Introduction
Phosphate diesters form part of the backbone of DNA and hence are extremely important. A variety of phosphate ester compounds such as sarin, soman, Tabun and Vx act as anticholinesterase agents “nerve gases”. Other phosphate compounds have wide application in agriculture and domestic use. In most hydrolysis reactions of phosphate esters, metalloenzymes are involved which usually require metal ions for activity [
1]. Unfortunately the specific roles played by different ligands and metal centers (mostly divalent) is not clearly understood. Divalent metal ions are substitutionally labile and form a variety of complexes in rapid equilibrium. As a result it has been quite difficult to define the binding mode of any particular complex as being responsible for the rate enhancement observed. In recent years there has been growing interest in the metal ion promoted hydrolysis of phosphate diesters as model systems for cleaving phosphate diesters hydrolytically [
2,
3]. The use of kinetically inert cobalt(III) complexes has led to very significant developments in our understanding of metal ion promoted hydrolysis of phosphate esters. It should be emphasized that lability of substitution is an essential requirement for efficient catalysis and it is for this reason that enzymes primarily contain the first row d-block metal ions as cofactors.
Cobalt (III) complexes offer two important advantages compared with divalent metal ions. First, the cobalt(III) complexes are prepared easily and are kinetically robust, thereby permitting characterization of all species present in solution. Second, the kinetics and mechanism of cobalt (III) complexes in substitution reactions are well understood. Hence this knowledge should facilitate in formulation of mechanisms for metal ion involvement in hydrolytic reactions. In the biosphere, mixed valence bimetallic/polymetallic centers are commonly employed as the catalytic sites in many phosphoryl-transfer enzymes.
Metallohydrolases that contain dinuclear active sites are involved in the degradation of agricultural neurotoxins, urea, B-lactam containing antibiotics, and several chemical weapons that contain phosphorous(V) compounds[
4,
5,
6]. The fact that some hydrolases utilize a mononuclear center while others can function with either a mononucear or dinuclear active site and still others require two metal ions to catalyze the same chemical reaction is not well understood. Hydrolases that contain dinuclear active sites utilize a wide range of first row transition metal ions whose structural properties (e.g. coordination geometry and ligand type) regulate their Lewis acidities, which in turn, controls the level of hydrolytic activity. Possible roles for one or both metal ions in hydrolases that contain co-catalytic active sites include: (i) binding and positioning the substrate; (ii) binding and activating a water molecule to yield an active hydroxide/water nucleophilic site; and (iii) stabilizing the transition state of the hydrolytic reaction. Temperature, pH and reaction media also play crucial roles in the hydrolysis of phosphate esters.
In this investigation prussian blue will be utilized as a model for a mixed valence bimetallic catalyst to mimic the enzymatic reactions. In order to understand the role of each metal ion in catalysis, it is important that a reaction mechanism of a model hydrolytic enzyme with a dinuclear active site be elucidated. With this in mind the investigation of hydrolysis of 4-nitrophenylphosphate assisted by prussian blue was undertaken.
Experimental
All reagents used were either analytical-reagent grade or the purest available commercially and were used without further purification. Measurement of pH was made with a Philip Harris (London, UK) pH meter model w87063, using a combination electrode. A Shimadzu (Kyoto, Japan) uv-2101pc UV/VIS spectrometer was used to obtain spectra and collect rate data. The pH of the reaction mixture was maintained by adding drops of NaOH or HClO
4 from a glass rod. The diaqua complexes [CoN
4(H
2O)
2]
3+, where N
4 = (tn)
2, (tme)
2 or trpn [ tn = trimethylenediamine; tme = 1,1,2,2-tetramethyl-1,2-diaminoethane; trpn = tris(3-aminopropyl)amine] were prepared in solution from the carbonato complexes [CoCO
3N
4] as described previously [
7,
8].
