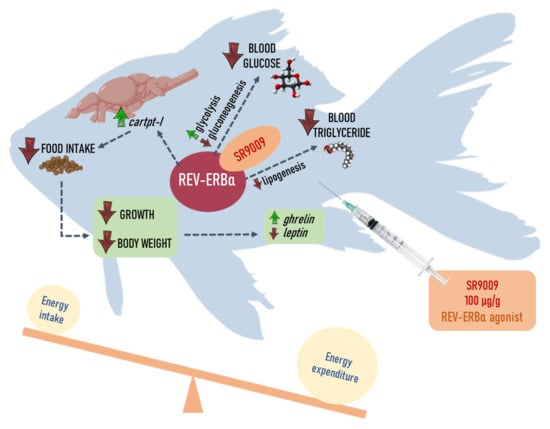
REV-ERBα Agonist SR9009 Promotes a Negative Energy Balance in Goldfish
Abstract
:1. Introduction
2. Results
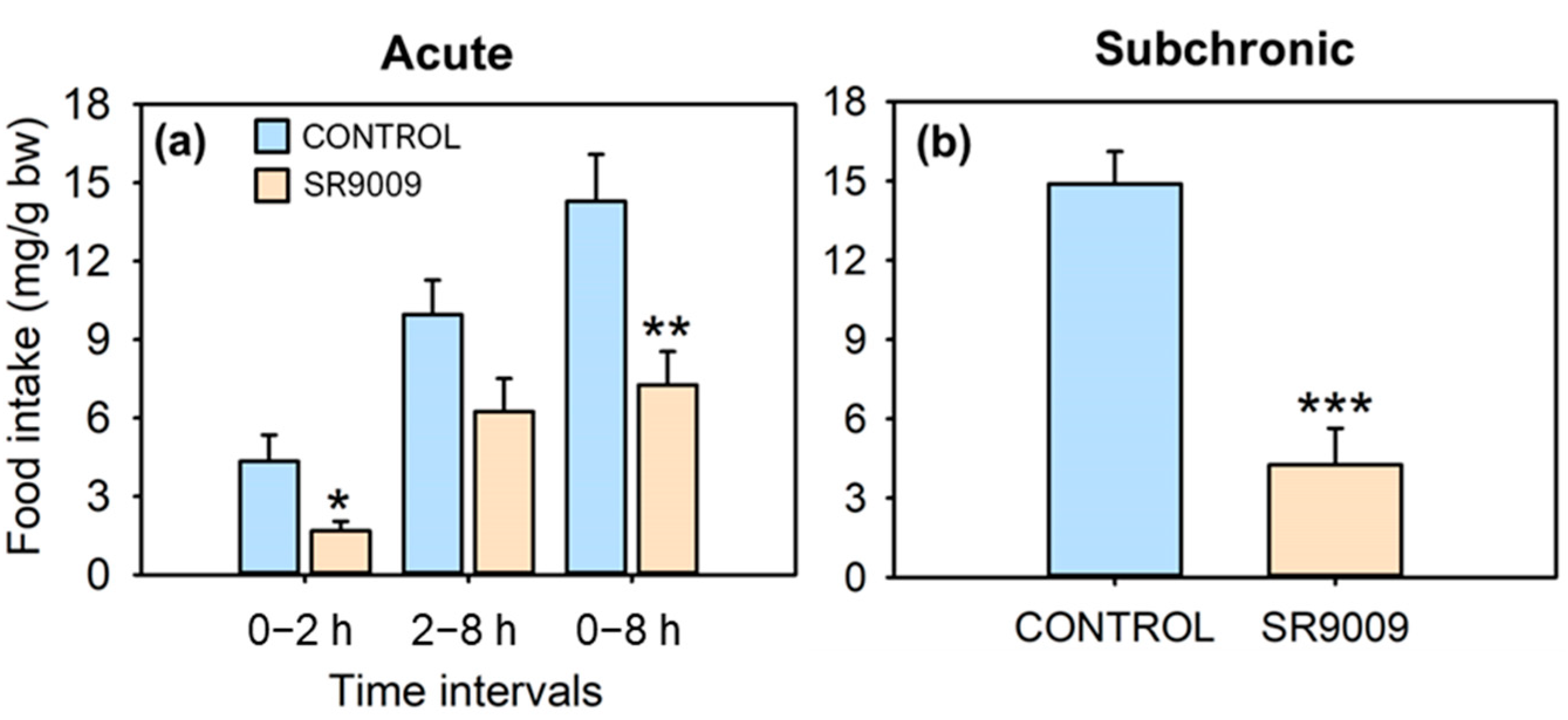
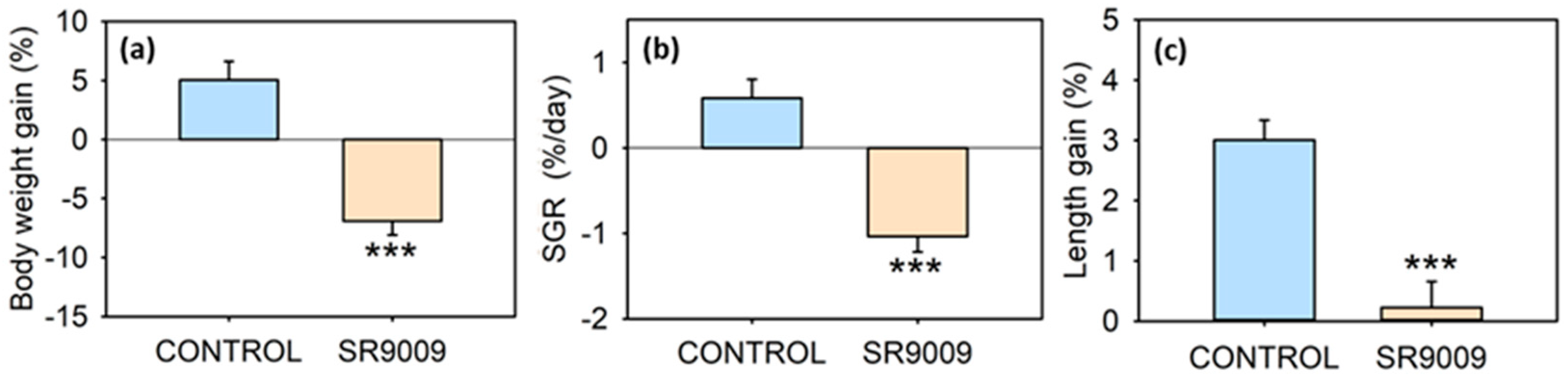
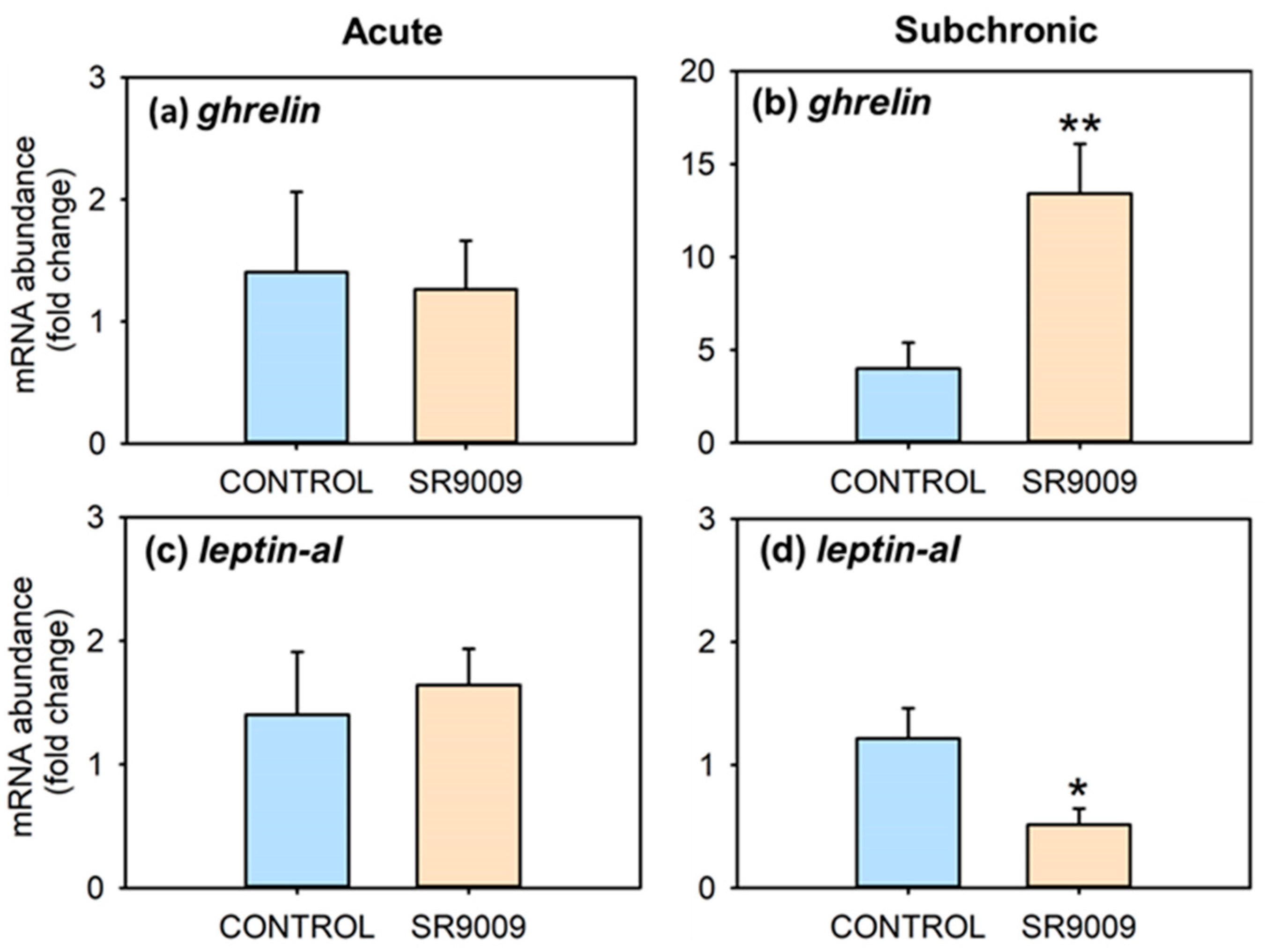
2.1. Effects of SR9009 on Food Intake, Growth, Feeding Regulators and Locomotor Activity
2.2. Effects of SR9009 on Metabolism
3. Discussion
4. Materials and Methods
4.1. Animals and Housing
4.2. SR9009 Administration
4.3. Experimental Designs
4.3.1. Acute Effect of SR9009 on Food Intake
4.3.2. Acute Effect of SR9009 on Intake Regulators and Metabolism
4.3.3. Effect of Subchronic SR9009 Administration on Feeding, Growth, Locomotor Activity, and Metabolism
4.4. Analytical Techniques
4.4.1. Biometric Parameters
4.4.2. Locomotor Activity Recordings
4.4.3. Plasma and Liver Metabolites
4.4.4. Liver Enzymatic Activity
4.4.5. Gene Expression Analysis
4.5. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Everett, L.J.; Lazar, M.A. Nuclear receptor Rev-erbα: Up, down, and all around. Trends Endocrinol. Metab. 2014, 25, 586–592. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Fang, B.; Emmett, M.J.; Damle, M.; Sun, Z.; Feng, D.; Armour, S.M.; Remsberg, J.R.; Jager, J.; Soccio, R.E.; et al. Discrete functions of nuclear receptor Rev-erbα couple metabolism to the clock. Science 2015, 348, 1488–1492. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Li, F.; Lin, Y.; Wu, B. Targeting REV-ERBα for therapeutic purposes: Promises and challenges. Theranostics 2020, 10, 4168–4182. [Google Scholar] [CrossRef] [PubMed]
- Hunter, A.L.; Pelekanou, C.E.; Adamson, A.; Downton, P.; Barron, N.J.; Cornfield, T.; Poolman, T.M.; Humphreys, N.; Cunningham, P.S.; Hodson, L.; et al. Nuclear receptor REVERBα is a state-dependent regulator of liver energy metabolism. Proc. Natl. Acad. Sci. USA 2020, 117, 25869–25879. [Google Scholar] [CrossRef] [PubMed]
- Alenghat, T.; Meyers, K.; Mullican, S.E.; Leitner, K.; Adeniji-Adele, A.; Avila, J.; Bućan, M.; Ahima, R.S.; Kaestner, K.H.; Lazar, M.A. Nuclear receptor corepressor-histone deacetylase 3 governs circadian metabolic physiology. Nature 2008, 456, 997. [Google Scholar] [CrossRef]
