1. Introduction
The 1-alkyl-3-methylimidazolium salts have generated considerable excitement in recent years because of their unusual properties as room-temperature ionic liquids [
1,
2,
3,
4,
5,
6,
7]. Ionic liquids have found widespread application as new media for organic reactions and solvent extraction [
1,
2]. The key property is that the vapor pressure of ionic liquids is negligible small. Nevertheless, little has been known for liquid structures of ionic liquids. Whereas the results of crystal structures are highly informative on the relative geometry changes, crystallography does not provide direct information on the local structure in the liquid phase. It is not clear whether the solid-structure is equivalent to that found in the liquid or not. Ionic liquids have a melting temperature around room temperature generally achieved by employing a bulky asymmetric organic cation to prevent ions from packing easily. The structure of ionic liquids results from a competition between screening and packing. This means a balance between long-range electrostatic forces and geometric factors [
3,
4,
5,
6,
7,
8].
The question of hydrogen bonding in systems containing 1-alkyl-3-methylimidazolium cations has been addressed recently [
6,
7,
8,
9,
10,
11]. The physical-chemical properties of these ionic liquids are strongly influenced by the nature of the N-alkyl imidazolium substituents and that of the conteranions. Various studies have been made to elucidate the role of weak hydrogen bonds, such as C-H---O and C-H---X, in the structure of ionic liquids [
3,
6,
7,
8,
9,
10,
11]. The imidazolium C-H groups are more favorable site for hydrogen bonding than the alkyl C-H groups. For example, there is evidence of hydrogen bonding between 1-alkyl-3-methylimidazolium cations and the halides via imidazolium C
2-H---X interactions [
6,
7,
8,
9,
10]. Nevertheless, several studies indicated that the alkyl side chain of the 1-alkyl-3-methylimidazolium cation exists in several conformations and may control the melting/ solidification transition of these ionic salts [
12,
13,
14,
15,
16]. The 1-alkyl-3-methylimidazolium cation is a prototype organic cation that generates a variety of ionic liquids when combined with different anions. Therefore, structure determination of the alky side chain both in the crystalline and liquid states is highly important in the structural studies of ionic liquids. Our present study is an attempt to provide the experimental evidence of structural organization of the N-butyl moiety in 1-butyl-3-methylimidazolium chloride.
The polymorphism of 1-butyl-3-methylimidazolium chloride has been independently verified by two groups [
4,
5]. Previous studies into the structure of alkyl side chains have included the use of x-ray crystallography and vibrational spectroscopy [
4,
5,
16]. 1-Butyl-3-methylimidazolium chloride forms two different crystal polymorphs at room temperature, monoclinic (mp 41
oC) and orthorhombic (mp 66
oC). The conformational feature of the cation that differentiates these two types of crystals is the torsion angle around C
7-C
8 in the butyl chain. In the monoclinic form, the butyl chain is all anti, and in the orthorhombic form the chain is gauche around C
7-C
8. In a convenient notation, these conformers are referred to here as the AA and the GA forms. The Raman spectra of these conformers differ considerably, when recorded at room temperature and ambient pressure. The presence of two crystalline polymorphs suggests that the potential energy surface for 1-butyl-3-methylimidazolium chloride contains two, or more, local conformational energy minima [
4,
5]. Inhibition of crystallization through provision of a large number of similarly stabilized solid-state structures may lead to plasticity and low melting points exhibited by ionic liquids.
Generally, vibrational studies were performed at ambient pressure and mostly at room temperature, while interest in pressure as an experimental variable has been growing in physicochemical studies [
17,
18,
19,
20]. The combination of high pressure and spectroscopic methods is beginning to map the conformational landscapes of the aggregation states. Under high pressure conditions, the relative weights of the strong intramolecular interactions responsible for molecular bonding, and of the weaker intermolecular forces defining the aggregation state, are altered, and the repulsive side of the intermolecular potential is explored. Static pressures up to several megabar can be generated using diamond anvil cells. Higher pressures can be generated dynamically by use of shock-wave techniques. The static approach is of interest because it allows continuous tuning of the pressure and the possibility to employ a large number of probing techniques that allow in situ measurements. In the results and discussion section, we demonstrate that the high pressure technique is a sensitive method to probe the conformations of ionic liquids.