![Ijms 04 00362 i001]()
Microemulsions (ME) were prepared by mixing hexane, sodium dodecylsulfate (SDS) and butan-1-ol and titrating the slurry with water, agitating mildly to give a clear solution [
9]. The following compositions (by mass) were used: ME1, H
2O (90%)-hexane(2%)- SDS(2%)- butan-1-ol(6%); ME2,H
2O (82%)-hexane(3%)- SDS(5%)- butan-1-ol(10%); ME3, H
2O (60%)-hexane(4%)- SDS(18%)- butan-1-ol(18%); ME4, H
2O (43.2%)-hexane(10.4%)- SDS(14.8%)- butan-1-ol(31.6%). The ME2 formulation was chosen for this study since previous investigation showed maximum hydrolysis of organophosphates in metal ion catalysed hydrolysis reactions in ME2 reaction solutions [
10]. Prussian blue/Turnbull blue was synthesized from hexacyanoferrate (III)/hexacyanoferrate(II) using known procedures [
11]. Phosphate buffer (pH 7.4) was prepared by mixing 8.62 grams of sodium phosphate dibasic and 5.42 grams of sodium phosphate monobasic salts and making up to 100 mL with carbon-dioxide free distilled water. The protocol of the study consists of mixing 8 mL of a 5 x 10
-4 M solution of Nitrophenyl phosphate (NPP) in a thermostated reaction vessel with 4 mL of NaClO
4 (0.1 M) and 20 mL of the solvent. The solvent was either water or ME2. The pH of the solution was adjusted to 6.5. An 8 mL volume of temperature equilibrated metal ion solution (5 x 10
-4 M), pH 6.5, aquohydroxo form predominant , was then added making the total volume to 40 mL. Aliquots (2mL) were withdrawn from the reaction vessel at 0,1,3,5,10,and minutes respectively and mixed with 2 mL of phosphate buffer.The absorbance of the reaction solution was then measured at 400 nm immediately after mixing against a solvent blank. The amount of nitrophenolate produced was determined from the calibration curve and the percentage hydrolysis calculated assuming 100% nitrophenolate production for complete hydrolysis. Under slightly basic condition the nitrophenol is deprotonated to form the nitrophenolate ion which is brght yellow and has an absorbance maximum at 400 nm, quite distinct from nitrophenyl phosphate which has an absorbance maximum at 310 nm. The molar extinction coefficients at 310 and 400 nm repectively, are as follows: NP, 8.224 x 10
4 and 1.118 x 10
4 M
-1 cm
-1 : NPP 1.100 x 10
4 and 67 M
-1 cm
-1 . The system confirmed to Beer’s law over the concentration range 0-20 μg l
