Exquisitely Preserved Fossil Snakes of Messel: Insight into the Evolution, Biogeography, Habitat Preferences and Sensory Ecology of Early Boas
Abstract
:1. Introduction
2. Materials and Methods
2.1. Abbreviations
2.2. Computed Tomography (CT)
2.3. Taxonomy
2.4. Phylogenetic Analyses
2.5. Infrared Organs Survey
2.6. Habitat Preference Survey
3. Results
3.1. Systematic Palaeontology
3.1.1. Genus-level taxonomy
3.1.2. Species-level taxonomy
3.2. Brief Anatomical Description
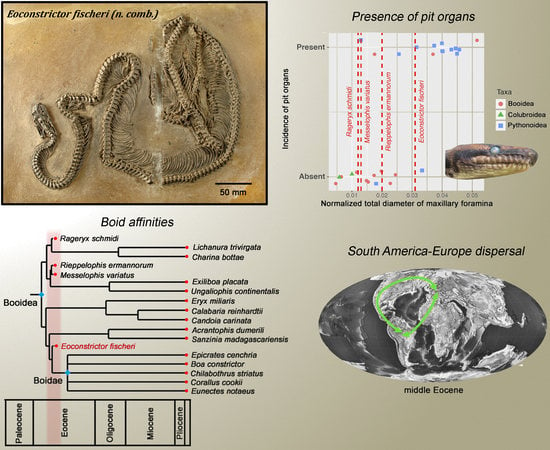
3.3. Phylogenetic Analysis and Biogeographic Implications
3.4. Infrared Reception in Messel Booids
3.5. Macrohabitat Preferences in Messel Booids
4. Discussion
4.1. Phylogeny and Biogeographic History
4.2. Labial Pits in Extant Snakes
4.3. Eoconstrictor and the Evolution of Labial Pits
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Moon, B.R.; Mehta, R.S. Constriction strength in snakes. In Biology of the Boas and Pythons; Henderson, R.W., Powell, R., Eds.; Eagle Mountain Publishing: Eagle Mountain, UT, USA, 2007; pp. 207–212. [Google Scholar]
- Scanferla, C.A. Postnatal ontogeny and the evolution of macrostomy in snakes. R. Soc. Open Sci. 2016, 3, 160612. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goris, R.C.; Nakano, M.; Atobe, Y.; Funakoshi, K. The infrared sight of boas and pythons. In Biology of the Boas and Pythons; Henderson, R.W., Powell, R., Eds.; Eagle Mountain Publishing, LC: Eagle Mountain, UT, USA, 2007; pp. 287–296. [Google Scholar]
- Goris, R.C. Infrared organs of snakes: An integral part of vision. J. Herpetol. 2011, 45, 2–14. [Google Scholar] [CrossRef]
- Underwood, G. A systematic analysis of boid snakes. In Morphology and Biology of Reptiles (Linnean Society Symposium Series Number 3); Bellairs, A.d.A., Cox, C.B., Eds.; Academic Press: London, UK, 1976; pp. 151–176. [Google Scholar]
- Kluge, A.G. Boine Snake Phylogeny and Research Cycles (Miscellaneous Publications, Museum of Zoology, University of Michigan, No. 178); Museum of Zoology, University of Michigan: Ann Arbor, MI, USA, 1991; pp. 1–58. [Google Scholar]
- Vences, M.; Glaw, F.; Kosuch, J.; Böhme, W.; Veith, M. Phylogeny of South American and Malagasy boine snakes: Molecular evidence for the validity of Sanzinia and Acrantophis and biogeographic implications. Copeia 2001, 2001, 1151–1154. [Google Scholar] [CrossRef]
- Noonan, B.P.; Chippindale, P.T. Dispersal and vicariance: The complex evolutionary history of boid snakes. Mol. Phylogenet. Evol. 2006, 40, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.G.; Niemiller, M.L.; Revell, L.J. Toward a Tree-of-Life for the boas and pythons: Multilocus species-level phylogeny with unprecedented taxon sampling. Mol. Phylogenet. Evol. 2014, 71, 201–213. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, R.G.; Henderson, R.W. Boas of the world (superfamily Booidae): A checklist with systematic, taxonomic, and conservation assessments. Bull. Mus. Comp. Zool. 2018, 162, 1–58. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.T.; Scanferla, A. A nearly complete skeleton of the oldest definitive erycine boid (Messel, Germany). Geodiversitas, in press.