2. Results and Discussion
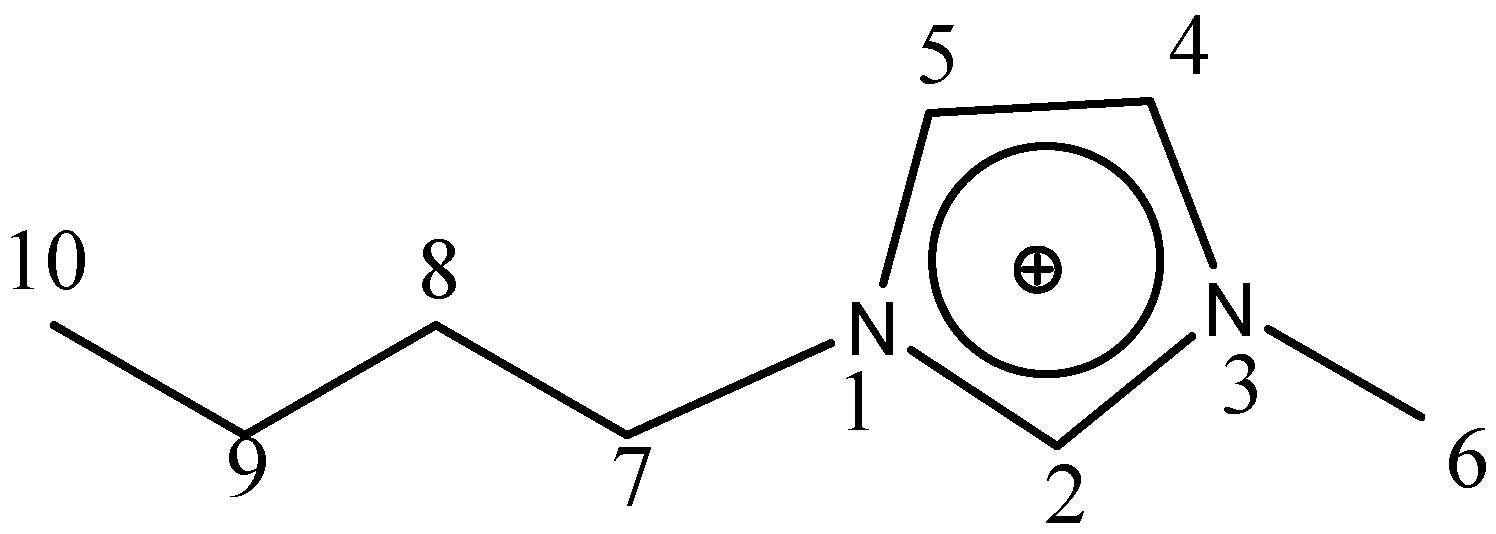
Figure 1 presents the numbering of the skeleton atoms for the 1-butyl-3-methylimidazolium cation.
Figure 1.
Numbering of the skeleton atoms for the 1-butyl-3-methylimidazolium cation.
Figure 1.
Numbering of the skeleton atoms for the 1-butyl-3-methylimidazolium cation.
In order to study the effect of anion, we measured the Raman spectrum of 1-butyl-3-methylimidazolium chloride in a supercooled liquid state (
Figure 2a) under ambient pressure. The Raman bands observed at 600 and 623 cm
-1 may be compared with the vibrational frequencies reported for the Crystal 2 (GA form) and Crystal 1 (AA form), respectively [
4,
21,
22]. Therefore, at least two rotational isomers of the 1-butyl-3-methylimidazolium cation, one having the trans conformation around the C
7-C
8 bond and the other having the gauche conformation, coexist in the ionic liquid state [
4,
16].
Figure 2b shows the Raman spectrum of 1-butyl-3-methylimidazolium chloride in the Crystal 1 form. The two characteristic bands of Crystal 1 at 626 and 733 cm
-1 are in agreement with the literature [
4]. As pointed out by Hamaguchi et al, Crystal 1 seems to be a more thermodynamically stable form under the condition of ambient pressure [
4].