-1 of nitrophenol when measured at 400 nm in a 1 cm cell.
Results and Discussion
The diaqua-, aquahydroxo-, and dihydroxo(tetraamine)cobalt(III)complexes are related by the acid-base equilibria shown below:
The values for pK
1 and pK
2 as determined by potentiometric titration under conditions where cis-trans isomerization had proceeded to equilibrium are 9.0, 12.1; 5.8, 8.0; 4.9, 8.6 for N
4 = (en)
2, (tn)
2, and (tme)
2 respectively. Diaquabis(ethylenediamine)cobalt(III) in solution will be a mixture of cis and trans isomers as reported by previous investigators. However both diaquabis(trimethylene-diamine)cobalt(III) and diaquabis(1,1,2,2-tetramethyl-1,2-diaminoethane)cobalt(III) will prevail as cis isomers in solution due to a fast cis-trans isomerization (t
1/2 = 1 s at 30
oC) under the experimental condition[
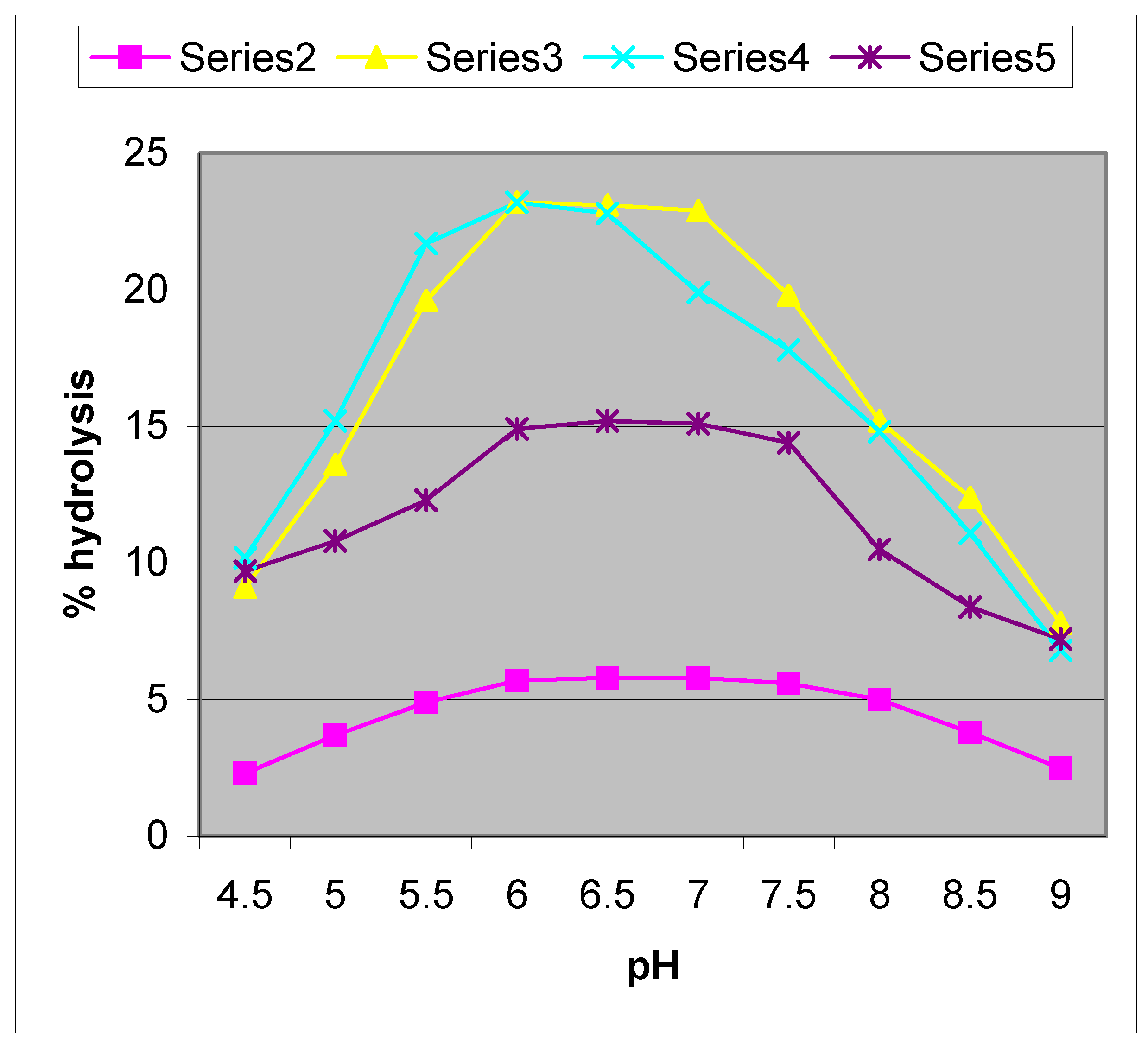
12]. The absorption maxima of this complexes was consistent with cis configuration.The pH rate profile for the cobalt complex ([N
4Co(OH
2)
2]
3+) promoted hydrolysis of NPP shows a bell shaped profile (
Table 1,
Figure 1). It can be seen that the pH corresponding to the rate maxima are all between pK
1 and pK
2 of the acid-base equilibrium for the cobalt complex, indicating that the aquahydroxo form is the active species that promotes the hydrolysis. It has been proposed in previous work that the mechanism of hydrolysis involves the coordination of the phosphate ester to the cobalt complex followed by intramolecular metal hydroxide attack on the coordinated phosphate [
13,
14]. Preliminary experiments have shown that nitrophenol is liberated rapidly and quantitatively from NPP in concentrated hydroxide solutions (2M). The investigation also reveals that metal complexes liberate nitrophenol from NPP with varying degrees of reactivity. The sequence of the reactivity of the metal chelates is [(tn)
2Co(OH
2)
2]
3+ < [(trpn)Co(OH
2)
2]
3+ [(tme)
2Co(OH
2)
2]
3+ < turnbull’s blue for 2:1 metal to NPP ratio in the prevailing experimental condition. The mode of hydrolysis reaction involves the formation of a monodentate chelate between the metal complex and NPP initially. This will be accompanied by the formation of a cyclic phosphate complex intermediate with a possibility of six or four membered chelates as shown previously.
