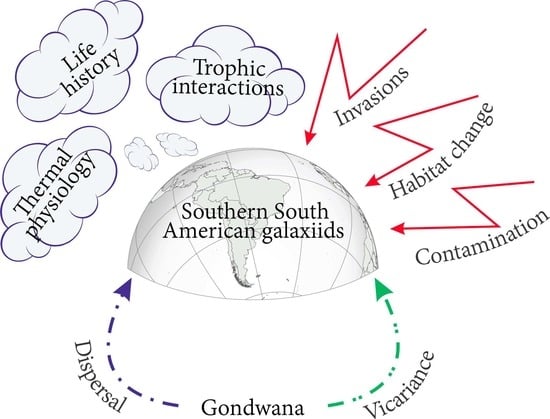
New Insights into the Distribution, Physiology and Life Histories of South American Galaxiid Fishes, and Potential Threats to This Unique Fauna
Abstract
:1. Introduction
2. Taxonomical Update
3. Changes in Distribution
4. Biological Traits and Environmental Constraints
4.1. Feeding
4.2. Physiological Ecology
4.3. Morphological Variation
4.4. Reproduction and Life History Patterns
4.5. Predation Risk
5. Climate Change, Invasions and Translocations
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Cussac, V.E.; Habit, E.; Ciancio, J.; Battini, M.A.; Riva Rossi, C.; Barriga, J.P.; Baigún, C.; Crichigno, S. Freshwater fishes of Patagonia: Conservation and fisheries. J. Fish Biol. 2016, 89, 1068–1097. [Google Scholar] [CrossRef]
- Dyer, B. Systematic review and biogeography of the freshwater fishes of Chile. Estud. Oceanol. 2000, 19, 77–98. [Google Scholar]
- Cussac, V.; Ortubay, S.; Iglesias, G.; Milano, D.; Lattuca, M.; Barriga, J.; Battini, M.; Gross, M. The distribution of South American galaxiid fishes: The role of biological traits and post glacial history. J. Biogeogr. 2004, 31, 103–122. [Google Scholar] [CrossRef]
- Habit, E.; Gonzalez, J.; Ruzzante, D.E.; Walde, S.J. Native and introduced fish species richness in Chilean Patagonian lakes: Inferences on invasion mechanisms using salmonid-free lakes. Divers. Distrib. 2012, 18, 1153–1165. [Google Scholar] [CrossRef]
- McDowall, R.M. The galaxiid fishes of South America. Zool. J. Linn. Soc. 1971, 50, 33–74. [Google Scholar] [CrossRef]
- Becker, L.A.; Crichigno, S.A.; Cussac, V.E. Climate change impacts on freshwater fishes: A Patagonian perspective. Hydrobiologia 2018, 816, 21–38. [Google Scholar] [CrossRef]
- Crichigno, S.; Cordero, P.; Blasetti, G.; Cussac, V. Dispersion of the invasive common carp Cyprinus carpio in Southern South America: Changes and expectations, westward and southward. J. Fish Biol. 2016, 89, 403–416. [Google Scholar] [CrossRef]
- Habit, E.; Dyer, B.; Vila, I. Estado de conocimiento de los peces dulceacuícolas de Chile. Gayana 2006, 70, 100–112. [Google Scholar] [CrossRef] [Green Version]
- Burridge, C.P.; McDowall, R.M.; Craw, D.; Wilson, M.V.H.; Waters, J.M. Marine dispersal as a prerequisite for Gondwanan vicariance among elements of the galaxiid fish fauna. J. Biogeogr. 2012, 39, 306–321. [Google Scholar] [CrossRef]
- Betancur, R.; Wiley, E.O.; Arratia, G.; Acero, A.; Bailly, N.; Miya, M.; Lecointre, G.; Ortí, G. Phylogenetic classification of bony fishes. BMC Evol. Biol. 2017, 17, 162. [Google Scholar] [CrossRef] [Green Version]
- Ruzzante, D.E.; Walde, S.J.; Gosse, J.C.; Cussac, V.E.; Habit, E.; Zemlak, T.S.; Adams, E.D.M. Climate control on ancestral population dynamics: Insight from patagonian fish phylogeography. Mol. Ecol. 2008, 17, 2234–2244. [Google Scholar] [CrossRef] [PubMed]
- Zemlak, T.S.; Habit, E.M.; Walde, S.J.; Battini, M.A.; Adams, E.D.M.; Ruzzante, D.E. Across the southern Andes on fin: Glacial refugia, drainage reversals and a secondary contact zone revealed by the phylogeographical signal of Galaxias platei in Patagonia. Mol. Ecol. 2008, 17, 5049–5061. [Google Scholar] [CrossRef] [PubMed]
