A New Species of Terrestrial-Breeding Frog (Amphibia, Strabomantidae, Noblella) from the Upper Madre De Dios Watershed, Amazonian Andes and Lowlands of Southern Peru
Abstract
:1. Introduction
2. Materials and Methods
2.1. Fieldwork and Data Collection
2.2. Morphological Characters
2.3. Micro-Computed Tomography
2.4. Molecular Phylogenetic Analysis
2.5. Registration of New Nomenclatural Acts
3. Results
3.1. Molecular Phylogenetic Analysis
3.2. Taxonomy
3.2.1. Holotype
3.2.2. Paratypes
3.2.3. Generic Placement
3.2.4. Diagnosis
3.2.5. Comparisons with Described Species
3.2.6. Description of Holotype
3.2.7. Coloration of Holotype
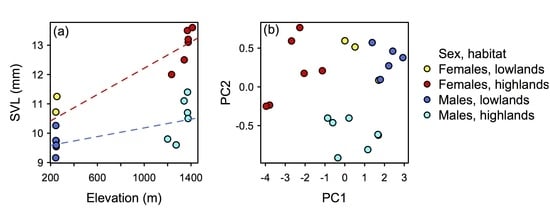
3.2.8. Variation
3.2.9. Etymology
3.3. Distribution, Natural History, and Threats
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
References
- Barbour, T. A list of Antillean reptiles and amphibians. Zoologica 1930, 11, 61–116. [Google Scholar]
- De la Riva, I.; Köhler, J. A new minute leptodactylid frog, genus Phyllonastes, from humid montane forests of Bolivia. J. Herpetol. 1998, 32, 325–329. [Google Scholar] [CrossRef]
- Guayasamin, J.M.; Terán-Valdez, A. A new species of Noblella (Amphibia: Strabomantidae) from the western slopes of the Andes of Ecuador. Zootaxa 2009, 47–59. [Google Scholar] [CrossRef]
- Lehr, E.; Aguilar, C.; Lundberg, M. A new species of Phyllonastes from Peru (Amphibia, Anura, Leptodactylidae). J. Herpetol. 2004, 38, 214–218. [Google Scholar] [CrossRef]
- Lynch, J.D. New species of minute leptodactylid frogs from the Andes of Ecuador and Peru. J. Herpetol. 1986, 20, 423–431. [Google Scholar] [CrossRef]
- Lynch, J.D. Two new species of frogs of the genus Euparkerella (Amphibia: Leptodactylidae) from Ecuador and Perú. Herpetologica 1976, 32, 48–53. [Google Scholar]
- Duellman, W.E. A new species of leptodactylid frog, genus Phyllonastes, from Peru. Herpetologica 1991, 47, 9–13. [Google Scholar]
- Catenazzi, A.; Uscapi, V.; von May, R. A new species of Noblella (Amphibia, Anura, Craugastoridae) from the humid montane forests of Cusco, Peru. ZooKeys 2015, 516, 71–84. [Google Scholar] [CrossRef]
- Reyes-Puig, J.P.; Reyes-Puig, C.; Ron, S.; Ortega, J.A.; Guayasamin, J.M.; Goodrum, M.; Recalde, F.; Vieira, J.J.; Koch, C.; Yánez-Muñoz, M.H. A new species of terrestrial frog of the genus Noblella Barbour, 1930 (Amphibia: Strabomantidae) from the Llanganates-Sangay Ecological Corridor, Tungurahua, Ecuador. PeerJ 2019, 7, e7405. [Google Scholar] [CrossRef]
- Harvey, M.B.; Almendáriz, A.; Brito, M.J.; Batallas, R.D. A new species of Noblella (Anura: Craugastoridae) from the Amazonian slopes of the Ecuadorian Andes with comments on Noblella lochites (Lynch). Zootaxa 2013, 3635, 1–14. [Google Scholar] [CrossRef]
- Noble, G.K. Five new species of Salientia from South America. Am. Mus. Novit. 1921, 29, 1–7. [Google Scholar]
- Lehr, E.; Catenazzi, A. A new species of minute Noblella (Anura: Strabomantidae) from southern Peru: The smallest frog of the Andes. Copeia 2009, 148–156. [Google Scholar] [CrossRef]
