PlatyphyllenoneExerts Anti-Metastatic Effects on Human Oral Cancer Cells by Modulating Cathepsin L Expression, MAPK Pathway and Epithelial–Mesenchymal Transition
Abstract
:1. Introduction
2. Results
2.1. Platyphyllenone Does Not Affect the Viability of Human Oral Cancer Cells
2.2. Platyphyllenone Inhibits the Motility of Human Oral Cancer Cells
2.3. Platyphyllenone Inhibits Migration and Invasion of Human Oral Cancer Cells
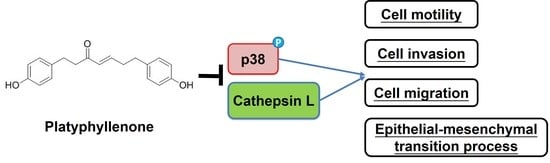
2.4. Platyphyllenone Reduces Motility of Human Oral Cancer Cells by Modulating p38 Pathway
2.5. Platyphyllenonealters Expression of Epithelial–Mesenchymal Transition Proteins
2.6. Platyphyllenone Alters the Expression of Cathepsin L in Human Oral Cancer Cells
3. Discussion
4. Materials and Methods
4.1. Cell Culture
4.2. Compound
4.3. MTT Assay
4.4. In Vitro Wound Closure
4.5. Cell Migration and Invasion Assay
4.6. Western Blot Assay
4.7. Transfection Assay
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- D’Souza, S.; Addepalli, V. Preventive measures in oral cancer: An overview. Biomed. Pharmacother. 2018, 107, 72–80. [Google Scholar] [CrossRef]
- Montero, P.H.; Patel, S.G. Cancer of the oral cavity. Surg. Oncol. Clin. N. Am. 2015, 24, 491–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wong, T.; Wiesenfeld, D. Oral cancer. Aust. Dent. J. 2018, 63 (Suppl. S1), S91–S99. [Google Scholar] [CrossRef]
- Peng, Q.; Deng, Z.; Pan, H.; Gu, L.; Liu, O.; Tang, Z. Mitogen-activated protein kinase signaling pathway in oral cancer. Oncol. Lett. 2018, 15, 1379–1388. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ahmed, T.A.; Hayslip, J.; Leggas, M. Simvastatin interacts synergistically with tipifarnib to induce apoptosis in leukemia cells through the disruption of RAS membrane localization and ERK pathway inhibition. Leuk. Res. 2014, 38, 1350–1357. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Steelman, L.S.; Franklin, R.A.; Abrams, S.L.; Chappell, W.; Kempf, C.R.; Basecke, J.; Stivala, F.; Donia, M.; Fagone, P.; Nicoletti, F.; et al. Roles of the Ras/Raf/MEK/ERK pathway in leukemia therapy. Leukemia 2011, 25, 1080–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pelaia, G.; Gallelli, L.; Renda, T.; Fratto, D.; Falcone, D.; Caraglia, M.; Busceti, M.T.; Terracciano, R.; Vatrella, A.; Maselli, R.; et al. Effects of statins and farnesyl transferase inhibitors on ERK phosphorylation, apoptosis and cell viability in non-small lung cancer cells. Cell Prolif. 2012, 45, 557–565. [Google Scholar] [CrossRef]
- Lee, J.C.; Chung, L.C.; Chen, Y.J.; Feng, T.H.; Chen, W.T.; Juang, H.H. Upregulation of B-cell translocation gene 2 by epigallocatechin-3-gallate via p38 and ERK signaling blocks cell proliferation in human oral squamous cell carcinoma cells. Cancer Lett. 2015, 360, 310–318. [Google Scholar] [CrossRef] [PubMed]
- Tang, F.Y.; Chiang, E.P.; Chung, J.G.; Lee, H.Z.; Hsu, C.Y. S-allylcysteine modulates the expression of E-cadherin and inhibits the malignant progression of human oral cancer. J. Nutr. Biochem. 2009, 20, 1013–1020. [Google Scholar] [CrossRef]
- Ko, C.P.; Lin, C.W.; Chen, M.K.; Yang, S.F.; Chiou, H.L.; Hsieh, M.J. Pterostilbene induce autophagy on human oral cancer cells through modulation of Akt and mitogen-activated protein kinase pathway. Oral Oncol. 2015, 51, 593–601. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.Y.; Hsieh, Y.H.; Yang, S.F.; Chen, C.T.; Tang, C.H.; Chou, M.Y.; Chuang, Y.T.; Lin, C.W.; Chen, M.K. Resveratrol suppresses TPA-induced matrix metalloproteinase-9 expression through the inhibition of MAPK pathways in oral cancer cells. J. Oral Pathol. Med. 2015, 44, 699–706. [Google Scholar] [CrossRef] [PubMed]
