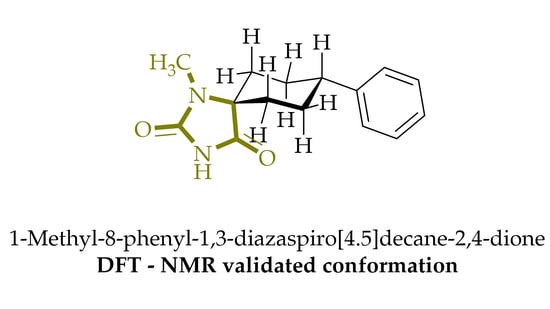
1-Methyl-8-phenyl-1,3-diazaspiro[4.5]decane-2,4-dione
Abstract
:1. Introduction
2. Results
3. Materials and Methods
3.1. Chemistry
3.2. Computational
3.3. Synthesis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cho, S.H.; Kim, S.-H.; Shin, D. Recent applications of hydantoin and thiohydantoin in medicinal chemistry. Eur. J. Med. Chem. 2019, 164, 517–545. [Google Scholar] [CrossRef] [PubMed]
- Trišović, N.; Valentić, N.; Ušćumlić, G. Solvent effects on the structure-property relationship of anticonvulsant hydantoin derivatives: A solvatochromic analysis. Chem. Cent. J. 2011, 5, 62. [Google Scholar] [CrossRef] [Green Version]
- Iqbal, Z.; Ali, S.; Iqbal, J.; Abbas, Q.; Qureshi, I.Z.; Hameed, S. Dual action spirobicycloimidazolidine-2,4-diones: Antidiabetic agents and inhibitors of aldose reductase-an enzyme involved in diabetic complications. Bioorg. Med. Chem. Lett. 2013, 23, 488–491. [Google Scholar] [CrossRef]
- Zhang, M.; Liang, Y.R.; Li, H.; Liu, M.M.; Wang, Y. Design, synthesis, and biological evaluation of hydantoin bridged analogues of combretastatin A-4 as potential anticancer agents. Bioorg. Med. Chem. 2017, 25, 6623–6634. [Google Scholar] [CrossRef]
- Czopek, A.; Byrtus, H.; Zagórska, A.; Siwek, A.; Kazek, G.; Bednarski, M.; Sapa, J.; Pawłowski, M. Design, synthesis, anticonvulsant, and antiarrhythmic properties of novel N-Mannich base and amide derivatives of β-tetralinohydantoin. Pharmacol. Rep. 2016, 68, 886–893. [Google Scholar] [CrossRef]
- Wang, Z.D.; Sheikh, S.O.; Zhang, Y. A Simple Synthesis of 2-Thiohydantoins. Molecules 2006, 11, 739–750. [Google Scholar] [CrossRef] [Green Version]
- Kopsky, D.J.; Keppel Hesselink, J.M. Phenytoin Cream for the Treatment of Neuropathic Pain: Case Series. Pharmaceuticals 2018, 11, 53. [Google Scholar] [CrossRef] [Green Version]
- Hosoya, T.; Aoyama, H.; Ikemoto, T.; Hiramatsu, T.; Kihara, Y.; Endo, M.; Suzuki, M. Dantrolene analogues revisited: General synthesis and specific functions capable of discriminating two kinds of Ca2+ release from sarcoplasmic reticulum of mouse skeletal muscle. Bioorg. Med. Chem. 2003, 11, 663–673. [Google Scholar] [CrossRef]
- Last-Barney, K.; Davidson, W.; Cardozo, M.; Frye, L.L.; Grygon, C.A.; Hopkins, J.L.; Jeanfavre, D.D.; Pav, S.; Stevenson, J.M.; Tong, L.; et al. Binding Site Elucidation of Hydantoin-Based Antagonists of LFA-1 Using Multidisciplinary Technologies: Evidence for the Allosteric Inhibition of a Protein−Protein Interaction. J. Am. Chem. Soc. 2001, 123, 5643–5650. [Google Scholar] [CrossRef]
- Giannakopoulou, E.; Pardali, V.; Skrettas, I.; Zoidis, G. Transesterification instead of N-Alkylation: An Intriguing Reaction. ChemistrySelect 2019, 4, 3195–3198. [Google Scholar] [CrossRef]
- Giannakopoulou, E.; Pardali, V.; Frakolaki, E.; Siozos, V.; Myrianthopoulos, V.; Mikros, E.; Taylor, M.C.; Kelly, J.M.; Vassilaki, N.; Zoidis, G. Scaffold hybridization strategy towards potent hydroxamate-based inhibitors of Flaviviridae viruses and Trypanosoma species. Med. Chem. Commun. 2019, 10, 991–1006. [Google Scholar] [CrossRef]
- Dragojlovic, V. Conformational analysis of cycloalkanes. ChemTexts 2015, 1, 1–30. [Google Scholar] [CrossRef]
- Frisch, M.J.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A.; et al. Gaussian 09, Revision E.01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Becke, A.D. Density-functional thermochemistry. III. The role of exact exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef] [Green Version]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti correlation-energy formula into a functional of the electron density. Phys. Rev. B Condens. Matter 1988, 37, 785–789. [Google Scholar] [CrossRef] [Green Version]