- Zemlak, T.S.; Habit, E.M.; Walde, S.J.; Carrea, C.; Ruzzante, D.E. Surviving historical Patagonian landscapes and climate: Molecular insights from Galaxias maculatus. BMC Evol. Biol. 2010, 10, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macchi, P.J.; Pascual, M.A.; Vigliano, P.H. Differential piscivory of the native Percichthys trucha and exotic salmonids upon the native forage fish Galaxias maculatus in Patagonian Andean lakes. Limnologica 2007, 37, 76–87. [Google Scholar] [CrossRef] [Green Version]
- Juncos, R.; Beauchamp, D.A.; Vigliano, P.H. Modeling prey consumption by native and nonnative piscivorous fishes: Implications for competition and impacts on shared prey in an ultraoligotrophic lake in Patagonia. Trans. Am. Fish. Soc. 2013, 142, 268–281. [Google Scholar] [CrossRef]
- Juncos, R.; Milano, D.; Macchi, P.J.; Vigliano, P.H. Niche segregation facilitates coexistence between native and introduced fishes in a deep Patagonian lake. Hydrobiologia 2015, 747, 53–67. [Google Scholar] [CrossRef]
- Aigo, J.; Cussac, V.; Peris, S.; Ortubay, S.; Gómez, S.; López, H.; Gross, M.; Barriga, J.; Battini, M. Distribution of introduced and native fish in Patagonia (Argentina): Patterns and changes in fish assemblages. Rev. Fish Biol. Fish. 2008, 18, 387–408. [Google Scholar] [CrossRef]
- Barriga, J.P.; Battini, M.A.; Cussac, V.E. Annual dynamics variation of landlocked Galaxias maculatus (Jenyns 1842) population in a northern Patagonia river: Occurrence of juvenile upstream migration. J. Appl. Ichthyol. 2007, 23, 128–135. [Google Scholar] [CrossRef]
- Carrea, C.; Cussac, V.E.; Ruzzante, D.E. Genetic and phenotypic variation among Galaxias maculatus populations reflects contrasting landscape effects between northern and southern Patagonia. Freshw. Biol. 2013, 58, 36–49. [Google Scholar] [CrossRef]
- Macchi, P.J.; Vigliano, P.H.; Pascual, M.A.; Alonso, M.; Denegri, M.A.; Milano, D.; Garcia Asorey, M.; Lippolt, G. Historical policy goals for fish management in northern continental Patagonia Argentina: A structuring force of actual fish assemblages? Am. Fish. Soc. Symp. 2008, 49, 331–348. [Google Scholar]
- Alò, D.; Correa, C.; Arias, C.; Cárdenas, L. Diversity of Aplochiton fishes (Galaxiidea) and the taxonomic resurrection of A. marinus. PLoS ONE 2013, 8, e71577. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alò, D.; Correa, C.; Samaniego, H.; Krabbenhoft, C.A.; Turner, T.F. Otolith microchemistry and diadromy in Patagonian river fishes. PeerJ 2019, 7, e6149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vera-Escalona, I.; Habit, E.; Ruzzante, D.E. Echoes of a distant time: Effects of historical processes on contemporary genetic patterns in Galaxias platei in Patagonia. Mol. Ecol. 2015, 24, 4112–4128. [Google Scholar] [CrossRef] [PubMed]
- Habit, E.; Piedra, P.; Ruzzante, D.; Walde, S.; Belk, M.; Cussac, V.; Gonzalez, J.; Colin, N. Changes in the distribution of native fishes in response to introduced species and other anthropogenic effects. Glob. Ecol. Biogeogr. 2010, 19, 697–710. [Google Scholar] [CrossRef]
- Maldonado-Márquez, A.; Contador, T.; Rendoll-Cárcamo, J.; Moore, S.; Pérez-Troncoso, C.; Gomez-Uchida, D.; Harrod, C. Southernmost distribution limit for endangered Peladillas (Aplochiton taeniatus) and non-native coho salmon (Oncorhynchus kisutch) coexisting within the Cape Horn biosphere reserve, Chile. J. Fish Biol. 2020, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Milano, D.; Vigliano, P.H. Nuevos registros de Galaxias platei Steindachner, 1898 en lagos andinos-patagónicos (Teleostei: Osmeriformes: Galaxiidade). Neotropica 1997, 43, 109–111. [Google Scholar]