- Larkin, M.A.; Blackshields, G.; Brown, N.P.; Chenna, R.; McGettigan, P.A.; McWilliam, H.; Valentin, F.; Wallace, I.M.; Wilm, A.; Lopez, R.; et al. Clustal W and Clustal X version 2.0. Bioinformatics 2007, 23, 2947–2948. [Google Scholar] [CrossRef] [Green Version]
- Goloboff, P.A.; Catalano, S.A. TNT version 1.5, including a full implementation of phylogenetic morphometrics. Cladistics 2016, 32, 221–238. [Google Scholar] [CrossRef]
- Stadler, T. Sampling-through-time in birth-death trees. J. Theor. Biol. 2010, 267, 396–404. [Google Scholar] [CrossRef]
- Ronquist, F.; Teslenko, M.; van der Mark, P.; Ayres, D.L.; Darling, A.; Höhna, S.; Larget, B.; Liu, L.; Suchard, M.A.; Huelsenbeck, J.P. MrBayes 3.2: Efficient Bayesian phylogenetic inference and model choice across a large model space. Syst. Biol. 2012, 61, 539–542. [Google Scholar] [CrossRef] [Green Version]
- Bapst, D.W. paleotree: An R package for paleontological and phylogenetic analyses of evolution. Methods Ecol. Evol. 2012, 3, 803–807. [Google Scholar] [CrossRef]
- Bapst, D.W. A stochastic rate-calibrated method for time-scaling phylogenies of fossil taxa. Methods Ecol. Evol. 2013, 4, 724–733. [Google Scholar] [CrossRef]
- Team, R.C. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2016. [Google Scholar]
- Muchlinski, M.N. The relationship between the infraorbital foramen, infraorbital nerve, and maxillary mechanoreception: Implications for interpreting the paleoecology of fossil mammals based on infraorbital foramen size. Anat. Rec. 2008, 291, 1221–1226. [Google Scholar] [CrossRef] [PubMed]
- Young, B.A. The cephalic vascular anatomy of three species of sea snakes. J. Morphol. 1988, 196, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Porter, W.R.; Witmer, L.M. Vascular patterns in iguanas and other squamates: Blood vessels and sites of thermal exchange. PLoS ONE 2015, 10, e0139215. [Google Scholar] [CrossRef]
- Kluge, A.G. Aspidites and the phylogeny of pythonine snakes. Rec. Aust. Mus. 1993, 19, 1–77. [Google Scholar] [CrossRef] [Green Version]
- Barrett, R.; Maderson, P.F.A.; Meszler, R.M. The pit organs of snakes. In Biology of the Reptilia, v2 (Morphology B); Gans, C., Parsons, T.J., Eds.; Academic Press: London, UK; New York, NY, USA, 1970; pp. 277–314. [Google Scholar]
- Sheehy, C.M., III; Albert, J.S.; Lillywhite, H.B. The evolution of tail length in snakes associated with different gravitational environments. Funct. Ecol. 2016, 30, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Schaal, S. Palaeopython fischerin. sp. (Serpentes: Boidae), eine Riesenschlange aus dem Eozän (MP 11) von Messel. Cour. Forsch. Senckenberg 2004, 252, 35–45. [Google Scholar]
- Schaal, S.; Baszio, S. Messelophis ermannorumn. sp., eine neue Zwergboa (Serpentes: Boidae: Tropidopheinae) aus dem Mittel-Eozän von Messel. Cour. Forsch. Senckenberg 2004, 252, 67–77. [Google Scholar]
- Baszio, S. Messelophis variatusn. gen. n. sp. from the Eocene of Messel: A tropidopheine snake with affinities to Erycinae (Boidae). Cour. Forsch. Senckenberg 2004, 252, 47–66. [Google Scholar]