Table 1.
Hydrolysis of Nitrophenylphosphate promoted by metal ions and complexes in aqueous and microemulsion media (10-4 M, I = 0.1 M NaClO4, 25oC, pH 6.5)
Table 1.
Hydrolysis of Nitrophenylphosphate promoted by metal ions and complexes in aqueous and microemulsion media (10-4 M, I = 0.1 M NaClO4, 25oC, pH 6.5)
| Reaction | Media | percentage nitrophenola |
| 0 | 1 | 3 | 5 | 10 | 30 |
| NPP + 2Co(III)(tn)2(aq) | Water | 2.9 | 3.6 | 4.9 | 5.1 | 5.3 | 5.8 |
| ME2 | 3.0 | 3.9 | 5.7 | 6.2 | 6.8 | 7.3 |
| NPP + 2Co(III)(trpn)(aq) | Water | 7.7 | 8.2 | 8.5 | 10.2 | 14.3 | 15.2 |
| ME2 | 8.7 | 9.1 | 9.3 | 12.1 | 17.2 | 21.4 |
| NPP + 2Co(III)(tme)2(aq) | Water | 8.3 | 10.2 | 13.1 | 18.5 | 22.8 | 23.2 |
| ME2 | 10.9 | 13.8 | 17.2 | 24.3 | 28.5 | 29.5 |
| NPP + 2Fe(III)(aq) | Water | 14.4 | 14.7 | 15.3 | 15.6 | 15.8 | 15.8 |
| ME2 | 16.3 | 17.1 | 17.8 | 17.8 | 18.1 | 18.2 |
| NPP + 2Fe(II)(aq) | Water | 10.5 | 10.7 | 10.8 | 10.9 | 11.1 | 11.2 |
| ME2 | 11.1 | 11.3 | 11.5 | 11.7 | 11.7 | 11.9 |
| NPP + Turnbull’s blue(aq) | Water | 18.4 | 19.5 | 20.1 | 21.0 | 22.2 | 22.8 |
| ME2 | 18.9 | 19.8 | 22.9 | 26.9 | 28.2 | 28.8 |
Figure 1.
pH-rate profile for [CoN4(H2O)2]3+, (where N4 = (tn)2, [series2]; (tme)2 [series3]; trpn [series5] ) and Turnbull’s blue[series4] promoted hydrolysis of NPP in aqueous media at 25 0C, c = 5 x 10-4 M, I = 0.1M NaClO4.
Figure 1.
pH-rate profile for [CoN4(H2O)2]3+, (where N4 = (tn)2, [series2]; (tme)2 [series3]; trpn [series5] ) and Turnbull’s blue[series4] promoted hydrolysis of NPP in aqueous media at 25 0C, c = 5 x 10-4 M, I = 0.1M NaClO4.
The formation of the cyclic phosphate complex intermediate creates a ring strain on the chelate therby aiding the hydrolysis of the NPP. In general four membered rings are unstable. However, four membered rings involving Co(III) are relatively stable[
15,
16].
An attack of the four membered chelate by the coordinated hydroxide or water of the second metal complex promotes the hydrolytic reaction envisaged. In [(tme)2Co(OH2)2]3+, the four methyls would lead to an increased electron density thereby enhancing the nucleophilic character of the chelate towards the formation of the 2:1 metal NPP complex. Similarly an attack of coordinated hydroxide or water on the complex at the phosphorous center of NPP will result in hydrolytic reactions. The enhanced reactivity of the [(trpn)Co(OH2)2]3+ over the [(tn)2Co(OH2)2]3+ is attributed to activation of the nitrophenyl phosphate substrate in a strained chelate.
