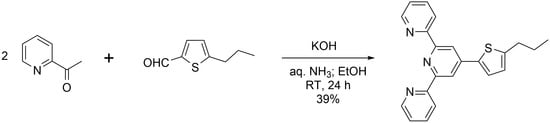
4′-(5-N-Propylthiophen-2-yl)-2,2′:6′,2″-terpyridine
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Schubert, U.S.; Hofmeier, H.; Newkome, G.R. Modern Terpyridine Chemistry; Wiley-VCH: Weinheim, Germany, 2006. [Google Scholar]
- Husson, J.; Knorr, M. 2,2′:6′,2″-Terpyridines Functionalized with Thienyl Substituents: Synthesis and Applications. J. Heterocycl. Chem. 2012, 49, 453–478. [Google Scholar] [CrossRef]
- Vincent Joseph, K.L.; Anthonysamy, A.; Easwaramoorthi, R.; Shinde, D.V.; Ganapathy, V.; Karthikeyan, S.; Lee, J.; Park, T.; Rhee, S.-W.; Kim, K.S.; et al. Cyanoacetic acid tethered thiophene for well-matched LUMO level in Ru(II)-terpyridine dye sensitized solar cells. Dyes Pigment. 2016, 126, 270–278. [Google Scholar] [CrossRef]
- Dehaudt, J.; Husson, J.; Guyard, L.; Oswald, F.; Martineau, D. A simple access to “Black-Dye” analogs with good efficiencies in dye-sensitized solar cells. Renew. Energy 2014, 66, 588–595. [Google Scholar] [CrossRef]
- Caramori, S.; Husson, J.; Beley, M.; Bignozzi, C.A.; Argazzi, R.; Gros, P.C. Combination of Cobalt and Iron Polypyridine Complexes for Improving the Charge Separation and Collection in Ru(terpyridine)2-Sensitised Solar Cells. Chem. Eur. J. 2010, 16, 2611–2618. [Google Scholar] [CrossRef] [PubMed]
- Pruskova, M.; Sutrova, V.; Slouf, M.; Vlckova, B.; Vohlidal, J.; Sloufova, I. Arrays of Ag and Au Nanoparticles with Terpyridine- and Thiophene-Based Ligands: Morphology and Optical Responses. Langmuir 2017, 33, 4146–4156. [Google Scholar] [CrossRef]
- Shen, Y.; Shao, T.; Fang, B.; Du, W.; Zhang, M.; Liu, J.; Liu, T.; Tian, X.; Zhang, Q.; Wang, A.; et al. Visualization of mitochondrial DNA in living cells with super-resolution microscopy using thiophene-based terpyridine Zn(II) complexes. Chem. Commun. 2018, 54, 11288–11291. [Google Scholar] [CrossRef]
- Feng, Z.; Li, D.; Zhang, M.; Shao, T.; Shen, T.; Tian, X.; Zhang, Q.; Li, S.; Wu, J.; Tian, Y. Enhanced three-photon activity triggered by the AIE behavior of a novel terpyridine-based Zn(II) complex bearing a thiophene bridge. Chem. Sci. 2019, 10, 7228–7232. [Google Scholar] [CrossRef]
- Njogu, E.M.; Martincigh, B.S.; Omondi, B.; Nyamori, V.O. Synthesis, characterization, antimicrobial screening and DNA binding of novel silver(I)-thienylterpyridine and silver(I)-furylterpyridine. Appl. Organomet. Chem. 2018, 32, e4554. [Google Scholar] [CrossRef]
- Liang, Y.W.; Strohecker, D.; Lynch, V.; Holliday, B.J.; Jones, R.A. A Thiophene-Containing Conductive Metallopolymer Using an Fe(II) Bis(terpyridine) Core for Electrochromic Materials. ACS Appl. Mater. Interfaces 2016, 8, 34568–34580. [Google Scholar] [CrossRef]
- Fernandes, S.S.M.; Besley, M.; Ciarrocchi, C.; Licchelli, M.; Raposo, M.M.M. Terpyridine derivatives functionalized with (hetero)aromatic groups and the corresponding Ru complexes: Synthesis and characterization as SHG chromophores. Dyes Pigment. 2018, 150, 49–58. [Google Scholar] [CrossRef]
- Mukherjee, S.; Torres, D.E.; Jakubikova, E. HOMO inversion as a strategy for improving the light-absorption properties of Fe(II) chromophores. Chem. Sci. 2017, 8, 8115–8126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heller, M.; Schubert, U.S. Syntheses of functionalized 2,2′:6′,2″-terpyridines. Eur. J. Org. Chem. 2003, 6, 947–961. [Google Scholar] [CrossRef]
- Fallahpour, R.A. Synthesis of 4′-substituted-2,2′:6′,2″-terpyridines. Synthesis 2003, 2, 155–184. [Google Scholar] [CrossRef]
- Thompson, A.M.W.C. The synthesis of 2,2′:6′,2″-terpyridine ligands- versatile building blocks for supramolecular chemistry. Coord. Chem. Rev. 1997, 160, 1–52. [Google Scholar] [CrossRef]
- Wang, J.; Hanan, G.S. A Facile Route to Sterically Hindered and Non-Hindered 4′-Aryl-2,2′:6′,2″-Terpyridines. Synlett 2005, 8, 1251–1254. [Google Scholar] [CrossRef]
- Organic Syntheses. Available online: http://www.orgsyn.org/instructions.aspx (accessed on 9 October 2020).
