Synthesis and Characterization of Molecularly Imprinted Polymers for Phenoxyacetic Acids
Abstract
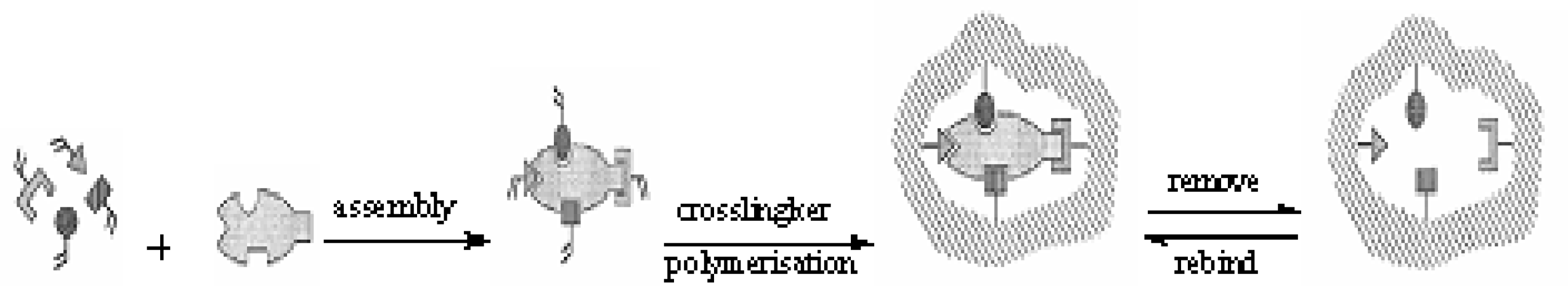
:1. Introduction
2. Results and Discussion
2.1 HPLC and Cross-selectivity
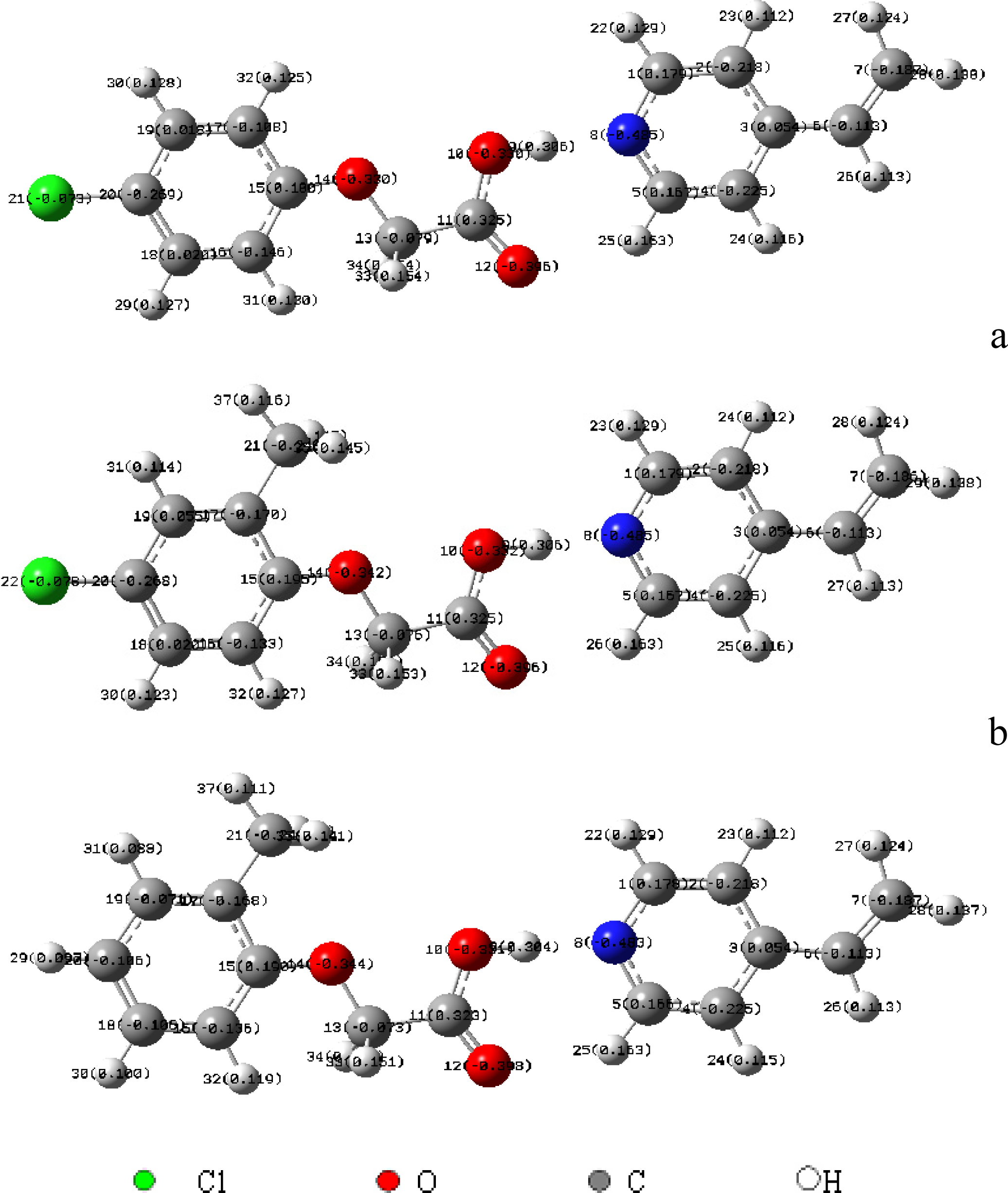
2.2 Computational molecular modeling
3. Experimental Section
3.1 Chemicals and apparatus
3.2 Preparation of Polymers
3.3 HPLC experiments
3.4 Computational molecular modeling
Acknowledgements
References and Notes
- Garrison, AW; Schmitt, P; Martens, D; Kettrup, A. Enatiomeric selectivity in the environ mental degradation of dichlorprop as determined by high performance capillary electrophoresis. Environ Sci & Technol 1996, 30, 2449–2455. [Google Scholar]
- Nilsson, T; Baglio, D; Galdo-Miguez, I; Ogaard-Madsen, J; Facchetti, S. Derivatisation/solidphase microextraction followed by gas chromatography-mass spectrometry for the analysis of phenoxy acid herbicides in aqueous samples. J Chromatogr A 1998, 826, 211–216. [Google Scholar]
- Eitzer, BD; Chevalier, A. Landscape care pesticide residues in residential drinking water wells. Bull Environ Contam Toxicol 1999, 62, 420–427. [Google Scholar]
- Haupt, K; Dzgoev, A; Mosbach, K. Assay system for the herbicide 2,4-dichlorophenoxyacetic acid using a molecularly imprinted polymer as an artificial recognition element. Anal Chem 1998, 70, 628–631. [Google Scholar]
- Haupt, K; Dzgoev, A; Mosbach, K. Assay system for the herbicide 2,4-Dichlorophenoxyacetic acid using a molecularly imprinted polymer as an artificial recognition element. Anal Chem 1998, 70, 3936–3939. [Google Scholar]
- Baggiani, C; Giraudi, G; Giovannoli, C. Chromatographic characterization of molecularly imprinted polymers bnding the herbicide 2,4,5-trichlorophenoxyacetic acid. J Chromatogr A 2000, 883, 119–126. [Google Scholar]