The reaction of Fe
3+ (aq) and [Fe(CN)
6]
4- gives the intensely colored Prussian blue. Similarly the reaction of Fe
2+ (aq) and [Fe(CN)
6]
3- produces Turnbull’s blue. The chemical and physical properties of Prussian blue and Turnbull’s blue seem to be identical. The essential structural element of the three dimensional polymeric framework is the sequence of Fe(III)-N-C-Fe(II). The formation of Prussian blue may be written as:
The cyanide ions act as bridges and link the two structurally distinct iron ions. The assignment of the oxidation states, Fe(II) in a carbon octahedron and Fe(III) in a nitrogen-water environment has been unambiguosly established by a variety of physical techniques[
17]. The studies confirmed the existence of two crystallographically and chemically distinct kinds of water molecules within the relatively open cubic Fe(III)-N-C-Fe(II) framework of prussian blue. The first kind of water molecules is part of the coordination shell of Fe(III) and fills empty nitrogen sites of the Fe(CN)
6 vacancies. The second kind occupies interstitial positions and represents uncoordinated water. For the ideal stoichiometry Fe
4[Fe(CN)
6]
3 .14H
2O, the unit cell contains six coordinated and eight uncoordinated water molecules. The very low solubility presents evidence for the polymeric nature of the product. High spin iron(III) is a spherically symmetrical tripositive cation of radius 0.65 Å, and as such is classified as a hard lewis acid by virtue of its high charge density. It is expected to form most stable bonds with hard ligands such as the charged oxygen atoms of the phosphate esters. In contrast the iron(II) cation, which has a lower charge density, prefers soft ligands. It is also known that ligands that prefer iron (II) retain an appreciable affinity for other biologically important bivalent metals such as copper(II) and zinc(II) ions[
18]. The chelation of Fe(III) of Prussian blue to NPP will produce template in such a way that coordinated hydroxide or water on Fe(II) will attack the phosphorous center thereby effecting hydrolysis. The presence of a large number of coordinated water molecules in the lattice of Prussian blue increases the chance of attack on the phosphorous center which is depicted by an increased degree of hydrolysis. It is established in the literature that hydrolases all have at least one bound water molecule for effective hydrolysis [