Figure 2c shows the Raman spectrum of 1-butyl-3-methylimidazolium bromide in the crystalline form obtained under ambient pressure. It is well known that the structure of the 1-butyl-3-methylimidazolium cation in 1-butyl-3-methylimidazolium bromide is essentially the same as that in 1-butyl-3-methylimidazolium chloride in Crystal 2 form [
12]. Raman bands characteristic of the GA conformers appeared at 604 cm
-1 as shown in
Figure 2c.
Figure 2.
Raman spectra of (a) 1-butyl-3-methylimidazolium chloride in a supercooled state, (b) 1-butyl-3-methylimidazolium chloride in the Crystal 1 form, and (c) 1-butyl-3-methylimidazolium bromide in the crystalline form obtained under ambient pressure. The Raman intensity (counts) was labeled on the top left corner.
Figure 2.
Raman spectra of (a) 1-butyl-3-methylimidazolium chloride in a supercooled state, (b) 1-butyl-3-methylimidazolium chloride in the Crystal 1 form, and (c) 1-butyl-3-methylimidazolium bromide in the crystalline form obtained under ambient pressure. The Raman intensity (counts) was labeled on the top left corner.
Figure 3 displays Raman spectra of a 1-butyl-3-methylimidazolium chloride in a supercooled liquid state obtained under ambient pressure (curve a) and at 0.3 (curve b), 0.9 (curve c), 1.5 (curve d), 1.9 (curve e), and 2.3 GPa (curve f). As the sample was compressed, i.e., increasing the pressure from ambient (
Figure 3a) to 0.3 GPa (
Figure 3b), we observed blue shifts in frequency for the modes at ca. 600 and 623 cm
-1 corresponding to GA and AA conformers, respectively. The blue shift may originate from the combined effect of the overlap repulsion enhanced by hydrostatic pressure, phase transition, and so forth. We note that the modes of the GA conformer decrease in intensity as the pressure was elevated, cf.,
Figure 3a-c. The contribution of the GA conformer becomes almost negligible under the pressure of 0.9 GPa as revealed in
Figure 3c. The spectral profiles underwent dramatic changes as the pressure was elevated to 1.5 GPa in
Figure 3d. The observation of the characteristic bands at 621 and 701 cm
-1 in
Figure 3d indicates that a new high pressure phase of 1-butyl-3-methylimidazolium chloride is formed. As the high pressure phase sample was further compressed, i.e., increasing the pressure from 1.5 GPa (
Figure 3d) to 2.3 GPa (
Figure 3f), we observed a monotonic blue shift in frequency for the characteristic bands at ca. 621 and 701 cm
-1, respectively. The characteristic bands were blue-shifted to 626 and 705 cm
-1 in
Figure 3f. These results indicate that the new high phase seems to be thermodynamically stable up to the pressure of 2.3 GPa.
Figure 3g displays Raman spectrum of 1-butyl-3-methylimidazolium bromide in the Crystal 2 form obtained under the pressure of 2.3 GPa. The similarity between
Figure 3f and 3g suggests that
Figure 3f originates from a Crystal 2-like structure.
Figure 3.
Raman spectra of 1-butyl-3-methylimidazolium chloride in a supercooled state under the following pressures: (a) ambient, (b) 0.3, (c) 0.9, (d) 1.5, (e) 1.9, and (f) 2.3 GPa. Curve g displays Raman spectrum of 1-butyl-3-methylimidazolium bromide obtained under the pressure of 2.3 GPa.
Figure 3.
Raman spectra of 1-butyl-3-methylimidazolium chloride in a supercooled state under the following pressures: (a) ambient, (b) 0.3, (c) 0.9, (d) 1.5, (e) 1.9, and (f) 2.3 GPa. Curve g displays Raman spectrum of 1-butyl-3-methylimidazolium bromide obtained under the pressure of 2.3 GPa.
We obtained a complementary insight into the new high pressure phase by measuring the pressure-dependent variations in the Raman spectra of 1-butyl-3-methylimidazolium chloride in Crystal 1 form.