- Sati, S.C.; Sati, N.; Sati, O.P. Bioactive constituents and medicinal importance of genus Alnus. Pharmacogn. Rev. 2011, 5, 174–183. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryu, M.; Sung, C.K.; Im, Y.J.; Chun, C. Activation of JNK and p38 in MCF-7 Cells and the in vitro anticancer activity of Alnus hirsuta extract. Molecules 2020, 25, 1073. [Google Scholar] [CrossRef] [Green Version]
- Monroe, J.D.; Hodzic, D.; Millay, M.H.; Patty, B.G.; Smith, M.E. Anti-cancer and ototoxicity characteristics of the curcuminoids, CLEFMA and EF24, in combination with cisplatin. Molecules 2019, 24, 3889. [Google Scholar] [CrossRef] [Green Version]
- Thongon, N.; Boonmuen, N.; Suksen, K.; Wichit, P.; Chairoungdua, A.; Tuchinda, P.; Suksamrarn, A.; Winuthayanon, W.; Piyachaturawat, P. Selective estrogen receptor modulator (SERM)-like Activities of diarylheptanoid, a phytoestrogen from Curcuma comosa, in breast cancer cells, pre-osteoblast cells, and rat uterine tissues. J. Agric. Food Chem. 2017, 65, 3490–3496. [Google Scholar] [CrossRef]
- Liu, Q.; Yin, W.; Han, L.; Lv, J.; Li, B.; Lin, Y.; Mi, Q.; He, R.; Lu, C. Diarylheptanoid from rhizomes of Curcuma kwangsiensis (DCK) inhibited imiquimod-induced dendritic cells activation and Th1/Th17 differentiation. Int. Immunopharmacol. 2018, 56, 339–348. [Google Scholar] [CrossRef]
- Ilic-Tomic, T.; Sokovic, M.; Vojnovic, S.; Ciric, A.; Veljic, M.; Nikodinovic-Runic, J.; Novakovic, M. Diarylheptanoids from Alnus viridis ssp. viridis and Alnus glutinosa: Modulation of quorum sensing activity in Pseudomonas aeruginosa. Planta Med. 2017, 83, 117–125. [Google Scholar] [CrossRef] [Green Version]
- Dong, G.Z.; Jeong, J.H.; Lee, Y.I.; Lee, S.Y.; Zhao, H.Y.; Jeon, R.; Lee, H.J.; Ryu, J.H. Diarylheptanoids suppress proliferation of pancreatic cancer PANC-1 cells through modulating shh-Gli-FoxM1 pathway. Arch. Pharm. Res. 2017, 40, 509–517. [Google Scholar] [CrossRef]
- Huh, J.Y.; Lee, S.; Ma, E.B.; Eom, H.J.; Baek, J.; Ko, Y.J.; Kim, K.H. The effects of phenolic glycosides from Betula platyphylla var. japonica on adipocyte differentiation and mature adipocyte metabolism. J. Enzyme Inhib. Med. Chem. 2018, 33, 1167–1173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dinic, J.; Novakovic, M.; Podolski-Renic, A.; Vajs, V.; Tesevic, V.; Isakovic, A.; Pesic, M. Structural differences in diarylheptanoids analogues from Alnus viridis and Alnus glutinosa influence their activity and selectivity towards cancer cells. Chem. Biol. Interact. 2016, 249, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.B.; Lin, S.H.; Mahalakshmi, B.; Lo, Y.S.; Lin, C.C.; Chuang, Y.C.; Hsieh, M.J.; Chen, M.K. Sodium Danshensu inhibits oral cancer cell migration and invasion by modulating p38 signaling pathway. Front. Endocrinol. 2020, 11, 568436. [Google Scholar] [CrossRef] [PubMed]
