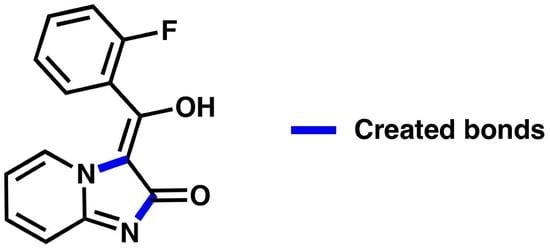
(E)-3-((2-Fluorophenyl)(hydroxy)methylene)imidazo[1,2-a]pyridin-2(3H)-one
Abstract
:1. Introduction
2. Results and Discussion
3. Materials and Methods
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goel, R.; Luxami, V.; Paul, K. Imidazo[1,2-a]pyridines: Promising drug candidates for antitumor therapy. Curr. Top. Med. Chem. 2016, 16, 3590–3616. [Google Scholar] [CrossRef] [PubMed]
- Yu, Y.; Han, Y.; Zhang, F.; Gao, Z.; Zhu, T.; Dong, S.; Ma, M. Design, synthesis, and biological evaluation of imidazo[1,2-a]pyridine derivatives as novel PI3K/mTOR dual inhibitors. J. Med. Chem. 2020, 63, 3028–3046. [Google Scholar] [CrossRef] [PubMed]
- Foda, N.H.; Ali, S.M. Zolpidem tartrate. Profiles Drug Subst. Excip. Relat. Methodol. 2012, 37, 413–438. [Google Scholar] [PubMed]
- Skolnick, P. Anxioselective anxiolytics: On a quest for the holy grail. Trends Pharmacol. Sci. 2012, 33, 611–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, D.; Bhutia, Z.; Panjikar, P.C.; Chatterjee, A.; Banerjee, M. A simple and efficient route to 2-arylimidazo[1,2-a]pyridines and zolimidine using automated grindstone chemistry. J. Heterocycl. Chem. 2020, 57, 4099–4107. [Google Scholar] [CrossRef]
- Mizushige, K.; Ueda, T.; Yukiiri, K.; Suzuki, H. Olprinone: A phosphodiesterase III inhibitor with positive inotropic and vasodilator effects. Cardiovasc. Drug Rev. 2006, 20, 163–174. [Google Scholar] [CrossRef] [PubMed]
- Kumar Bagdi, A.; Santra, S.; Monir, K.; Hajra, A. Synthesis of imidazo[1,2-a]pyridines: A decade update. Chem. Commun. 2015, 51, 1555–1575. [Google Scholar] [CrossRef] [PubMed]
- Tashrifi, Z.; Mohammadi-Khanaposhtani, M.; Larijani, B.; Mahdav, M. C3-functionalization of imidazo[1,2-a]pyridines. Eur. J. Org. Chem. 2020, 269–284. [Google Scholar] [CrossRef]
- Hemasrilatha, S.; Sruthi, K.; Manjula, A.; Harinadha Babu, V.; Vittal Rao, B. Synthesis and anti-inflammatory activity of imidazo[1,2-a]pyridinyl/pyrazinyl benzamides and acetamides. Indian J. Chem. 2012, 51B, 981–987. [Google Scholar]
- Heil, M.; Hoffmeister, L.; Webber, M.; Ilg, K.; Goergens, U.; Turberg, A. Preparation of Mesoionic Imidazopyridines for Use as Insecticides. WO Patent 2018192872, 25 October 2018. [Google Scholar]
- Liu, Z.; Li, Q.X.; Song, B. Recent research progress in and perspectives of mesoionic insecticides: Nicotinic acetylcholine receptor inhibitors. J. Agric. Food Chem. 2020, 68, 11039–11053. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tenti, G.; Cores, Á.; Ramos, M.T.; Menéndez, J.C. (E)-3-((2-Fluorophenyl)(hydroxy)methylene)imidazo[1,2-a]pyridin-2(3H)-one. Molbank 2021, 2021, M1212. https://0-doi-org.brum.beds.ac.uk/10.3390/M1212
Tenti G, Cores Á, Ramos MT, Menéndez JC. (E)-3-((2-Fluorophenyl)(hydroxy)methylene)imidazo[1,2-a]pyridin-2(3H)-one. Molbank. 2021; 2021(2):M1212. https://0-doi-org.brum.beds.ac.uk/10.3390/M1212
Chicago/Turabian StyleTenti, Giammarco, Ángel Cores, María Teresa Ramos, and J. Carlos Menéndez. 2021. "(E)-3-((2-Fluorophenyl)(hydroxy)methylene)imidazo[1,2-a]pyridin-2(3H)-one" Molbank 2021, no. 2: M1212. https://0-doi-org.brum.beds.ac.uk/10.3390/M1212