Figure 4a and 4b presents Raman spectra of Crystal 1 form obtained under ambient pressure and 0.3 GPa, respectively. The two characteristic bands of Crystal 1 at 626 and 733 cm
-1 in
Figure 4a are blue-shifted to 631 and 734 cm
-1, respectively, in
Figure 4b. Upon leaving Crystal 1 under 0.3 GPa for more than 24 hours, Crystal 1 was converted to Crystal 2-like (GA form-like) as revealed in
Figure 4c. The characteristic bands appear at 613 and 700 cm
-1 in
Figure 4c., while the Crystal 2 bands (GA form) locate at 600 cm
-1 and 700 cm
-1 as shown in
Figure 2a. Therefore, we suggest that the spectral features in
Figure 4c originate from the GA conformer in a perturbed form. AA form, being a thermodynamically stable form at ambient pressure, is switched to a metastable state under the condition of high pressure, cf.
Figure 4b-c. At pressure of 0.9 GPa (
Figure 4d), the spectral features showed further evolution through the observation of bandwidth narrowing for the peaks at ca. 830 and 900 cm
-1. Analysis of the pressure dependence shows that the characteristic band at 613 cm
-1 in
Figure 4c was blue-shifted to 619 cm
-1 in
Figure 4d, but the characteristic band at 700 cm
-1 in
Figure 4c was slightly red-shifted to 698 cm
-1 in
Figure 4d. These results suggest that a structural relaxation or a second phase transition is taking place. As the pressure was elevated to 1.5 GPa (
Figure 4e), both of the characteristic bands were blue-shifted again to 622 and 701 cm
-1, respectively. We noted that
Figure 3d and
Figure 4e are almost identical in spectral features.
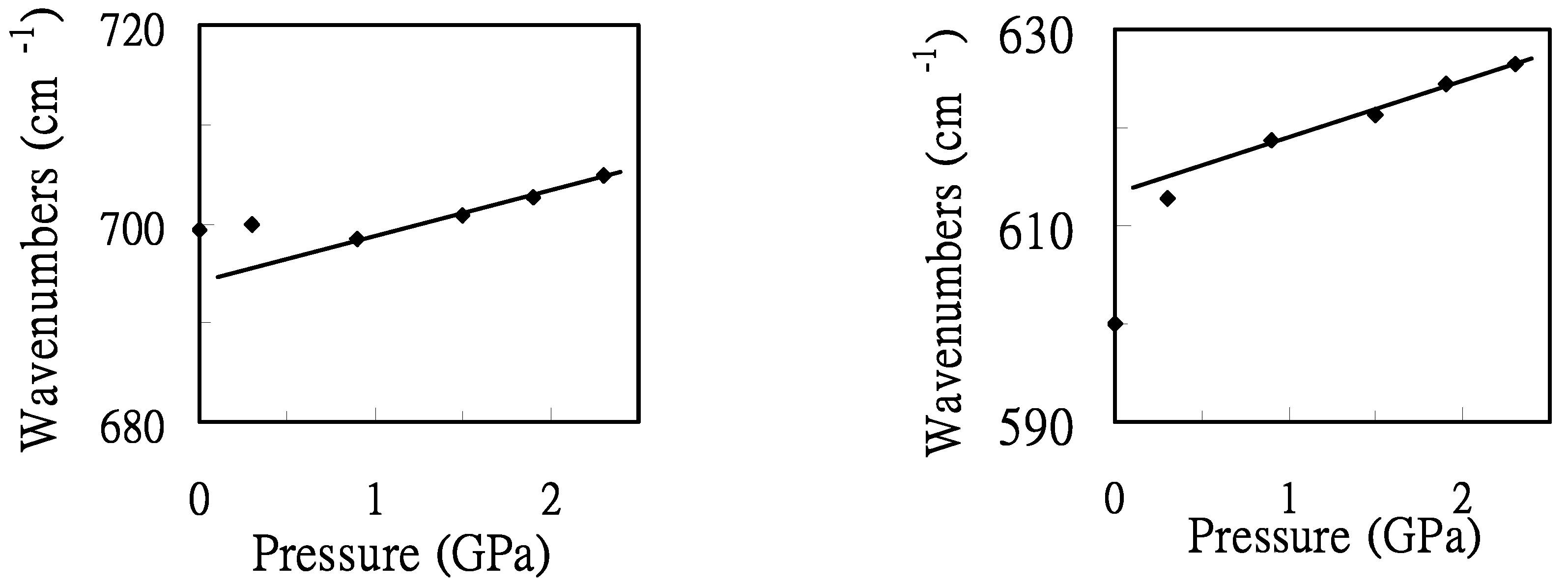
To illustrate the frequency shift, the pressure dependence of the maximum positions of the characteristic bands of the GA conformer (Crystal 2-like) was plotted in
Figure 5. The application of external pressure hardly alters the frequencies of the 700 cm
-1 band for the pressures less than 0.9 GPa. Nevertheless, the pressure-induced frequency-shifts of the 700 cm
-1 band are relatively large under the pressure above 0.9 GPa. This may indicate a pressure-induced structural transformation above the pressure of 0.9 GPa. The increase in the vibrational frequencies with pressure for the 600 cm
-1 band under the pressures < 0.9 GPa, is contrasts to the trend observed for the 700 cm
-1 band. The major changes induced by the pressure are the changes in intermolecular distances. Frequencies of the characteristic bands, i.e., 600 and 700 cm