- Raghuram, S.; Stayrook, K.R.; Huang, P.; Rogers, P.M.; Nosie, A.K.; McClure, D.B.; Burris, L.L.; Khorasanizadeh, S.; Burris, T.P.; Rastinejad, F. Identification of heme as the ligand for the orphan nuclear receptors REV-ERBα and REV-ERBβ. Nat. Struct. Mol. Biol. 2007, 14, 1207–1213. [Google Scholar] [CrossRef]
- Yin, L.; Wu, N.; Curtin, J.C.; Qatanani, M.; Szwergold, N.R.; Reid, R.A.; Waitt, G.M.; Parks, D.J.; Pearce, K.H.; Wisely, G.B.; et al. Rev-erbα, a heme sensor that coordinates metabolic and circadian pathways. Science 2007, 318, 1786–1789. [Google Scholar] [CrossRef]
- Kojetin, D.J.; Burris, T.P. A role for REV-ERBα ligands in the regulation of adipogenesis. Curr. Pharm. Des. 2011, 17, 320–324. [Google Scholar] [CrossRef]
- Rogers, P.M.; Ying, L.; Burris, T.P. Relationship between circadian oscillations of REV-ERBα expression and intracellular levels of its ligand, heme. Biochem. Biophys. Res. Commun. 2008, 368, 955–958. [Google Scholar] [CrossRef]
- Duez, H.; Staels, B. REV-ERBα gives a time cue to metabolism. FEBS Lett. 2008, 582, 19–25. [Google Scholar] [CrossRef]
- Cho, H.; Zhao, X.; Hatori, M.; Yu, R.T.; Barish, G.D.; Lam, M.T.; Chong, L.W.; Ditacchio, L.; Atkins, A.R.; Glass, C.K.; et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature 2012, 485, 123–127. [Google Scholar] [CrossRef] [PubMed]
- Delezie, J.; Dumont, S.; Dardente, H.; Oudart, H.; Gréchez-Cassiau, A.; Klosen, P.; Teboul, M.; Delaunay, F.; Pévet, P.; Challet, E. The nuclear receptor REV-ERBα is required for the daily balance of carbohydrate and lipid metabolism. FASEB J. 2012, 26, 3321–3335. [Google Scholar] [CrossRef] [PubMed]
- Borba, T.K.F.; Toscano, A.E.; Costa de Santana, B.J.R.; Silva, C.d.A.; Lagranha, C.J.; Quevedo, O.G.; Manhães-de-Castro, R. Central administration of REV-ERBα agonist promotes opposite responses on energy balance in fasted and fed states. J. Neuroendocrinol. 2020, 32, e12833. [Google Scholar] [CrossRef] [PubMed]