- Pyron, R.A.; Burbrink, F.T.; Wiens, J.J. A phylogeny and revised classification of Squamata, including 4161 species of lizards and snakes. BMC Evol. Biol. 2013, 13, 93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Revell, L.J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 2012, 3, 217–223. [Google Scholar] [CrossRef]
- Pyron, R.A.; Reynolds, R.E.; Burbrink, F.T. A taxonomic revision of boas (Serpentes: Boidae). Zootaxa 2014, 3846, 249–260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Georgalis, G.L.; Scheyer, T.M. A new species of Palaeopython (Serpentes) and other extinct squamates from the Eocene of Dielsdorf (Zurich, Switzerland). Swiss J. Geosci. 2019, 112, 383–417. [Google Scholar] [CrossRef] [Green Version]
- Smith, K.T.; Scanferla, A. Fossil snake preserving three trophic levels and evidence for an ontogenetic dietary shift. Palaeobiodiv. Palaeoenv. 2016, 96, 589–599. [Google Scholar] [CrossRef]
- Messel: An Ancient Greenhouse Ecosystem; Smith, K.T.; Schaal, S.F.K.; Habersetzer, J. (Eds.) Schweizerbart: Stuttgart, Germany, 2018; p. 355. [Google Scholar]
- Lenz, O.K.; Wilde, V.; Mertz, D.F.; Riegel, W. New palynology-based astronomical and revised40Ar/39Ar ages for the Eocene maar lake of Messel (Germany). Int. J. Earth Sci. 2015, 104, 873–889. [Google Scholar] [CrossRef]
- Hsiang, A.Y.; Field, D.J.; Webster, T.H.; Behlke, A.D.B.; Davis, M.B.; Racicot, R.A.; Gauthier, J.A. The origin of snakes: Revealing the ecology, behavior, and evolutionary history of early snakes using genomics, phenomics, and the fossil record. BMC Evol. Biol. 2015, 15, 87. [Google Scholar] [CrossRef] [Green Version]
- Scanferla, C.A.; Smith, K.T.; Schaal, S.F.K. Revision of the cranial anatomy and phylogenetic relationships of the Eocene minute boas Messelophis variatus and Messelophis ermannorum (Serpentes, Booidea). Zool. J. Linn. Soc. 2016, 176, 182–206. [Google Scholar] [CrossRef] [Green Version]
- Colston, T.J.; Grazziotin, F.G.; Shepard, D.B.; Colli, G.R.; Henderson, R.W.; Hedges, S.B.; Bonatto, S.; Zaher, H.; Noonan, B.P.; Burbrink, F.T. Molecular systematics and historical biogeography of tree boas (Corallus spp.). Mol. Phylogenet. Evol. 2013, 66, 953–959. [Google Scholar] [CrossRef]
- Smith, K.T. A new lizard assemblage from the earliest Eocene (zone Wa0) of the Bighorn Basin, Wyoming, USA: Biogeography during the warmest interval of the Cenozoic. J. Syst. Palaeontol. 2009, 7, 299–358. [Google Scholar] [CrossRef]
- Henderson, R.W.; Waller, T.; Micucci, P.; Puorto, G.; Bourgeois, R.W. Ecological correlates and the patterns in the distribution of the New World boines (Serpentes: Boidae): A preliminary assessment. Herpetol. Nat. Hist. 1995, 3, 15–27. [Google Scholar]
- Pizzatto, L.; Marques, O.A.V.; Martins, M. Ecomorphology of boine snakes, with emphasis on south american forms. In Biology of the Boas and Pythons; Henderson, R.W., Powell, R., Eds.; Eagle Mountain Publishing, LC: Eagle Mountain, UT, USA, 2007; pp. 35–48. [Google Scholar]
- Lillywhite, H.B.; Henderson, R.W. Behavioral and functional ecology of arboreal snakes. In Snakes—Ecology and Behavior; Seigel, R.A., Collins, J.T., Eds.; McGraw-Hill, Inc.: London, UK, 1993; pp. 1–48. [Google Scholar]