- Pinciroli, V.; Biancardi, V.; Visentin, G.; Rizzo, V. The Well-Characterized Synthetic Molecule: A Role for Quantitative 1H NMR. Org. Process. Res. Dev. 2004, 8, 381–384. [Google Scholar] [CrossRef]
- Husson, J.; Guyard, L. 4′-(5-Methylfuran-2-yl)-2,2′:6′,2″-terpyridine: A New Ligand Obtained from a Biomass-Derived Aldehyde with Potential Application in Metal-Catalyzed Reactions. Molbank 2018, 2018, M1032. [Google Scholar] [CrossRef] [Green Version]
- Beley, M.; Delabouglise, D.; Houppy, G.; Husson, J.; Petit, J.-P. Preparation and properties of ruthenium (II) complexes of 2,2′:6′,2″-terpyridines substituted at the 4′-position with heterocyclic groups. Inorg. Chim. Acta 2005, 358, 3075–3083. [Google Scholar] [CrossRef]
- Husson, J.; Dehaudt, J.; Guyard, L. Preparation of carboxylate derivatives of terpyridine via the furan pathway. Nat. Protoc. 2014, 9, 21–26. [Google Scholar] [CrossRef]
- Et Taouil, A.; Husson, J.; Guyard, L. Synthesis and characterization of electrochromic [Ru(terpy)2 selenophene]-based polymer film. J. Electroanal. Chem. 2014, 728, 81–85. [Google Scholar] [CrossRef]
- Husson, J.; Abdeslam, E.T.; Guyard, L. A missing member in the family of chalcogenophene-substituted 2,2′:6′,2″-terpyridine: 4′-(tellurophen-2-yl)-2,2′:6′,2″-terpyridine, its Ru(II) complex and its electropolymerization as a thin film. J. Electroanal. Chem. 2019, 855, 113594. [Google Scholar] [CrossRef]
- Zheng, C.; Pu, S.; Xu, J.; Luo, M.; Huang, D.; Shen, L. Synthesis and the effect of alkyl chain length onoptoelectronic properties of diarylethene derivatives. Tetrahedron Lett. 2007, 63, 5437–5449. [Google Scholar] [CrossRef]
- Howbert, J.J.; Mohamadi, F.; Spees, M.M. Antitumor Compositions and methods of Treatment. U.S. Patent 5,302,724, 12 April 1994. [Google Scholar]
- Zhang, S.; Huang, S.; Feng, C.; Cai, J.; Chen, J.; Ji, M. Novel Preparation of Tiaprofenic Acid. J. Chem. Res. 2013, 37, 406–408. [Google Scholar] [CrossRef]



 | ||||||
| X= | S | O | Se | Te | ||
| R= | nC6H13 | nC3H7 | H | H | H | H |
| Mp (°C) | 70–72 a | 98–99 | 197–199 a | 219 a | 215–218 a | 196–200 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Husson, J.; Guyard, L. 4′-(5-N-Propylthiophen-2-yl)-2,2′:6′,2″-terpyridine. Molbank 2021, 2021, M1183. https://0-doi-org.brum.beds.ac.uk/10.3390/M1183
Husson J, Guyard L. 4′-(5-N-Propylthiophen-2-yl)-2,2′:6′,2″-terpyridine. Molbank. 2021; 2021(1):M1183. https://0-doi-org.brum.beds.ac.uk/10.3390/M1183
Chicago/Turabian StyleHusson, Jérôme, and Laurent Guyard. 2021. "4′-(5-N-Propylthiophen-2-yl)-2,2′:6′,2″-terpyridine" Molbank 2021, no. 1: M1183. https://0-doi-org.brum.beds.ac.uk/10.3390/M1183