- Vieira, E.; Marroquí, L.; Batista, T.M.; Caballero-Garrido, E.; Carneiro, E.M.; Boschero, A.C.; Nadal, A.; Quesada, I. The clock gene Rev-erbα regulates pancreatic β-cell function: Modulation by leptin and high-fat diet. Endocrinology 2012, 153, 592–601. [Google Scholar] [CrossRef]
- Kojetin, D.J.; Burris, T.P. REV-ERB and ROR nuclear receptors as drug targets. Nat. Rev. Drug Discov. 2014, 13, 197–216. [Google Scholar] [CrossRef]
- Wang, J.; Lazar, M.A. Bifunctional Role of Rev-erbα in Adipocyte Differentiation. Mol. Cell. Biol. 2008, 28, 2213–2220. [Google Scholar] [CrossRef]
- Raspé, E.; Duez, H.; Mansén, A.; Fontaine, C.; Fiévet, C.; Fruchart, J.C.; Vennström, B.; Staels, B. Identification of REV-ERBα as a physiological repressor of apoC-III gene transcription. J. Lipid Res. 2002, 43, 2172–2179. [Google Scholar] [CrossRef]
- Feng, D.; Liu, T.; Sun, Z.; Bugge, A.; Mullican, S.E.; Alenghat, T.; Liu, X.S.; Lazar, M.A. A circadian rhythm orchestrated by histone deacetylase 3 controls hepatic lipid metabolism. Science 2011, 331, 1315–1319. [Google Scholar] [CrossRef]
- Le Martelot, G.; Claudel, T.; Gatfield, D.; Schaad, O.; Kornmann, B.; Lo Sasso, G.; Moschetta, A.; Schibler, U. REV-ERBα participates in circadian SREBP signaling and bile acid homeostasis. PLoS Biol. 2009, 7, e1000181. [Google Scholar] [CrossRef]
- Downes, M.; Carozzi, A.J.; Muscat, G.E.O. Constitutive expression of the orphan receptor, Rev-erbAα, inhibits muscle differentiation and abrogates the expression of the myoD gene family. Mol. Endocrinol. 1995, 9, 1666–1678. [Google Scholar] [CrossRef]
- Woldt, E.; Sebti, Y.; Solt, L.A.; Duhem, C.; Lancel, S.; Eeckhoute, J.; Hesselink, M.K.C.; Paquet, C.; Delhaye, S.; Shin, Y.; et al. Rev-erb-α modulates skeletal muscle oxidative capacity by regulating mitochondrial biogenesis and autophagy. Nat. Med. 2013, 19, 1039–1046. [Google Scholar] [CrossRef] [PubMed]
- Banerjee, S.; Wang, Y.; Solt, L.A.; Griffett, K.; Kazantzis, M.; Amador, A.; El-Gendy, B.M.; Huitron-Resendiz, S.; Roberts, A.J.; Shin, Y.; et al. Pharmacological targeting of the mammalian clock regulates sleep architecture and emotional behaviour. Nat. Commun. 2014, 5, 5759. [Google Scholar] [CrossRef] [PubMed]