- Baggiani, C; Giovannoli, C; Anfossi, L; Tozzi, C. Molecularly imprinted solid-phase extraction sorbent for the clean-up of chlorinated phenoxyacids from aqueous samples. J Chromatogr A 2001, 938, 35–44. [Google Scholar]
- Baggiani, C; Giraudi, G; Giovannoli, C; Tozzi, C; Anfossi, L. Adsorption isotherms of a molecular imprinted polymer prepared in the presence of a polymerisable template indirect evidence of the formation of template clusters in the binding site. Anal Chim Acta 2004, 504, 43–52. [Google Scholar]
- Baggiani, C; Anfossi, L; Giovannoli, C; Tozzi, C. Binding properties of 2,4,5- trichlorophenoxyacetic acid-imprinted polymers prepared with different molar ratios between template and functional monomer. Talanta 2004, 62, 1029–1034. [Google Scholar]
- O'Mahony, J; Molinelli, A; Mizaikoff, B. Towards the rational development of molecularly imprinted polymers: 1H NMP studies on hydrophobicity and ion-pair interactions as driving forces for selectivity. Biosens Bioelect 2005, 20, 1884–1893. [Google Scholar]
- Kroger, S; Turner, APF; Mosbach, K; Haupt, K. Imprinted polymer-based sensor system for herbicides using differential-pulse voltammetry on screen-printed electrodes. Anal Chem 1999, 71, 3698–3702. [Google Scholar]
- Jakusch, M; Janotta, M; Mizaikoff, B. Molecularly imprinted polymers and infrared evanescent wave spectroscopy. A chemical sensors approach. Anal Chem 1999, 71, 4786–4791. [Google Scholar]
- Liang, C; Peng, H; Nie, LH. Bulk acoustic wave sensor for herbicide assaybased on molecularly imprinted polymer. Fresenius J Anal Chem 2000, 367, 551–555. [Google Scholar]
- Leung, M; Chow, C; Lam, M. A sol–gel derived molecular imprinted luminescent PET sensing material for 2,4-dichlorophenoxyacetic acid. J Mater Chem 2001, 11, 2985–2991. [Google Scholar]
- Svitel, J; Surugiu, I; Dzgoev, A. Functionalized surfaces for optical biosensors: applications to in vitro pesticide residual analysis. J Mater Sci - Mater Med 2001, 12, 1075–1078. [Google Scholar]