- Köhler, J. A new species of Phyllonastes Heyer from the Chapare region of Bolivia, with notes on Phyllonastes carrascoicola. Spixiana 2000, 23, 47–53. [Google Scholar]
- Catenazzi, A.; Ttito, A. Noblella thiuni sp. n., a new (singleton) species of minute terrestrial-breeding frog (Amphibia, Anura, Strabomantidae) from the montane forest of the Amazonian Andes of Puno, Peru. PeerJ 2019, 7, e6780. [Google Scholar] [CrossRef] [PubMed]
- Hedges, S.B.; Duellman, W.E.; Heinicke, M.P. New World direct-developing frogs (Anura: Terrarana): Molecular phylogeny, classification, biogeography, and conservation. Zootaxa 2008, 1737, 1–182. [Google Scholar] [CrossRef] [Green Version]
- De la Riva, I.; Chaparro, J.C.; Castroviejo-Fisher, S.; Padial, J.M. Underestimated anuran radiations in the high Andes: Five new species and a new genus of Holoadeninae, and their phylogenetic relationships (Anura: Craugastoridae). Zool. J. Linn. Soc. 2017, 182, 129–172. [Google Scholar] [CrossRef]
- Catenazzi, A.; Lehr, E.; von May, R. The amphibians and reptiles of Manu National Park and its buffer zone, Amazon basin and eastern slopes of the Andes, Peru. Biota Neotropica 2013, 13, 269–283. [Google Scholar] [CrossRef] [Green Version]
- Duellman, W.E.; Lehr, E. Terrestrial-breeding frogs (Strabomantidae) in Peru; Natur und Tier: Münster, Germany, 2009; p. 382. [Google Scholar]
- Lynch, J.D.; Duellman, W.E. Frogs of the genus Eleutherodactylus in western Ecuador: Systematics, ecology, and biogeography. Univ. Kans. Spec. Publ. 1997, 23, 1–236. [Google Scholar]
- Duellman, W.E.; Lehr, E.; Venegas, P.J. Two new species of Eleutherodactylus (Anura: Leptodactylidae) from the Andes of northern Peru. Zootaxa 2006, 1285, 51–64. [Google Scholar]
- Padial, J.M.; Grant, T.; Frost, D.R. Molecular systematics of terraranas (Anura: Brachycephaloidea) with an assessment of the effects of alignment and optimality criteria. Zootaxa 2014, 3825, 1–132. [Google Scholar] [CrossRef] [Green Version]
- Heinicke, M.P.; Lemmon, A.R.; Lemmon, E.M.; McGrathc, K.; Hedges, S.B. Phylogenomic support for evolutionary relationships of New World direct-developing frogs (Anura: Terraranae). Mol. Phylogenetics Evol. 2018, 118, 145–155. [Google Scholar] [CrossRef]
- von May, R.; Catenazzi, A.; Corl, A.; Santa-Cruz, R.; Carnaval, A.C.; Moritz, C. Divergence of thermal physiological traits in terrestrial breeding frogs along a tropical elevational gradient. Ecol. Evol. 2017, 7, 3257–3267. [Google Scholar] [CrossRef]
- Biomatters. Geneious R6, version 6.1.5. 2013. Available online: http://www.geneious.com/ (accessed on 31 May 2019).
- Lanfear, R.; Calcott, B.; Ho, S.Y.W.; Guindon, S. PartitionFinder: Combined Selection of Partitioning Schemes and Substitution Models for Phylogenetic Analyses. Mol. Biol. Evol. 2012, 29, 1695–1701. [Google Scholar] [CrossRef] [Green Version]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [Green Version]
- Rambaut, A.; Drummond, A.J. Tracer. Version 1.5. 2007. Available online: http://tree.bio.ed.ac.uk/ software/tracer/ (accessed on 1 June 2019).
- Rambaut, A. FigTree, Version 1.4.2. 2009. Available online: http://tree.bio.ed.ac.uk/software/figtree/ (accessed on 1 June 2019).