- Jager, J.; Timothy O’Brien, W.; Manlove, J.; Krizman, E.N.; Fang, B.; Gerhart-Hines, Z.; Robinson, M.B.; Klein, P.S.; Lazar, M.A. Behavioral changes and dopaminergic dysregulation in mice lacking the nuclear receptor rev-erbα. Mol. Endocrinol. 2014, 28, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Solt, L.A.; Wang, Y.; Banerjee, S.; Hughes, T.; Douglas, J.; Lundasen, T.; Shin, Y.; Liu, J.; Cameron, M.D.; Yoo, S.; et al. Regulation of circadian behaviour and metabolism by synthetic REV-ERB agonists. Nature 2012, 485, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Viscarra, J.; Kim, S.-J.; Sul, H.S. Transcriptional regulation of hepatic lipogenesis. Nat. Rev. Mol. Cell Biol. 2015, 16, 678–689. [Google Scholar] [CrossRef]
- Vieira, E.; Merino, B.; Quesada, I. Role of the clock gene Rev-erbα in metabolism and in the endocrine pancreas. Diabetes Obes. Metab. 2015, 17, 106–114. [Google Scholar] [CrossRef]
- Betancor, M.B.; McStay, E.; Minghetti, M.; Migaud, H.; Tocher, D.R.; Davie, A. Daily rhythms in expression of genes of hepatic lipid metabolism in Atlantic salmon (Salmo salar L.). PLoS ONE 2014, 9, e106739. [Google Scholar] [CrossRef]
- Kopp, R.; Billecke, N.; Legradi, J.; Den Broeder, M.; Parekh, S.H.; Legler, J. Bringing obesity to light: REV-ERBα, a central player in light-induced adipogenesis in the zebrafish? Int. J. Obes. 2016, 40, 824–832. [Google Scholar] [CrossRef]
- Wang, H.; Yang, Z.; Li, X.; Huang, D.; Yu, S.; He, J.; Li, Y.; Yan, J. Single-cell in vivo imaging of cellular circadian oscillators in zebrafish. PLoS Biol. 2020, 18, e3000435. [Google Scholar] [CrossRef]
- Amaral, I.P.G.; Johnston, I.A. Circadian expression of clock and putative clock-controlled genes in skeletal muscle of the zebrafish. J. Physiol. Regul. Integr. Comp. Physiol. 2012, 302, 193–206. [Google Scholar] [CrossRef]
- Wu, P.; Chu, W.; Liu, X.; Guo, X.; Zhang, J. The influence of short-term fasting on muscle growth and fiber hypotrophy regulated by the rhythmic expression of clock genes and myogenic factors in Nile Tilapia. Mar. Biotechnol. 2018, 20, 750–768. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Cheng, J.; Chen, L.; Xiang, J.; Pan, Y.; Zhang, Y.; Zheng, T.; Liu, N.; Chu, W.; Zhang, J. Nr1d1 affects autophagy in the skeletal muscles of juvenile Nile tilapia by regulating the rhythmic expression of autophagy-related genes. Fish Physiol. Biochem. 2020, 46, 891–907. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.; Li, Y.-L.; Cheng, J.; Chen, L.; Zhu, X.; Feng, Z.-G.; Zhang, J.-S.; Chu, W.-Y. Daily rhythmicity of clock gene transcript levels in fast and slow muscle fibers from Chinese perch (Siniperca chuatsi). BMC Genom. 2016, 17, 1008. [Google Scholar] [CrossRef] [PubMed]
- Lazado, C.C.; Kumaratunga, H.P.S.; Nagasawa, K.; Babiak, I.; Giannetto, A.; Fernandes, J.M.O. Daily rhythmicity of clock gene transcripts in Atlantic cod fast skeletal muscle. PLoS ONE 2014, 9, e99172. [Google Scholar] [CrossRef]
- Gómez-Boronat, M.; de Pedro, N.; Delgado, M.J.; Isorna, E. Nuclear receptors expression rhythms are triggered by feeding time in the liver but not in the hypothalamus of goldfish (Carassius auratus). In Advances in Comparative Endocrinology; Soengas, J.L., Míguez, J.M., López-Patiño, M.A., Álvarez-Otero, R., Eds.; Universidade do Vigo: Vigo, Spain, 2017; Volume IX, pp. 182–184. [Google Scholar]
- Wang, M.; Zhong, Z.; Zhong, Y.; Zhang, W.; Wang, H. The zebrafish period2 protein positively regulates the circadian clock through mediation of retinoic acid receptor (RAR)-related orphan receptor α (Rorα). J. Biol. Chem. 2015, 290, 4367–4382. [Google Scholar] [CrossRef]
- Blanco, A.M.; Sundarrajan, L.; Bertucci, J.I.; Unniappan, S. Why goldfish? Merits and challenges in employing goldfish as a model organism in comparative endocrinology research. Gen. Comp. Endocrinol. 2018, 257, 13–28. [Google Scholar] [CrossRef]
- Delgado, M.J.; Cerdá-Reverter, J.M.; Soengas, J.L. Hypothalamic integration of metabolic, endocrine, and circadian signals in fish: Involvement in the control of food intake. Front. Neurosci. 2017, 11, 354. [Google Scholar] [CrossRef]
- Soengas, J.L.; Cerdá-Reverter, J.M.; Delgado, M.J. Central regulation of food intake in fish: An evolutionary perspective. J. Mol. Endocrinol. 2018, 60, R171–R199. [Google Scholar] [CrossRef]