- Weetall, HH; Rogers, KR. Preparation and characterization of molecularly imprinted electropoly-merized carbon electrodes. Talanta 2004, 62, 329–335. [Google Scholar]
- Kawaguchi, M; Hayatsu, Y; Nakata, H; Ishii, Y; Ito, R; Saito, K; Nakazawa, H. Molecularly imprinted solid phase extraction using stable isotope labeled compounds as template and liquid chromatography-mass spectrometry for trace analysis of bisphenol A in water sample. Anal Chim Acta 2005, 539, 83–89. [Google Scholar]
- Theodoridis, G; Kantifes, A; Manesiotis, P; Raikos, N; Tsoukali-Papadopoulou, H. Preparation of a molecularly imprinted polymer for the solid-phase extraction of scopolamine with hyoscyamine as a dummy template molecule. J Chromatogr A 2003, 987, 103–109. [Google Scholar]
- Wang, J; Guo, R; Chen, J; Zhang, Q; Liang, X. Phenylurea herbicides-selective polymer prepared by molecular imprinting using N-(4-isopropylphenyl)-N′-butyleneurea as dummy template. Anal Chim Acta 2005, 540, 307–315. [Google Scholar]
- Chianella, I; Karim, K; Piletska, EV; Preston, C; Piletsky, SA. Computational design and synthesis of molecularly imprinted polymers with high binding capacity for pharmaceutical applications-model case: adsorbent for abacavir. Anal Chim Acta 2006, 559, 73–78. [Google Scholar]
- Breton, F; Rouillon, R; Piletska, EV; Piletsky, SA. Virtual imprinteing as a tool design efficient MIPs for photosynthesis-inhibiting herbicides. Biosens Bioelect 2006, 22, 1948–1954, DOI: 10.1016/j.bios.2006.08.017. [Google Scholar]
- Dineiro, Y; Menendez, MI; Blanco-Lopez, MC; Lobo-Castanon, MJ; Miranda-Ordieres, AJ; Tunon-Blanco, P. Computational predictions and experimental affinity distributions for a homovanillic acid molecularly imprinted polymer. Biosens Bioelect 2006, 22, 364–371. [Google Scholar]