- Rodríguez, L.O.; Cadle, J.E. A preliminary overview of the herpetofauna of Cocha Cashu, Manu National Park, Peru. In Four Neotropical Rainforests; Gentry, A.H., Ed.; Yale University Press: New Haven, CT, USA, 1990; pp. 410–425. [Google Scholar]
- Rodríguez, L.O. Structure et organization du peuplement d’anoures de Cocha Cashu, Parc National Manu, Amazonie Péruvienne. Rev. De Ecol. 1992, 47, 151–197. [Google Scholar]
- Morales, V.R.; McDiarmid, R.W. Annotated checklist of the amphibians and reptiles of Pakitza, Manu National Park Reserve Zone, with comments on the herpetofauna of Madre de Dios, Peru. In Manu: The Biodiversity of Southeastern Peru; Wilson, D.E., Sandoval, A., Eds.; Smithsonian Institution: Lima, Peru, 1996; pp. 503–522. [Google Scholar]
- Doan, T.M.; Arizabal, W. Microgeographic variation in species composition of the herpetofaunal communities of Tambopata Region, Peru. Biotropica 2002, 34, 101–117. [Google Scholar] [CrossRef]
- von May, R.; Siu-Ting, K.; Jacobs, J.M.; Medina-Müller, M.; Gagliardi, G.; Rodríguez, L.O.; Donnelly, M.A. Species diversity and conservation status of amphibians in Madre de Dios, Peru. Herpetol. Conserv. Biol. 2009, 4, 14–29. [Google Scholar]
- von May, R.; Jacobs, J.M.; Santa-Cruz, R.; Valdivia, J.; Huamán, J.; Donnelly, M.A. Amphibian community structure as a function of forest type in Amazonian Peru. J. Trop. Ecol. 2010, 26, 509–519. [Google Scholar] [CrossRef]
- von May, R.; Jacobs, J.M.; Jennings, R.D.; Catenazzi, A.; Rodríguez, L.O. Anfibios de Los Amigos, Manu y Tambopata, Perú; Rapid Color Guide # 236; The Field Museum: Chicago, IL, USA, 2010; p. 12. [Google Scholar]
- Whitworth, A.; Downie, R.; von May, R.; Villacampa, J.; McLeod, R. How much potential biodiversity and conservation value can a regenerating rainforest provide? A “best-case scenario” approach from the Peruvian Amazon. Trop. Conserv. Sci. 2016, 9, 224–245. [Google Scholar] [CrossRef]
- Villacampa, J.; Serrano-Rojas, S.; Whitworth, A. Amphibians of the Manu Learning Centre and Other Areas of the Manu Region; The Crees Foundation: Cusco, Peru, 2017; p. 282. [Google Scholar]
- von May, R.; Catenazzi, A.; Santa-Cruz, R.; Kosch, T.A.; Vredenburg, V.T. Microhabitat temperatures and prevalence of the pathogenic fungus Batrachochytrium dendrobatidis in lowland Amazonian frogs. Trop. Conserv. Sci. 2018, 11, 1–13. [Google Scholar] [CrossRef]
- von May, R.; Catenazzi, A.; Santa-Cruz, R.; Gutiérrez, A.; Moritz, C.; Rabosky, D.L. Thermal physiological traits in tropical lowland amphibians: Vulnerability to climate warming and cooling. PLoS ONE 2019, 14, e0219759. [Google Scholar] [CrossRef]
- De la Riva, I.; Chaparro, J.C.; Padial, J.M. A new, long-standing misidentified species of Psychrophrynella Hedges, Duellman and Heinicke from Departamento Cusco, Peru (Anura: Strabomantidae). Zootaxa 2008, 1823, 42–50. [Google Scholar] [CrossRef]
- De la Riva, I.; Chaparro, J.C.; Padial, J.M. The taxonomic status of Phyllonastes Heyer and Phrynopus peruvianus (Noble) (Lissamphibia, Anura): Resurrection of Noblella Barbour. Zootaxa 2008, 1685, 67–68. [Google Scholar] [CrossRef]
- von May, R.; Donnelly, M.A. Do trails affect relative abundance estimates of rainforest frogs and lizards? Austral Ecol. 2009, 34, 613–620. [Google Scholar] [CrossRef]
- Catenazzi, A.; Lehr, E.; Vredenburg, V.T. Thermal physiology, disease and amphibian declines in the eastern slopes of the Andes. Conserv. Biol. 2014, 28, 509–517. [Google Scholar] [CrossRef]
- IUCN. Guidelines for Using the IUCN Red List Categories and Criteria—Version 10.1. Prepared by the Standards and Petitions Subcommittee. 2013. Available online: http://www. iucnredlist.org/documents/RedListGuidelines.pdf (accessed on 5 April 2015).
