mice. PLoS ONE 2018, 13, e0197751. [Google Scholar] [CrossRef]
- E O’Sullivan, S.; A Kendall, D.; Randall, M.D. Heterogeneity in the mechanisms of vasorelaxation to anandamide in resistance and conduit rat mesenteric arteries. Br. J. Pharmacol. 2004, 142, 435–442. [Google Scholar] [CrossRef]
- Wheal, A.J.; Cipriano, M.; Fowler, C.J.; Randall, M.D.; O’Sullivan, S.E. Cannabidiol Improves Vasorelaxation in Zucker Diabetic Fatty Rats through Cyclooxygenase Activation. J. Pharmacol. Exp. Ther. 2014, 351, 457–466. [Google Scholar] [CrossRef] [Green Version]
- Wheal, A.J.; Jadoon, K.; Randall, M.D.; O’Sullivan, S.E. In Vivo Cannabidiol Treatment Improves Endothelium-Dependent Vasorelaxation in Mesenteric Arteries of Zucker Diabetic Fatty Rats. Front. Pharmacol. 2017, 8, 248. [Google Scholar] [CrossRef] [Green Version]
- Schulz, P.; Hryhorowicz, S.; Rychter, A.M.; Zawada, A.; Słomski, R.; Dobrowolska, A.; Krela-Kaźmierczak, I. What Role Does the Endocannabinoid System Play in the Pathogenesis of Obesity? Nutrients 2021, 13, 373. [Google Scholar] [CrossRef]
- Schiffrin, E.L. Vascular remodeling in hypertension: Mechanisms and treatment. Hypertension 2012, 59, 367–374. [Google Scholar] [CrossRef] [Green Version]
- Mulvany, M.J. Small Artery Remodelling in Hypertension. Basic Clin. Pharmacol. Toxicol. 2011, 110, 49–55. [Google Scholar] [CrossRef]
- Wenzel, D.; Matthey, M.; Bindila, L.; Lerner, R.; Lutz, B.; Zimmer, A.; Fleischmann, B.K. Endocannabinoid anandamide mediates hypoxic pulmonary vasoconstriction. Proc. Natl. Acad. Sci. USA 2013, 110, 18710–18715. [Google Scholar] [CrossRef] [Green Version]
- Stanke-Labesque, F.; Mallaret, M.; Lefebvre, B.; Hardy, G.; Caron, F.; Bessard, G. 2-Arachidonoyl glycerol induces contraction of isolated rat aorta: Role of cyclooxygenase-derived products. Cardiovasc. Res. 2004, 63, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Kilkenny, C.; Browne, W.; Cuthill, I.C.; Emerson, M.; Altman, D.G.; Group NCRRGW. Animal research: Reporting in vivo experiments: The ARRIVE guidelines. Br. J. Pharmacol. 2010, 160, 1577–1579. [Google Scholar] [CrossRef] [PubMed]
- Luque-Córdoba, D.; Calderón-Santiago, M.; de Castro, M.L.; Priego-Capote, F. Study of sample preparation for determination of endocannabinoids and analogous compounds in human serum by LC–MS/MS in MRM mode. Talanta 2018, 185, 602–610. [Google Scholar] [CrossRef] [PubMed]







| Group | WKY | WKY + URB | SHR | SHR + URB |
|---|---|---|---|---|
| Phe | (6) | (6) | (6) | (6) |
| pEC50 | 5.3 ± 0.10 | 5.4 ± 0.10 | 5.6 ± 0.07 * | 5.5 ± 0.10 |
| Rmax (%) | 129.9 ± 13.4 | 113.0 ± 4.6 | 122.8 ± 6.9 | 116.0 ± 6.3 |
| Phe + AM251 | (6) | (6) | (6) | (6) |
| pEC50 | 5.7 ± 0.10 # | 5.6 ± 0.10 | 6.1 ± 0.07 *,### | 5.7 ± 0.08 ∆ |
| Rmax (%) | 158.4 ± 16.2 | 142.2 ± 12.6 | 144.9 ± 14.3 | 148.1 ± 22.3 |
| U46619 | (6) | (6) | (6) | (6) |
| pEC50 | 6.1 ± 0.05 | 6.2 ± 0.04 | 6.5 ± 0.05 *** | 7.0 ± 0.07 ∆∆∆ |
| Rmax (%) | 76.8 ± 9.1 | 72.7 ± 7.6 | 75.5 ± 5.6 | 88.9 ± 7.0 |
| U46619 + AM251 | (6) | (6) | (6) | (6) |
| pEC50 | 6.8 ± 0.03 &&& | 6.5 ± 0.06 && | 6.9 ± 0.10 && | 7.2 ± 0.09 |