- Pizzatto, L.; Marques, O.A.V.; Facure, K. Food habits of Brazilian boid snakes: Overview and new data, with special reference to Corallus hortulanus. Amphib. Reptil. 2009, 30, 533–544. [Google Scholar] [CrossRef] [Green Version]
- Matzke, N. Probabilistic historical biogeography: new models for founder-event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Front. Biogeogr. 2015, 5, 242–248. [Google Scholar]
- Rage, J.-C.; Augé, M. Squamates from the Cainozoic of the western part of Europe: A review. Rev. Paléobiol. Vol. Spéc. 1993, 7, 199–216. [Google Scholar]
- Szyndlar, Z.; Rage, J.-C. Non-Erycine Booidea from the Oligocene and Miocene of Europe; Institute of Systematics and Evolution of Animals, Polish Academy of Sciences: Kraków, Poland, 2003; p. 111. [Google Scholar]
- Holman, J.A. Fossil Snakes of North America: Origin, Evolution, Distribution, Paleoecology; Indiana University Press: Bloomington, IN, USA, 2000; p. 357. [Google Scholar]
- McCartney, J.A.; Seiffert, E.R. A late Eocene snake fauna from the Fayum Depression, Egypt. J. Vertebr. Paleontol. 2016, 36, e1029580. [Google Scholar] [CrossRef]
- Rage, J.-C. Fossil snakes from the Palaeocene of São José de Itaboraí, Brazil. Part III. Ungaliophiinae, booid sincertae sedis, and Caenophidia. Summary, update, and discussion of the snake fauna from the locality. Palaeovert 2008, 36, 37–73. [Google Scholar] [CrossRef]
- Smith, K.T. New constraints on the evolution of the snake clades Ungaliophiinae, Loxocemidae and Colubridae (Serpentes), with comments on the fossil history of erycine boids in North America. Zool. Anz. 2013, 252, 157–182. [Google Scholar] [CrossRef]
- Rage, J.-C. An erycine snake (Boidae) of the genus Calamagras from the French lower Eocene, with comments on the phylogeny of the Erycinae. Herpetologica 1977, 33, 459–463. [Google Scholar]
- Head, J.J.; Bloch, J.I.; Hastings, A.K.; Bourque, J.R.; Cadena, E.A.; Herrera, F.A.; Polly, P.D.; Jaramillo, C.A. Giant boid snake from the Palaeocene neotropics reveals hotter past equatorial temperatures. Nature 2009, 457, 715–717. [Google Scholar] [CrossRef]
- Head, J.J.; Bloch, J.I.; Moreno-Bernal, J.; Rincon Burbano, A.F.; Bourque, J.R. Cranial osteology, body size, systematics, and ecology of the giant Paleocene snake Titanoboa cerrejonensis. J. Vertebr. Paleontol. 2013, 33, 140–141. [Google Scholar]
- Zachos, J.; Dickens, G.R.; Zeebe, R.E. An early Cenozoic perspective on greenhouse warming and carbon-cycle dynamics. Nature 2008, 451, 279–283. [Google Scholar] [CrossRef]
- Gavrilets, S.; Vose, A. Dynamic patterns of adaptive radiation. Proc. Natl. Acad. Sci. USA 2005, 102, 18040–18045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grande, L. The Lost World of Fossil Lake: Snapshots from Deep Time; University of Chicago Press: Chicago, IL, USA, 2013; p. 425. [Google Scholar]
- Rage, J.-C. The fossil snake Cheilophis huerfanoensis Gilmore, 1938, from Eocene of Colorado: Redescription and reappraisal of relationships. J. Vertebr. Paleontol. 1984, 3, 219–222. [Google Scholar] [CrossRef]
- Bhullar, B.-A.S.; Pauly, G.; Scanferla, C.A.; Bever, G.S.; Smith, K.T. The first fossil Sunbeam Snake [sic] and the antiquity of modern snake clades. J. Vertebr. Paleontol. 2009, 29, 63A. [Google Scholar]