- Padial, J.M.; De la Riva, I. Integrative taxonomy reveals cryptic Amazonian species of Pristimantis (Anura: Strabomantidae). Zool. J. Linn. Soc. 2009, 155, 97–122. [Google Scholar] [CrossRef]
- Lehr, E.; von May, R. New species of Pristimantis (Anura: Strabomantidae) from the Amazonian lowlands of southern Peru. J. Herpetol. 2009, 43, 485–494. [Google Scholar] [CrossRef]
- Shepack, A.; von May, R.; Ttito, A.; Catenazzi, A. A new species of Pristimantis (Amphibia, Anura, Craugastoridae) from the foothills of the Andes in Manu National Park, southeastern Peru. ZooKeys 2016, 594, 143–164. [Google Scholar]
- Jungfer, K.-H.; Hoogmoed, M.; Angulo, A.; Reynolds, R.; Icochea, J.; Azevedo-Ramos, C. Noblella Myrmecoides. The IUCN Red List of Threatened Species 2010: e.T57235A11606716. 2010. Available online: http://0-dx-doi-org.brum.beds.ac.uk/10.2305/IUCN.UK.2010-2.RLTS.T57235A11606716.en (accessed on 29 May 2019).
- Hedges, S.B. Distribution patterns of amphibians in the West Indies. In Regional Patterns of Amphibian Distribution: A Global Perspective; Duellman, W.E., Ed.; Johns Hopkins University Press: Baltimore, MD, USA, 1999; pp. 211–254. [Google Scholar]
- González-Voyer, A.; Padial, J.M.; Castroviejo-Fisher, S.; De la Riva, I.; Vilà, C. Correlates of species richness in the largest Neotropical amphibian radiation. J. Evol. Biol. 2011, 24, 931–942. [Google Scholar] [CrossRef] [Green Version]
- Narins, P.M.; Meenderink, S.W.F. Climate change and frog calls: Long-term correlations along a tropical altitudinal gradient. Proc. R. Soc. B 2014, 281, 20140401. [Google Scholar] [CrossRef]
- Lehr, E.; von May, R. A new species of terrestrial-breeding frog (Amphibia, Craugastoridae, Pristimantis) from high elevations of the Pui Pui Protected Forest in central Peru. ZooKeys 2017, 660, 17–42. [Google Scholar] [CrossRef]
- von May, R.; Lehr, E.; Rabosky, D.L. Evolutionary radiation of earless frogs in the Andes: Molecular phylogenetics and habitat shifts in high-elevation terrestrial breeding frogs. PeerJ 2018, 6, e4313. [Google Scholar] [CrossRef]
- Bergmann, C. Über die Verhältnisse der Wärmeökonomie der Thiere zu ihrer Größe. Göttingen Stud. 1847, 1, 595–708. [Google Scholar]
- Mayr, E. Geographic character gradients and climatic adaptation. Evolution 1956, 10, 105–108. [Google Scholar] [CrossRef]
- Lehr, E.; Coloma, L.A. A minute new Ecuadorian Andean frog (Anura: Strabomantidae, Pristimantis). Herpetologica 2008, 64, 354–367. [Google Scholar] [CrossRef]
- Kraus, F. At the lower size limit for tetrapods, two new species of the miniaturized frog genus Paedo-phryne (Anura, Microhylidae). ZooKeys 2011, 154, 71–88. [Google Scholar] [CrossRef]
- Rittmeyer, E.N.; Allison, A.; Gründler, M.C.; Thompson, D.K.; Austin, C.C. Ecological guild evolution and the discovery of the world’s smallest vertebrate. PLoS ONE 2012, 7, e29797. [Google Scholar] [CrossRef]