- O'Mahony, J; Molinelli, A; Mizaikoff, B. Anatomy of a successful imprint: Analysing the recognition mechanisms of a molecularly imprinted polymer for quercetin. Biosens Bioelect 2006, 21, 1383–1392. [Google Scholar]
- Ernzerhof, M; Perdew, JP; Burke, K. Coupling-constant dependence of atomization energies. International Journal of Quantum Chemistry 1997, 64, 285–295. [Google Scholar]
- Ernzernof, M; Scuseria, GE. Assessment of the Perdew–Burke–Ernzerhof exchange-correlation functional. The Journal of Chemical Physics 1999, 110, 5029–5036. [Google Scholar]
- Rabuck, AD; Scuseria, GE. Performance of recently developed kinetic energy density functionals for the calculation of hydrogen binding strengths and hydrogen-bonded structures. Theoretical Chemistry Accounts 2000, 104, 439–444. [Google Scholar]
- Frisch, MJ; Trucks, GW; Schlegel, HB; Scuseria, GE; Robb, MA; Cheeseman, JR; Montgomery, JA; Vreven, T, Jr; Kudin, KN; Burant, JC; Millam, JM; Iyengar, SS; Tomasi, J; Barone, V; Mennucci, B; Cossi, M; Scalmani, G; Rega, N; Petersson, GA; Nakatsuji, H; Hada, M; Ehara, M; Toyota, K; Fukuda, R; Hasegawa, J; Ishida, M; Nakajima, T; Honda, Y; Kitao, O; Nakai, H; Klene, M; Li, X; Knox, JE; Hratchian, HP; Cross, JB; Adamo, C; Jaramillo, J; Gomperts, R; Stratmann, RE; Yazyev, O; Austin, AJ; Cammi, R; Pomelli, C; Ochterski, JW; Ayala, PY; Morokuma, K; Voth, GA; Salvador, P; Dannenberg, JJ; Zakrzewski, VG; Dapprich, S; Daniels, AD; Strain, MC; Farkas, O; Malick, DK; Rabuck, AD; Raghavachari, K; Foresman, JB; Ortiz, JV; Cui, Q; Baboul, AG; Clifford, S; Cioslowski, J; Stefanov, BB; Liu, G; Liashenko, A; Piskorz, P; Komaromi, I; Martin, RL; Fox, DJ; Keith, T; Al-Laham, MA; Peng, CY; Nanayakkara, A; Challacombe, M; Gill, PMW; Johnson, B; Chen, W; Wong, MW; Gonzalez, C; Pople, JA. GAUSSIAN03, Revision B02 2003.
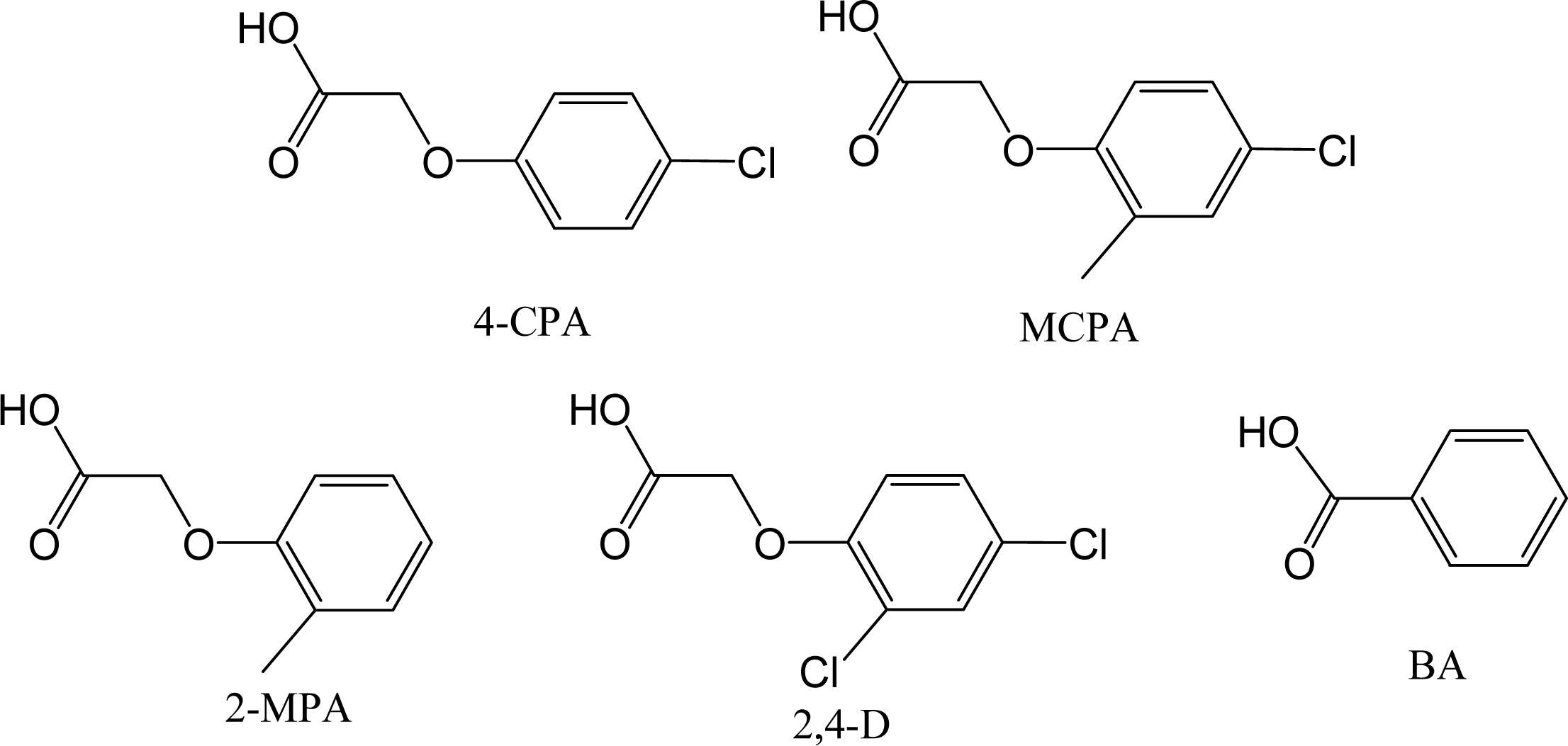

| k′ blank | k′ MIPs | αblank | αMIPs | RI | |