- Gheerbrant, E.; Rage, J.-C. Paleobiogeography of Africa: How distinct from Gondwana and Laurasia? Palaeogeogr. Palaeoclimatol. Palaeoecol. 2006, 241, 224–246. [Google Scholar] [CrossRef]
- Ezcurra, M.D.; Agnolín, F.L. A new global palaeobiogeographical model for the late Mesozoic and early Tertiary. Syst. Biol. 2012, 61, 553–566. [Google Scholar] [CrossRef] [PubMed]
- Krause, D.W.; Sertich, J.J.W.; O’Connor, P.M.; Curry Rogers, K.; Rogers, R.R. The Mesozoic biogeographic history of Gondwanan terrestrial vertebrates: Insights from Madagascar’s fossil record. Annu. Rev. Earth Planet. Sci. 2019, 47, 519–553. [Google Scholar] [CrossRef]
- McKenna, M.C. Cenozoic paleogeography of North Atlantic land bridges. In Structure and Development of the Greenland–Scotland Ridge: New Methods and Concepts; Bott, M.H.P., Saxov, S., Talwani, M., Thiede, J., Eds.; Plenum Press: New York, NY, USA, 1983; pp. 351–399. [Google Scholar]
- Krishtalka, L.; West, R.M.; Black, C.C.; Dawson, M.R.; Flynn, J.J.; Turnbull, W.D.; Stucky, R.K.; McKenna, M.C.; Bown, T.M.; Golz, D.J.; et al. Eocene (Wasatchian through Duchesnean) biochronology of North America. In Cenozoic Mammals of North America: Geochronology and Biostratigraphy; Woodburne, M.O., Ed.; University of California Press: Berkeley, CA, USA, 1987; pp. 77–117. [Google Scholar]
- Augé, M. Évolution des lézards du Paléogène en Europe. Mém. Mus. Natl. Hist. Nat. Paris 2005, 192, 1–369. [Google Scholar]
- Smith, K.T. Eocene lizards of the clade Geiseltaliellus from Messel and Geiseltal, Germany, and the early radiation of Iguanidae (Squamata: Iguania). Bull. Peabody Mus. Nat. Hist. 2009, 50, 219–306. [Google Scholar] [CrossRef]
- Grace, M.S.; Matsushita, A. Neural correlates of complex behavior: Vision and infrared imaging in boas and pythons. In Biology of the Boas and Pythons; Henderson, R.W., Powell, R., Eds.; Eagle Mountain Publishing: Eagle Mountain, UT, USA, 2007; pp. 271–285. [Google Scholar]
- Molenaar, G.J. An additional trigeminal system in certain snakes possessing infrared receptors. Brain Res. 1974, 78, 340–344. [Google Scholar] [CrossRef] [Green Version]
- Bullock, T.H.; Barrett, R. Radiant heat reception in snakes. Commun. Behav. Biol. 1968, 1, 19–29. [Google Scholar]
- Noble, G.K.; Schmidt, A. The structure and function of the facial and labial pits of snakes. Proc. Am. Philos. Soc. 1937, 77, 263–288. [Google Scholar]
- Greene, H.W. The ecological and behavioral context for pitviper evolution. In Biology of the Pitvipers; Campbell, J.A., Brodie, E.D., Eds.; Selva: Tyler, TX, USA, 1992; pp. 107–117. [Google Scholar]
- Krochmal, A.R. Heat in evolution’s kitchen: Evolutionary perspectives on the functions and origin of the facial pit of pitvipers (Viperidae: Crotalinae). J. Exp. Biol. 2004, 207, 4231–4238. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shine, R.; Sun, L.-X.; Kearney, M.; Fitzgerald, M. Thermal correlates of foraging-site selection by Chinese Pit-vipers (Gloydius shedaoensis, Viperidae). J. Therm. Biol. 2002, 27, 405–412. [Google Scholar] [CrossRef]
- Greene, H.W. Dietary correlates of the origin and radiation of snakes. Am. Zool. 1983, 23, 431–441. [Google Scholar] [CrossRef]