- Volkoff, H. Fish as models for understanding the vertebrate endocrine regulation of feeding and weight. Mol. Cell. Endocrinol. 2019, 497, 110437. [Google Scholar] [CrossRef]
- Gómez-Boronat, M.; Isorna, E.; Conde-Sieira, M.; Delgado, M.J.; Soengas, J.L.; de Pedro, N. First evidence on the role of palmitoylethanolamide in energy homeostasis in fish. Horm. Behav. 2020, 117, 104609. [Google Scholar] [CrossRef]
- Amador, A.; Wang, Y.; Banerjee, S.; Kameneka, T.M.; Solt, L.A.; Burris, T.P. Pharmacological and genetic modulation of REV-ERB activity and expression affects orexigenic gene expression. PLoS ONE 2016, 11, e0156367. [Google Scholar] [CrossRef]
- Dar, S.A.; Srivastava, P.P.; Varghese, T.; Gupta, S.; Gireesh-Babu, P.; Krishna, G. Effects of starvation and refeeding on expression of ghrelin and leptin gene with variations in metabolic parameters in Labeo rohita fingerlings. Aquaculture 2018, 484, 219–227. [Google Scholar] [CrossRef]
- de Pedro, N.; Martínez-Álvarez, R.; Delgado, M.J.; de Pedro, N.; Martínez-Álvarez, R.; Delgado, M.J. Acute and chronic leptin reduces food intake and body weight in goldfish (Carassius auratus). J. Endocrinol. 2006, 188, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Yan, A.F.; Chen, T.; Chen, S.; Ren, C.H.; Hu, C.Q.; Cai, Y.M.; Liu, F.; Tang, D.S. Goldfish leptin-AI and leptin-AII: Function and central mechanism in feeding control. Int. J. Mol. Sci. 2016, 17, 783. [Google Scholar] [CrossRef] [PubMed]
- Kristensen, P.; Judge, M.E.; Thim, L.; Ribel, U.; Christjansen, K.N.; Wulff, B.S.; Clausen, J.T.; Jensen, P.B.; Madsen, O.D.; Vrang, N.; et al. Hypothalamic CART is a new anorectic peptide regulated by leptin. Nature 1998, 393, 72–76. [Google Scholar] [CrossRef]
- Nakhate, K.T.; Subhedar, N.K.; Kokare, D.M. Involvement of neuropeptide CART in the central effects of insulin on feeding and body weight. Pharmacol. Biochem. Behav. 2019, 181, 101–109. [Google Scholar] [CrossRef]
- Koch, C.E.; Begemann, K.; Kiehn, J.T.; Griewahn, L.; Mauer, J.; Hess, M.E.; Moser, A.; Schmid, S.M.; Brüning, J.C.; Oster, H. Circadian regulation of hedonic appetite in mice by clocks in dopaminergic neurons of the VTA. Nat. Commun. 2020, 11, 3071. [Google Scholar] [CrossRef]