- Milano, D.; Cussac, V.E.; Macchi, P.J.; Ruzzante, D.E.; Alonso, M.F.; Vigliano, P.H.; Denegri, M.A. Predator associated morphology in Galaxias platei in Patagonian lakes. J. Fish Biol. 2002, 61, 138–156. [Google Scholar] [CrossRef]
- Correa, C.; Hendry, A. Invasive salmonids and lake order interact in the decline of puye grande Galaxias platei in western Patagonia lakes. Ecol. Appl. 2012, 22, 828–842. [Google Scholar] [CrossRef] [Green Version]
- Díaz, G. Revealing the Effects of Loss of Longitudinal Connectivity on Freshwater Fish in Andean River Networks. Ph.D. Thesis, University of Concepción, Concepción, Chile, 2019. [Google Scholar]
- Delgado, M.L.; Górski, K.; Habit, E.; Ruzzante, D.E. The effects of diadromy and its loss on genomic divergence: The case of amphidromous Galaxias maculatus populations. Mol. Ecol. 2019. [Google Scholar] [CrossRef]
- Górski, K.; Habit, E.M.; Pingram, M.A.; Manosalva, A.J. Variation of the use of marine resources by Galaxias maculatus in large Chilean rivers. Hydrobiologia 2018, 814, 61–73. [Google Scholar] [CrossRef]
- McDowall, R.M.; Nakaya, K. Morphological divergence in the two species of Aplochiton Jenyns (Salmoniformes: Aplochitonidae): A generalist and a specialist. Copeia 1988, 1, 233–236. [Google Scholar] [CrossRef]
- Lattuca, M.E.; Battini, M.; Macchi, P.J. Trophic interaction among fishes in a northern Patagonian oligotrophic lake. J. Fish Biol. 2008, 72, 1306–1320. [Google Scholar] [CrossRef]
- Lattuca, M.E.; Ortubay, S.; Battini, M.A.; Barriga, J.P.; Cussac, V.E. Presumptive environmental effects on body shape of Aplochiton zebra (Pisces, Galaxiidae) in Northern Patagonian lakes. J. Appl. Ichthyol. 2007, 23, 25–33. [Google Scholar] [CrossRef]
- Elgueta, A.; González, J.; Ruzzante, D.E.; Walde, S.J.; Habit, E. Trophic interference by Salmo trutta on Aplochiton zebra and Aplochiton taeniatus in southern Patagonian lakes. J. Fish Biol. 2013, 82, 430–443. [Google Scholar] [CrossRef]
- Cervellini, P.M.; Battini, M.A.; Cussac, V.E. Ontogenetic shifts in the feeding of Galaxias maculatus (Galaxiidae) and Odontesthes microlepidotus (Atherinidae). Environ. Biol. Fish. 1993, 36, 283–290. [Google Scholar] [CrossRef]
- Modenutti, B.E.; Balseiro, E.G.; Cervellini, P.M. Effect of the selective feeding of Galaxias maculatus (Salmoniformes Galaxiidae) on zooplankton of a South Andes lake. Aquat. Sci. 1993, 55, 65–75. [Google Scholar] [CrossRef]
- Ferriz, A. Alimentación del puyen Galaxias maculatus (Jenyns) en el río Limay, Provincia de Neuquén. Physica B 1984, 42, 29–32. [Google Scholar]
- Barriga, J.P.; Battini, M.A.; García-Asorey, M.; Carrea, C.; Macchi, P.J.; Cussac, V.E. Intraspecific variation in growth and morphology of landlocked Galaxias maculatus during the limnetic period of its life history. Hydrobiologia 2012, 679, 27–41. [Google Scholar] [CrossRef]
- Ferriz, R.A. Alimentación de Galaxias platei (Pisces, Galaxiidae) en siete ambientes lénticos de la provincia de Chubut, Argentina. Rev. Mus. Argentino Ciencias Nat. 2003, 5, 79–85. [Google Scholar] [CrossRef] [Green Version]
- Belk, M.C.; Habit, E.; Ortiz-Sandoval, J.J.; Sobenes, C.; Combs, E.A. Ecology of Galaxias platei in a depauperate lake. Ecol. Freshw. Fish 2014, 23, 615–621. [Google Scholar] [CrossRef]
- Ortiz-Sandoval, J.; Górski, K.; Sobenes, C.; González, J.; Manosalva, A.; Elgueta, A.; Habit, E. Invasive trout affect trophic ecology of Galaxias platei in Patagonian lakes. Hydrobiologia 2017, 790, 201–212. [Google Scholar] [CrossRef]