|---|---|---|---|---|---|
| MCPA | 0.668 | 1.411 | 1 | 1 | 1 |
| 4-CPA | 0.778 | 1.581 | 0.859 | 0.892 | 0.962 |
| 2-MPA | 0.468 | 0.864 | 1.427 | 1.633 | 0.874 |
| BA | 0.504 | 0.535 | 1.325 | 2.637 | 0.503 |
| 2,4-D | 0.982 | 2.404 | 0.680 | 0.587 | 1.158 |
| k′ blank | k′ MIPs | αblank | αMIPs | RI | |
|---|---|---|---|---|---|
| 4-CPA | 0.778 | 2.241 | 1 | 1 | 1 |
| MCPA | 0.668 | 1.732 | 1.165 | 1.294 | 0.900 |
| 2-MPA | 0.468 | 1.072 | 1.662 | 2.090 | 0.795 |
| BA | 0.504 | 0.590 | 1.544 | 3.798 | 0.406 |
| 2,4-D | 0.982 | 3.170 | 0.792 | 0.707 | 1.121 |
| k′ blank | k′ MIPs | αblank | αMIPs | RI | |
|---|---|---|---|---|---|
| 2-MPA | 0.468 | 0.943 | 1 | 1 | 1 |
| 4-CPA | 0.778 | 1.702 | 0.602 | 0.554 | 1.086 |
| MCPA | 0.668 | 1.410 | 0.700 | 0.669 | 1.048 |
| BA | 0.504 | 0.605 | 0.928 | 1.559 | 0.596 |
| 2,4-D | 0.982 | 2.475 | 0.476 | 0.381 | 1.251 |
| ΔE (kcal/mol) | rA(Å) | rB(Å) | rC (Å) | |
|---|---|---|---|---|
| 4-CPA | -14.47979 | 0.967 | 1.016 | 1.664 |
| MCPA | -14.56388 | 0.967 | 1.016 | 1.663 |
| 2-MPA | -14.15600 | 0.967 | 1.013 | 1.666 |
Share and Cite
Zhang, H.; Song, T.; Zong, F.; Chen, T.; Pan, C. Synthesis and Characterization of Molecularly Imprinted Polymers for Phenoxyacetic Acids. Int. J. Mol. Sci. 2008, 9, 98-106. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms9010098
Zhang H, Song T, Zong F, Chen T, Pan C. Synthesis and Characterization of Molecularly Imprinted Polymers for Phenoxyacetic Acids. International Journal of Molecular Sciences. 2008; 9(1):98-106. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms9010098
Chicago/Turabian StyleZhang, Huiting, Tao Song, Fulin Zong, Tiechun Chen, and Canping Pan. 2008. "Synthesis and Characterization of Molecularly Imprinted Polymers for Phenoxyacetic Acids" International Journal of Molecular Sciences 9, no. 1: 98-106. https://0-doi-org.brum.beds.ac.uk/10.3390/ijms9010098