- Chung, S.; Lee, E.J.; Yun, S.; Choe, H.K.; Park, S.B.; Son, H.J.; Kim, K.S.; Dluzen, D.E.; Lee, I.; Hwang, O.; et al. Impact of circadian nuclear receptor REV-ERBα on midbrain dopamine production and mood regulation. Cell 2014, 157, 858–868. [Google Scholar] [CrossRef]
- Mayeuf-Louchart, A.; Thorel, Q.; Delhaye, S.; Beauchamp, J.; Duhem, C.; Danckaert, A.; Lancel, S.; Pourcet, B.; Woldt, E.; Boulinguiez, A.; et al. Rev-erb-α regulates atrophy-related genes to control skeletal muscle mass. Sci. Rep. 2017, 7, 14383. [Google Scholar] [CrossRef]
- Yuan, X.; Dong, D.; Li, Z.; Wu, B. Rev-erbα activation down-regulates hepatic Pck1 enzyme to lower plasma glucose in mice. Pharmacol. Res. 2019, 141, 310–318. [Google Scholar] [CrossRef]
- McLeod, M.J.; Holyoak, T. Enyzmes|Phosphoenolpyruvate Carboxykinases. In Encyclopedia of Biological Chemistry, 3rd ed.; Joseph, J., Ed.; Elsevier: St Louis, MO, USA, 2021; Volume 3, pp. 400–412. [Google Scholar]
- Dierickx, P.; Emmett, M.J.; Jiang, C.; Uehara, K.; Liu, M.; Adlanmerini, M.; Lazar, M.A. SR9009 has REV-ERB–independent effects on cell proliferation and metabolism. Proc. Natl. Acad. Sci. USA 2019, 116, 12147–12152. [Google Scholar] [CrossRef] [PubMed]
- Parrino, V.; Kesbiç, O.S.; Acar, Ü.; Fazio, F. Hot pepper (Capsicum sp.) oil and its effects on growth performance and blood parameters in rainbow trout (Oncorhynchus mykiss). Nat. Prod. Res. 2019, 34, 3226–3230. [Google Scholar] [CrossRef] [PubMed]
- Saiz, N.; Gómez-Boronat, M.; De Pedro, N.; Delgado, M.J.; Isorna, E. The lack of light-dark and feeding-fasting cycles alters temporal events in the goldfish (Carassius auratus) stress axis. Animals 2021, 11, 669. [Google Scholar] [CrossRef] [PubMed]
- Keppler, D.; Decker, K. Glycogen. Determination with amyloglucosidase. In Methods of Enzymatic Analysis; Bergmeyer, H.U., Ed.; Academic Press: Cambridge, MA, USA, 1974; Volume 3, pp. 1127–1131. [Google Scholar]