- Clark, T.; Chandrasekhar, J.; Spitznagel, G.W.; Schleyer, P.V.R. Efficient diffuse function-augmented basis sets for anion calculations. III. The 3-21+G basis set for first-row elements, Li–F. J. Comput. Chem. 1983, 4, 294–301. [Google Scholar] [CrossRef]
- McLean, A.D.; Chandler, G.S. Contracted Gaussian basis sets for molecular calculations. I. Second row atoms, Z=11–18. J. Chem. Phys. 1980, 72, 5639–5648. [Google Scholar] [CrossRef]
- Wolinski, K.; Hinton, J.F.; Pulay, P. Efficient implementation of the gauge-independent atomic orbital method for NMR chemical shift calculations. J. Am. Chem. Soc. 2002, 112, 8251–8260. [Google Scholar] [CrossRef]
- Cheeseman, J.R.; Trucks, G.W.; Keith, T.A.; Frisch, M.J. A comparison of models for calculating nuclear magnetic resonance shielding tensors. J. Chem. Phys. 1996, 104, 5497–5509. [Google Scholar] [CrossRef]
- Ditchfield, R. Self-consistent perturbation theory of diamagnetism. Mol. Phys. 1974, 27, 789–807. [Google Scholar] [CrossRef]
- McWeeny, R. Perturbation Theory for the Fock-Dirac Density Matrix. Phys. Rev. 1962, 126, 1028–1034. [Google Scholar] [CrossRef]
- London, F. Théorie quantique des courants interatomiques dans les combinaisons aromatiques. J. Phys. Radium 1937, 8, 397–409. [Google Scholar] [CrossRef] [Green Version]
- Deng, W.; Cheeseman, J.R.; Frisch, M.J. Calculation of Nuclear Spin-Spin Coupling Constants of Molecules with First and Second Row Atoms in Study of Basis Set Dependence. J. Chem. Theory Comput. 2006, 2, 1028–1037. [Google Scholar] [CrossRef]
- Peralta, J.E.; Scuseria, G.E.; Cheeseman, J.R.; Frisch, M.J. Basis set dependence of NMR spin–spin couplings in density functional theory calculations: First row and hydrogen atoms. Chem. Phys. Lett. 2003, 375, 452–458. [Google Scholar] [CrossRef]
- Barone, V.; Peralta, J.E.; Contreras, R.H.; Snyder, J.P. DFT Calculation of NMR JFF Spin−Spin Coupling Constants in Fluorinated Pyridines. J. Phys. Chem. A 2002, 106, 5607–5612. [Google Scholar] [CrossRef]
- Helgaker, T.; Watson, M.; Handy, N.C. Analytical calculation of nuclear magnetic resonance indirect spin–spin coupling constants at the generalized gradient approximation and hybrid levels of density-functional theory. J. Chem. Phys. 2000, 113, 9402–9409. [Google Scholar] [CrossRef]
- Sychrovský, V.R.; Gräfenstein, J.; Cremer, D. Nuclear magnetic resonance spin–spin coupling constants from coupled perturbed density functional theory. J. Chem. Phys. 2000, 113, 3530–3547. [Google Scholar] [CrossRef] [Green Version]




| Compound | Conformation A | Conformation Β | Conformation C | Conformation D |
|---|---|---|---|---|
| 4 |  |  |  |  |
| Charge | 0 | 0 | 0 | 0 |
| Spin | Singlet | Singlet | Singlet | Singlet |
| Solvation | None | None | None | None |
| E(RB3LYP) | -842.498395 Hartree | -842.501986 Hartree | -842.492052 Hartree | -842.490242 Hartree |
| RMS Gradient Norm | - | - | - | - |
| Imaginary Freq | - | - | - | - |
| Dipole Moment | 3.364844 | 3.032529 Debye | 3.042446 Debye | 3.364416 Debye |
| Point Group | C1 | C1 | C1 | C1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pardali, V.; Katsamakas, S.; Giannakopoulou, E.; Zoidis, G. 1-Methyl-8-phenyl-1,3-diazaspiro[4.5]decane-2,4-dione. Molbank 2021, 2021, M1228. https://0-doi-org.brum.beds.ac.uk/10.3390/M1228
Pardali V, Katsamakas S, Giannakopoulou E, Zoidis G. 1-Methyl-8-phenyl-1,3-diazaspiro[4.5]decane-2,4-dione. Molbank. 2021; 2021(2):M1228. https://0-doi-org.brum.beds.ac.uk/10.3390/M1228
Chicago/Turabian StylePardali, Vasiliki, Sotirios Katsamakas, Erofili Giannakopoulou, and Grigoris Zoidis. 2021. "1-Methyl-8-phenyl-1,3-diazaspiro[4.5]decane-2,4-dione" Molbank 2021, no. 2: M1228. https://0-doi-org.brum.beds.ac.uk/10.3390/M1228