| Rmax (%) | 87.1 ± 6.3 | 97.0 ± 4.4& | 90.2 ± 4.4 | 111.2 ± 5.7 & |
| Ach | (6) | (6) | (6) | (6) |
| pEC50 | 6.8 ± 0.05 | 6.6 ± 0.09 | 7.0 ± 0.07 | 7.9 ± 0.07 ∆∆∆ |
| Rmax (%) | 87.4 ± 4.4 | 90.6 ± 3.4 | 86.1 ± 11.1 | 96.0 ± 3.1 |
| SNP | (6) | (6) | (6) | (6) |
| pEC50 | 6.8 ± 0.09 | 6.8 ± 0.10 | 7.0 ± 0.10 | 7.2 ± 0.10 |
| Rmax (%) | 71.2 ± 7.5 | 62.7 ± 6.9 | 66.7 ± 7.3 | 74.2 ± 3.1 |
| MethAEA | (10) | (10) | (8) | (8) |
| pEC50 | 6.1 ± 0.07 | 6.1 ± 0.10 | 5.6 ± 0.10 ** | 5.8 ± 0.10 |
| Rmax (%) | 96.5 ± 1.7 | 87.8 ± 7.7 | 97.9 ± 3.1 | 88.4 ± 4.4 |
| MethAEA + AM251 | (10) | (10) | (8) | (8) |
| pEC50 | 5.9 ± 0.04 | 5.8 ± 0.10 | 4.9 ± 0.07 ***,$$$ | 5.2 ± 0.10 ∆∆∆,$$ |
| Rmax (%) | 96.0 ± 1.3 | 92.6 ± 4.1 | 89.4 ± 3.2 | 91.3 ± 1.5 |
| Group | WKY | WKY + URB | SHR | SHR + URB |
|---|---|---|---|---|
| Phe | (7) | (7) | (7) | (7) |
| pEC50 | 7.3 ± 0.05 | 7.2 ± 0.03 | 7.3 ± 0.06 | 7.5 ± 0.05 |
| Rmax (%) | 82.9 ± 2.9 | 82.9 ± 4.1 | 88.8 ± 4.0 | 92.5 ± 2.8 |
| Phe + AM251 | (7) | (7) | (7) | (7) |
| pEC50 | 7.2 ± 0.03 | 7.4 ± 0.10 | 7.4 ± 0.06 | 7.4 ± 0.05 |
| Rmax (%) | 91.3 ± 2.6 | 83.3 ± 7.1 | 94.5 ± 5.7 | 91.6 ± 4.2 |
| U46619 | (7) | (7) | (7) | (7) |
| pEC50 | 7.4 ± 0.10 | 7.4 ± 0.03 | 8.1 ± 0.05 *** | 7.9 ± 0.04 |
| Rmax (%) | 97.2 ± 2.9 | 106.8 ± 2.5 | 108.4 ± 3.6 | 104.7 ± 1.9 |
| U46619 + AM251 | (7) | (7) | (7) | (7) |
| pEC50 | 7.7 ± 0.04 | 7.5 ± 0.06 | 8.0 ± 0.04 | 7.8 ± 0.06 |
| Rmax (%) | 98.7 ± 4.0 | 108.5 ± 2.2 | 106.4 ± 2.6 | 107.9 ± 1.1 |
| Ach | (6) | (6) | (6) | (6) |
| pEC50 | 7.2 ± 0.03 | 6.8 ± 0.02 *** | 7.3 ± 0.09 | 7.7 ± 0.06 ∆∆∆ |
| Rmax (%) | 100.0 ± 6.7 | 96.2 ± 7.0 | 91.3 ± 4.7 | 92.6 ± 1.7 |
| SNP | (6) | (6) | (6) | (6) |
| pEC50 | 8.4 ± 0.08 | 8.1 ± 0.04 * | 7.6 ± 0.05 *** | 7.7 ± 0.06 |
| Rmax (%) | 113.0 ± 4.8 | 103.9 ± 2.0 | 110.0 ± 3.6 | 100.2 ± 6.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baranowska-Kuczko, M.; Kozłowska, H.; Kloza, M.; Harasim-Symbor, E.; Biernacki, M.; Kasacka, I.; Malinowska, B. Beneficial Changes in Rat Vascular Endocannabinoid System in Primary Hypertension and under Treatment with Chronic Inhibition of Fatty Acid Amide Hydrolase by URB597. Int. J. Mol. Sci. 2021, 22, 4833. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22094833
Baranowska-Kuczko M, Kozłowska H, Kloza M, Harasim-Symbor E, Biernacki M, Kasacka I, Malinowska B. Beneficial Changes in Rat Vascular Endocannabinoid System in Primary Hypertension and under Treatment with Chronic Inhibition of Fatty Acid Amide Hydrolase by URB597. International Journal of Molecular Sciences. 2021; 22(9):4833. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22094833
Chicago/Turabian StyleBaranowska-Kuczko, Marta, Hanna Kozłowska, Monika Kloza, Ewa Harasim-Symbor, Michał Biernacki, Irena Kasacka, and Barbara Malinowska. 2021. "Beneficial Changes in Rat Vascular Endocannabinoid System in Primary Hypertension and under Treatment with Chronic Inhibition of Fatty Acid Amide Hydrolase by URB597" International Journal of Molecular Sciences 22, no. 9: 4833. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22094833