-1 bands, increase linearly with increasing pressure above the pressure of 0.9 GPa. Slopes (dν/dP) of 6 and 5 cm
-1/GPa were obtained for the bands at ca. 600 and 700 cm
-1, respectively. Based on the experimental results in
Figure 3,
Figure 4 and
Figure 5, we suggest that the new high pressure phase of 1-butyl-3-methylimidazolium chloride arises from perturbed GA conformer, i.e, distorted Crystal 2. Crystal 2 form (AA form) of 1-butyl-3-methylimidazolium chloride is known as a less thermodynamically stable form under ambient pressure. It appears that high pressure somehow stabilize this unusual conformation. Crystal 1 form is switched to a metastable state under the condition of high pressure.
3. Experimental Section
Samples were prepared using 1-butyl-3-methylimidazolium chloride (purity>90%, H2O< 0.02%) and 1-butyl-3-methylimidazolium bromide (purity>97%, H2O < 1%) supplied by Fluka. The compounds were received in the crystalline form. A diamond anvil cell (DAC) of Merril–Bassett design, having a diamond culet size of 0.6 mm, was used for generating pressures up to ca. 2 GPa. Two type-Ia diamonds were used for Raman measurements. The sample was contained in a 0.3-mm-diameter hole in a 0.25-mm-thick stainless-steel gasket mounted on the diamond anvil cell. A droplet of a sample filled the empty space of the entire hole of the gasket in the DAC, which was subsequently sealed when the opposed anvils were pushed toward one another.
Figure 4.
Pressure dependence of the Raman spectra of 1-butyl-3-methylimidazolium chloride in the Crystal 1 form under the following pressures: (a) ambient, (b) 0.3, (c) 0.3 (after 24 hours), (d) 0.9, and (e) 1.5 GPa.
Figure 4.
Pressure dependence of the Raman spectra of 1-butyl-3-methylimidazolium chloride in the Crystal 1 form under the following pressures: (a) ambient, (b) 0.3, (c) 0.3 (after 24 hours), (d) 0.9, and (e) 1.5 GPa.
Figure 5.
Pressure dependence of the maximum positions of the characteristic bands at ca. 700 and 600 cm-1.
Figure 5.
Pressure dependence of the maximum positions of the characteristic bands at ca. 700 and 600 cm-1.
The Raman spectra were measured using a 100-mw diode pumped solid state (DPSS) laser (λ= 532 nm) and a microscope-based Raman spectrometer having a 300-mm spectrograph (Acton SP308), equipped with an 1200 gr/mm holographic grating and a side window photon counting detector system. We chose 100 μm slit width corresponding to a resolution of ca. 5 cm-1.