19].The effect of the mixed valence bimetallic center on the hydrolysis of Nitrophenylphosphate is clearly demonstrated by comparing the magnitude of hydrolysis obtained by the Fe(II) and Fe(III) promoted hydrolysis of the compound. Since prussian blue is sparingly soluble in water, the mechanism proposed based on the solid structure is also assumed to be applicable in the reaction solutions. It is presumed that the bimetallic center aids in substrate binding and organizing it for effective intramolecular nucleophilic attack by correctly positioned coordinated hydroxide or water. A charge transfer between the Fe(III) and Fe(II) is also presumed to help in aiding complex forming and hydrolysis of the chelate.
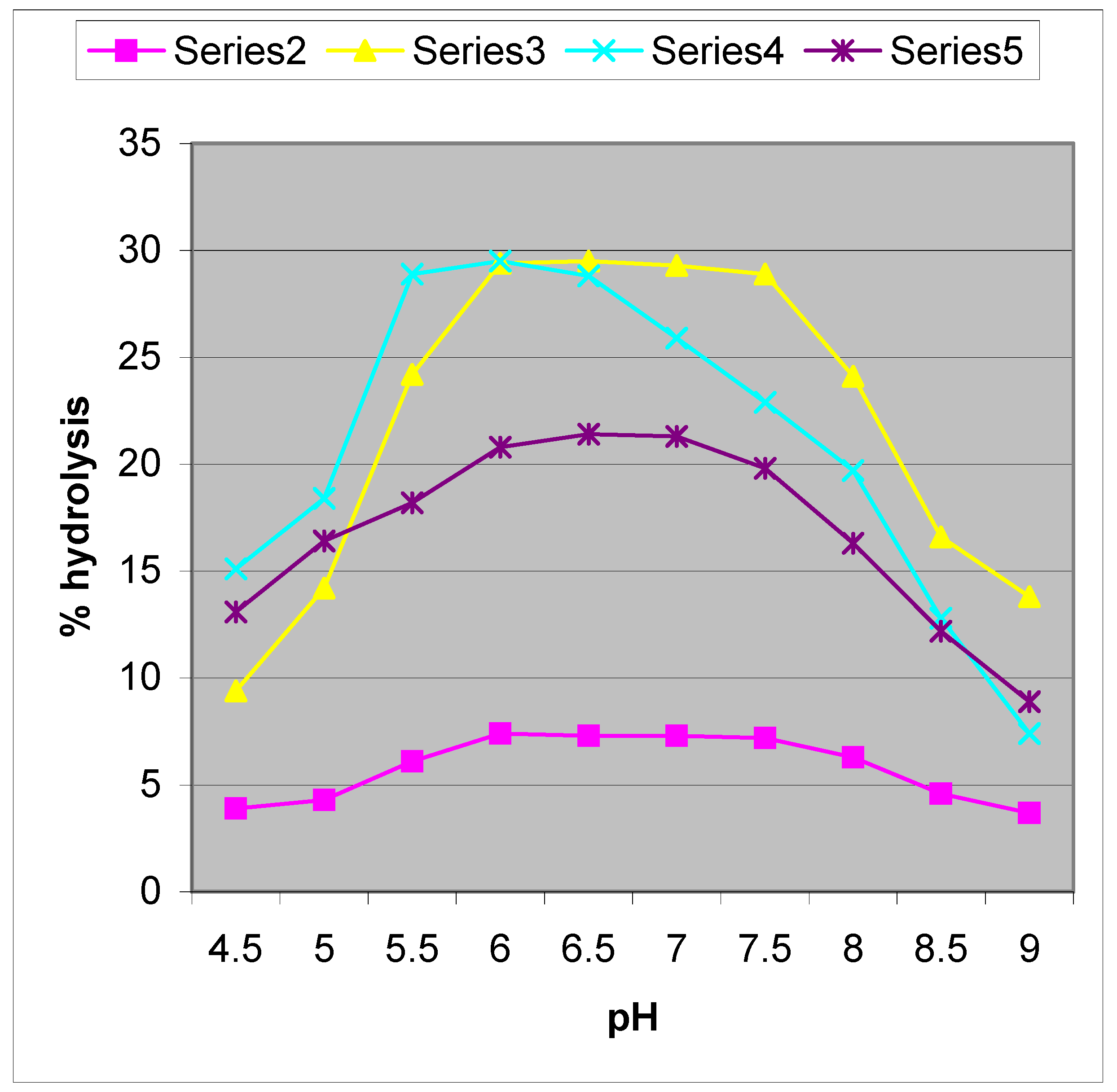
An increased degree of hydrolysis was noted for reactions in microemulsion media(ME2) compared to that of water (
Table 1,
Figure 2). This is not surprizing as microemulsions are thermodynamically stable transparent dispersions of two immiscible liquids, stabilized by an interfacial film of surfactants[
20]. They have the ability to solubilize and disperse the maximum amount of the substrate and the metal complex, enhancing the possibility of hydrolysis by the metal centers. The microemulsion droplets act as tiny microreactors at molecular levels.These features arise from the fact that each of the components of the reaction (water, hydrocarbon, surfactant, co-surfactant, substrate and the metal complex) has its own residence site and its own role in the reaction. This kind of self assembly, inherent to microemulsions, results in the formation of an organized system that helps in
Figure 2.
pH-rate profile for [CoN4(H2O)2]3+, (where N4 = (tn)2,[series2]; (tme)2 [series3] trpn [series5]) and Prussian blue [series4] promoted hydrolysis of NPP in microemulsion (ME2 at 25 0C; c=5 x 10-4 M, I = 0.1M NaClO4.
Figure 2.
pH-rate profile for [CoN4(H2O)2]3+, (where N4 = (tn)2,[series2]; (tme)2 [series3] trpn [series5]) and Prussian blue [series4] promoted hydrolysis of NPP in microemulsion (ME2 at 25 0C; c=5 x 10-4 M, I = 0.1M NaClO4.
assisting hydrolysis. Synergistic effects of the bimetallic active centers and the microemulsions are important in bringing about effective hydrolysis of the NPP. The experimental findings of this investigation establish that polymetallic active centers in oil in water microemulsion (O/W) are a potent means for destroying phosphate ester environmental contaminants.