- Gómez-Boronat, M.; Velasco, C.; Isorna, E.; De Pedro, N.; Delgado, M.J.; Soengas, J.L.; De Pedro, N.; Delgado, M.J.; Soengas, J.L. The satiety factor oleoylethanolamide impacts hepatic lipid and glucose metabolism in goldfish. J. Comp. Physiol. B 2016, 186, 1009–1021. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]





| Gene | Access Number (GeneBank) | Sense | Sequence | Product (bp) |
|---|---|---|---|---|
| actb | AB039726.2 | Forward | CGGGAGTGATGGTTGGCA | 168 |
| Reverse | AACACGCAGCTGTTGTAGA | |||
| eef1a1 | AB056104 | Forward | CCCTGGCCACAGAGATTTCA | 101 |
| Reverse | CAGCCTCGAACTCACCAACA | |||
| npy | M87297 | Forward | TTCGTCTGCTTGGGAACTCT | 151 |
| Reverse | TGGACCTTTTGCCATACCTC | |||
| pomc | AJ431209 | Forward | CTCACCACTGACGAGAACATCTTG | 121 |
| Reverse | CGGTTTGCTCCAGCTCAGA | |||
| cartpt-I | AY033816 | Forward | GTGCCGAGATGGACTTTGAC | 97 |
| Reverse | AGCTGCTTCTCGTTGGTCAG | |||
| hcrt | DQ923590.1 | Forward | ACTGCACAGCCAAGAGAGTTCA | 166 |
| Reverse | GTTATTAAAGCGGCCGATATGC | |||
| ghrelin | AF454389 | Forward | TTCATGATGAGTGCTCCGTTC | 124 |
| Reverse | GTCAGAATTCAAGTGGCGAATC | |||
| leptin-aI | FJ534535.1 | Forward | AGCTCCTCATAGGGGATC | 192 |
| Reverse | TAGATGTCGTTCTTTCCTTA | |||
| pepck | MK598557 | Forward | TAACTGGCGTCATGGTGTGT | 120 |
| Reverse | TAGCCGAAGAAAGGACGCAT |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saiz, N.; Herrera-Castillo, L.; Isorna, E.; Delgado, M.J.; Conde-Sieira, M.; Soengas, J.L.; de Pedro, N. REV-ERBα Agonist SR9009 Promotes a Negative Energy Balance in Goldfish. Int. J. Mol. Sci. 2022, 23, 2921. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms23062921
Saiz N, Herrera-Castillo L, Isorna E, Delgado MJ, Conde-Sieira M, Soengas JL, de Pedro N. REV-ERBα Agonist SR9009 Promotes a Negative Energy Balance in Goldfish. International Journal of Molecular Sciences. 2022; 23(6):2921. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms23062921
Chicago/Turabian StyleSaiz, Nuria, Lisbeth Herrera-Castillo, Esther Isorna, María Jesús Delgado, Marta Conde-Sieira, José Luis Soengas, and Nuria de Pedro. 2022. "REV-ERBα Agonist SR9009 Promotes a Negative Energy Balance in Goldfish" International Journal of Molecular Sciences 23, no. 6: 2921. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms23062921