- Correa, C.; Bravo, A.P.; Hendry, A.P. Reciprocal trophic niche shifts in native and invasive fish: Salmonids and galaxiids in Patagonian lakes. Freshw. Biol. 2012, 57, 1769–1781. [Google Scholar] [CrossRef]
- Boy, C.C.; Pérez, A.F.; Tagliaferro, M.; Lattuca, M.E.; Gutiérrez, M.; Vanella, F.A. Exploring bioenergetics of diadromous Galaxias maculatus in the southernmost extreme of its distribution: Summer is not always the better season. J. Exp. Mar. Biol. Ecol. 2017, 488, 102–110. [Google Scholar] [CrossRef]
- Rojo, J.H.; Figueroa, D.E.; Boy, C.C. Age and growth of diadromous Galaxias maculatus (Jenyns, 1842) in southernmost South America (54° S) including contribution of age classes to reproduction. Environ. Biol. Fish. 2018, 101, 1149–1160. [Google Scholar] [CrossRef]
- Boy, C.C.; Pérez, A.F.; Fernández, D.A.; Calvo, J.; Morriconi, E.R. Energy allocation in relation to spawning and overwintering of a diadromous puyen (Galaxias maculatus) population in the southernmost limit of the species distribution. Polar Biol. 2009, 32, 9–14. [Google Scholar] [CrossRef]
- Urbina, M.A.; Glover, C.N. Effect of salinity on osmoregulation, metabolism and nitrogen excretion in the amphidromous fish, inanga (Galaxias maculatus). J. Exp. Mar. Biol. Ecol. 2015, 473, 7–15. [Google Scholar] [CrossRef]
- Milano, D.; Aigo, J.C.; Macchi, P.J. Diel patterns in space use, food and metabolic activity of Galaxias maculatus (Pisces: Galaxiidae) in the littoral zone of a shallow Patagonian lake. Aquat. Ecol. 2013, 47, 277–290. [Google Scholar] [CrossRef]
- Milano, D.; Vigliano, P.H.; Beauchamp, D.A. Effect of body size and temperature on respiration of Galaxias maculatus (Pisces: Galaxiidae). N. Z. J. Mar. Freshw. Res. 2017, 51, 295–303. [Google Scholar] [CrossRef]
- Busse, K. On a green pigment in the blood-serum of subadult lacustrine Galaxias (Pisces; Galaxiidae). Bonn. Zool. Beitr. Bd. 1993, 44, 125–131. [Google Scholar]
- Heller, N.E.; Zavaleta, E.S. Biodiversity management in the face of climate change: A review of 22 years of recommendations. Biol. Conserv. 2009, 142, 14–32. [Google Scholar] [CrossRef]
- Gille, S.T. Warming of the Southern Ocean since the 1950s. Science 2002, 295, 1275–1277. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lattuca, M.E.; Boy, C.C.; Vanella, F.A.; Barrantes, M.E.; Fernández, D.A. Thermal responses of three native fishes from estuarine areas of the Beagle Channel, and their implications for climate change. Hydrobiologia 2018, 808, 235–249. [Google Scholar] [CrossRef]
- Barrantes, M.E.; Lattuca, M.E.; Vanella, F.A.; Fernández, D.A. Thermal ecology of Galaxias platei (Pisces, Galaxiidae) in South Patagonia: Perspectives under a climate change scenario. Hydrobiologia 2017, 802, 255–267. [Google Scholar] [CrossRef]
- Johnson, J.A.; Kelsch, S.W. Effects of evolutionary thermal environment on temperature-preference relationships in fishes. Environ. Biol. Fish. 1998, 4, 447–458. [Google Scholar] [CrossRef]
- Das, T.; Pal, A.K.; Chakraborty, S.K.; Manush, S.M.; Chatterjee, N.; Mukherjee, S.C. Thermal tolerance and oxygen consumption of Indian Major Carps acclimated to four temperatures. J. Therm. Biol. 2004, 29, 157–163. [Google Scholar] [CrossRef]
- Kita, J.; Tsuchida, S.; Setoguma, T. Temperature preference and tolerance, and oxygen consumption of the marbled rockfish, Sebastiscus marmoratus. Mar. Biol. 1996, 125, 467–471. [Google Scholar] [CrossRef]