- Scherz, M.D.; Hutter, C.R.; Rakotoarison, A.; Riemann, J.C.; Rödel, M.-O.; Ndriantsoa, S.H.; Glos, J.; Roberts, S.H.; Crottini, A.; Vences, M.; et al. Morphological and ecological convergence at the lower size limit for vertebrates highlighted by five new miniaturised microhylid frog species from three different Madagascan genera. PLoS ONE 2019, 14, e0213314. [Google Scholar] [CrossRef]
- Tracy, C.R.; Christian, K.A.; Tracy, C.R. Not just small, wet, and cold: Effects of body size and skin resistance on thermoregulation and arboreality of frogs. Ecology 2010, 91, 1477–1484. [Google Scholar] [CrossRef]












| Females (n = 9) | Range | Males (n = 12) | Range | |
|---|---|---|---|---|
| Character | Mean ± SD | (min–max) | Mean ± SD | (min–max) |
| SVL | 12.18 ± 1.36 | (9.74–13.60) | 10.08 ± 0.70 | (9.16–11.40) |
| TL | 6.16 ± 0.74 | (4.95–7.10) | 5.34 ± 0.60 | (4.50–6.20) |
| FL | 5.44 ± 0.92 | (4.52–7.00) | 4.56 ± 0.65 | (3.68–5.80) |
| HL | 3.56 ± 0.53 | (2.55–4.00) | 2.97 ± 0.30 | (2.44–3.40) |
| HW | 4.09 ± 0.34 | (3.55–4.40) | 3.62 ± 0.38 | (3.19–4.30) |
| IOD | 1.74 ± 0.26 | (1.29–2.10) | 1.49 ± 0.18 | (1.18–1.75) |
| EW | 1.01 ± 0.15 | (0.80–1.20) | 0.83 ± 0.14 | (0.57–1.00) |
| IND | 1.38 ± 0.27 | (1.03–1.78) | 1.14 ± 0.16 | (0.87–1.43) |
| E–N | 0.80 ± 0.07 | (0.67–0.93) | 0.66 ± 0.10 | (0.47–0.80) |
| S–N | 0.34 ± 0.14 | (0.05–0.50) | 0.24 ± 0.10 | (0.05–0.33) |
| ED | 1.55 ± 0.18 | (1.26–1.80) | 1.33 ± 0.19 | (1.02–1.65) |
| TY | 0.56 ± 0.05 | (0.45–0.63) | 0.52 ± 0.06 | (0.42–0.66) |
| E–TY | 0.38 ± 0.12 | (0.17–0.50) | 0.30 ± 0.10 | (0.13–0.41) |
| ForL | 2.95 ± 0.36 | (2.35–3.41) | 2.60 ± 0.29 | (2.16–2.94) |
| HaL | 2.37 ± 0.37 | (1.80–2.80) | 1.98 ± 0.42 | (1.35–2.48) |
| FIL | 0.93 ± 0.13 | (0.76–1.16) | 0.73 ± 0.07 | (0.60–0.80) |
| FIIL | 1.19 ± 0.16 | (1.03–1.53) | 0.97 ± 0.11 | (0.80–1.20) |
| TIL | 1.24 ± 0.20 | (0.94–1.43) | 0.88 ± 0.09 | (0.73–0.98) |
| TIIL | 1.88 ± 0.16 | (1.63–2.20) | 1.48 ± 0.26 | (1.05–1.90) |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santa-Cruz, R.; von May, R.; Catenazzi, A.; Whitcher, C.; López Tejeda, E.; Rabosky, D.L. A New Species of Terrestrial-Breeding Frog (Amphibia, Strabomantidae, Noblella) from the Upper Madre De Dios Watershed, Amazonian Andes and Lowlands of Southern Peru. Diversity 2019, 11, 145. https://0-doi-org.brum.beds.ac.uk/10.3390/d11090145
Santa-Cruz R, von May R, Catenazzi A, Whitcher C, López Tejeda E, Rabosky DL. A New Species of Terrestrial-Breeding Frog (Amphibia, Strabomantidae, Noblella) from the Upper Madre De Dios Watershed, Amazonian Andes and Lowlands of Southern Peru. Diversity. 2019; 11(9):145. https://0-doi-org.brum.beds.ac.uk/10.3390/d11090145
Chicago/Turabian StyleSanta-Cruz, Roy, Rudolf von May, Alessandro Catenazzi, Courtney Whitcher, Evaristo López Tejeda, and Daniel L. Rabosky. 2019. "A New Species of Terrestrial-Breeding Frog (Amphibia, Strabomantidae, Noblella) from the Upper Madre De Dios Watershed, Amazonian Andes and Lowlands of Southern Peru" Diversity 11, no. 9: 145. https://0-doi-org.brum.beds.ac.uk/10.3390/d11090145