- Arumugam, A.; Subramani, R.; Nandy, S.; Powell, S.; Velazquez, M.; Orozco, A.; Galvez, A.; Lakshmanaswamy, R. Desacetylnimbinene inhibits breast cancer growth and metastasis through reactive oxygen species mediated mechanisms. Tumour Biol. 2016, 37, 6527–6537. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.H.; Hwang, Y.P.; Jin, S.W.; Lee, G.H.; Kim, H.G.; Han, E.H.; Kim, S.K.; Kang, K.W.; Chung, Y.C.; Jeong, H.G. Suppression of PMA-induced human fibrosarcoma HT-1080 invasion and metastasis by kahweol via inhibiting Akt/JNK1/2/p38 MAPK signal pathway and NF-kappaB dependent transcriptional activities. Food Chem. Toxicol. 2019, 125, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Loh, C.Y.; Chai, J.Y.; Tang, T.F.; Wong, W.F.; Sethi, G.; Shanmugam, M.K.; Chong, P.P.; Looi, C.Y. The E-Cadherin and N-Cadherin switch in epithelial-to-mesenchymal transition: Signaling, therapeutic implications, and challenges. Cells 2019, 8, 1118. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bahrami, A.; Majeed, M.; Sahebkar, A. Curcumin: A potent agent to reverse epithelial-to-mesenchymal transition. Cell Oncol. 2019, 42, 405–421. [Google Scholar] [CrossRef]
- Pai, J.T.; Lee, Y.C.; Chen, S.Y.; Leu, Y.L.; Weng, M.S. Propolin C inhibited migration and invasion via suppression of EGFR-mediated epithelial-to-mesenchymal transition in human lung cancer cells. Evid. Based Complement. Alternat. Med. 2018, 2018, 7202548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, P.N.; Yang, S.F.; Yu, C.C.; Lin, C.Y.; Huang, S.H.; Chu, S.C.; Hsieh, Y.S. Duchesnea indica extract suppresses the migration of human lung adenocarcinoma cells by inhibiting epithelial-mesenchymal transition. Environ. Toxicol. 2017, 32, 2053–2063. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Xiong, Y.; Ding, X.; Wang, L.; Zhao, Y.; Fei, Y.; Zhu, Y.; Shen, X.; Tan, C.; Liang, Z. Cathepsin L activated by mutant p53 and Egr-1 promotes ionizing radiation-induced EMT in human NSCLC. J. Exp. Clin. Cancer Res 2019, 38, 61. [Google Scholar] [CrossRef]
- Han, M.L.; Zhao, Y.F.; Tan, C.H.; Xiong, Y.J.; Wang, W.J.; Wu, F.; Fei, Y.; Wang, L.; Liang, Z.Q. Cathepsin L upregulation-induced EMT phenotype is associated with the acquisition of cisplatin or paclitaxel resistance in A549 cells. Acta Pharmacol. Sin. 2016, 37, 1606–1622. [Google Scholar] [CrossRef]







Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bharath Kumar, V.; Lin, J.-T.; Mahalakshmi, B.; Chuang, Y.-C.; Ho, H.-Y.; Lin, C.-C.; Lo, Y.-S.; Hsieh, M.-J.; Chen, M.-K. PlatyphyllenoneExerts Anti-Metastatic Effects on Human Oral Cancer Cells by Modulating Cathepsin L Expression, MAPK Pathway and Epithelial–Mesenchymal Transition. Int. J. Mol. Sci. 2021, 22, 5012. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22095012
Bharath Kumar V, Lin J-T, Mahalakshmi B, Chuang Y-C, Ho H-Y, Lin C-C, Lo Y-S, Hsieh M-J, Chen M-K. PlatyphyllenoneExerts Anti-Metastatic Effects on Human Oral Cancer Cells by Modulating Cathepsin L Expression, MAPK Pathway and Epithelial–Mesenchymal Transition. International Journal of Molecular Sciences. 2021; 22(9):5012. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22095012
Chicago/Turabian StyleBharath Kumar, V., Jen-Tsun Lin, B. Mahalakshmi, Yi-Ching Chuang, Hsin-Yu Ho, Chia-Chieh Lin, Yu-Sheng Lo, Ming-Ju Hsieh, and Mu-Kuan Chen. 2021. "PlatyphyllenoneExerts Anti-Metastatic Effects on Human Oral Cancer Cells by Modulating Cathepsin L Expression, MAPK Pathway and Epithelial–Mesenchymal Transition" International Journal of Molecular Sciences 22, no. 9: 5012. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms22095012