- Delfino, M.; Smith, T. Reappraisal of the morphology and phylogenetic relationships of the middle Eocene alligatoroid Diplocynodon deponiae (Frey, Laemmert, and Riess, 1987) based on a three-dimensional specimen. J. Vertebr. Paleontol. 2012, 32, 1358–1369. [Google Scholar] [CrossRef]
- Gunnell, G.F.; Lehmann, T.; Ruf, I.; Habersetzer, J.; Morlo, M.; Rose, K.D. Ferae—Animals that eat animals. In Messel—An Ancient Greenhouse Ecosystem; Smith, K.T., Schaal, S.F.K., Habersetzer, J., Eds.; Schweizerbart: Stuttgart, Germany, 2018; pp. 270–283. [Google Scholar]
- Mayr, G.; Schaal, S.F.K. Gastric pellets with bird remains from the early Eocene of Messel. Palaios 2016, 31, 447–451. [Google Scholar] [CrossRef]
- Lehmann, T. With and without spines: The hedgehog kindred from Messel. In Messel: An Ancient Greenhouse Ecosystem; Smith, K.T., Schaal, S.F.K., Habersetzer, J., Eds.; Schweizerbart: Stuttgart, Germany, 2018; pp. 234–239. [Google Scholar]
- Mayr, G. The early Eocene birds of the Messel fossil site: A 48-million-year-old bird community adds a temporal perspective to the evolution of tropical avifaunas. Biol. Rev. 2017, 92, 1174–1188. [Google Scholar] [CrossRef]
- Morlo, M.; Schaal, S.; Mayr, G.; Seiffert, C. An annotated taxonomic list of the Middle Eocene (MP 11) Vertebrata of Messel. Cour. Forsch. Senckenberg 2004, 252, 95–108. [Google Scholar]
- Franzen, J.L. Eozäne Equoidea (Mammalia, Perissodactyla) aus der Grube Messel bei Darmstadt (Deutschland): Funde der Jahre 1969–2000. Schweiz. Paläont. Abh. 2007, 127, 1–245. [Google Scholar]
- Mayr, G. Birds—The most species-rich vertebrate group in Messel. In Messel: An Ancient Greenhouse Ecosystem; Smith, K.T., Schaal, S.F.K., Habersetzer, J., Eds.; Schweizerbart: Stuttgart, Germany, 2018; pp. 168–213. [Google Scholar]
- Habersetzer, J.; Rabenstein, R.; Gunnell, G.F. Bats—Highly specialized nocturnal hunters with echolocatin. In Messel: An Ancient Greenhouse Ecosystem; Smith, K.T., Schaal, S.F.K., Habersetzer, J., Eds.; Schweizerbart: Stuttgart, Germany, 2018; pp. 248–261. [Google Scholar]
- Smith, K.T.; Čerňanský, A.; Scanferla, A.; Schaal, S.F.K. Lizards and snakes: Warmth-loving sunbathers. In Messel: An Ancient Greenhouse Ecosystem; Smith, K.T., Schaal, S.F.K., Habersetzer, J., Eds.; Schweizerbart: Frankfurt am Main, Germany, 2018; pp. 122–147. [Google Scholar]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Scanferla, A.; Smith, K.T. Exquisitely Preserved Fossil Snakes of Messel: Insight into the Evolution, Biogeography, Habitat Preferences and Sensory Ecology of Early Boas. Diversity 2020, 12, 100. https://0-doi-org.brum.beds.ac.uk/10.3390/d12030100
Scanferla A, Smith KT. Exquisitely Preserved Fossil Snakes of Messel: Insight into the Evolution, Biogeography, Habitat Preferences and Sensory Ecology of Early Boas. Diversity. 2020; 12(3):100. https://0-doi-org.brum.beds.ac.uk/10.3390/d12030100
Chicago/Turabian StyleScanferla, Agustín, and Krister T. Smith. 2020. "Exquisitely Preserved Fossil Snakes of Messel: Insight into the Evolution, Biogeography, Habitat Preferences and Sensory Ecology of Early Boas" Diversity 12, no. 3: 100. https://0-doi-org.brum.beds.ac.uk/10.3390/d12030100