- McDowall, R.M. Falkland Islands biogeography: Converging trajectories in the South Atlantic Ocean. J. Biogeogr. 2005, 32, 49–62. [Google Scholar] [CrossRef]
- Pankhurst, N.W. Ocular morphology of the sweep Scorpis lineolatus and the spotty Pseudolabrus celidotus (Pisces: Teleostei) grown in low intensity light. Brain Behav. Evol. 1992, 39, 116–123. [Google Scholar] [CrossRef]
- McDowall, R.M.; Pankhurst, N.W. Loss of negative eye-size in a population of Aplochiton zebra (Teleostei: Galaxiidae) from the Falkland Islands. N. Z. J. Zool. 2005, 32, 17–22. [Google Scholar] [CrossRef]
- Rowe, D.K.; Dean, T.L. Effects of turbidity on the feeding ability of the juvenile migrant stage of six New Zealand freshwater fish species. N. Z. J. Mar. Freshw. Res. 1998, 32, 21–29. [Google Scholar] [CrossRef]
- Ferriz, R.A.; Gómez, S.E. Polymorphism of lacustrine and diadromous populations of Galaxias maculatus, Argentina, South America. Bioikos 2015, 29, 19–25. [Google Scholar]
- Rojo, J.H.; Fernández, D.A.; Figueroa, D.E.; Boy, C.C. Phenotypic and genetic differentiation between diadromous and landlocked puyen Galaxias maculatus. J. Fish Biol. 2020. [Google Scholar] [CrossRef] [PubMed]
- McDowall, R.M. New Zealand Freshwater Fishes: A Guide and Natural History; Heinemann Reed: Auckland, Australia, 1990; 232p. [Google Scholar]
- Carrea, C.; Barriga, J.P.; Cussac, V.E.; Ruzzante, D.E. Genetic and phenotypic differentiation among Galaxias maculatus populations in a Patagonian postglacial lake system. Biol. J. Linn. Soc. 2012, 107, 368–382. [Google Scholar] [CrossRef] [Green Version]
- McDowall, R.M. Variation in vertebral number in galaxiid fishes (Teleostei: Galaxiidae): A legacy of life history, latitude and length. Environ. Biol. Fish. 2003, 66, 361–381. [Google Scholar] [CrossRef]
- Milano, D.; Ruzzante, D.E.; Cussac, V.E.; Macchi, P.J.; Ferriz, R.A.; Barriga, J.P.; Aigo, J.C.; Lattuca, M.E.; Walde, S.J. Latitudinal and ecological correlates of morphological variation in Galaxias platei (Pisces, Galaxiidae) in Patagonia. Biol. J. Linn. Soc. 2006, 87, 69–82. [Google Scholar] [CrossRef] [Green Version]
- Barriga, J.P.; Milano, D.; Cussac, V.E. Variation in vertebral number and its morphological implication in Galaxias platei. J. Fish Biol. 2013, 83, 1321–1333. [Google Scholar] [CrossRef]
- Lattuca, M.E.; Brown, D.; Castiñeira, L.; Renzi, M.; Luizon, C.; Urbanski, J.; Cussac, V. Reproduction of landlocked Aplochiton zebra Jenyns (Pisces, Galaxiidae). Ecol. Freshw. Fish 2008, 17, 394–405. [Google Scholar] [CrossRef]
- Pollard, D.A. The biology of a landlocked form of the normally catadromous salmoniform fish Galaxias maculatus (Jenyns). I. Life cycle and origin. Aust. J. Mar. Freshw. Res. 1971, 22, 91–123. [Google Scholar] [CrossRef]
- Chapman, A.; Morgan, D.L.; Beatty, S.J.; Gill, H.S. Variation in life history of land-locked lacustrine and riverine populations of Galaxias maculatus (Jenyns 1842) in Western Australia. Environ. Biol. Fish. 2006, 77, 21–37. [Google Scholar] [CrossRef]
- Waters, J.M.; López, J.A.; Wallis, G.P. Molecular phylogenetics and biogeography of galaxiid fishes (Osteichthyes: Galaxiidae): Dispersal, vicariance, and the position of Lepidogalaxias salamandroides. Syst. Biol. 2000, 49, 777–795. [Google Scholar] [CrossRef] [Green Version]
- Boy, C.C.; Morriconi, E.; Calvo, J. Reproduction in puyen, Galaxias maculatus (Pisces: Galaxiidae), in the southernmost extreme of distribution. J. Appl. Ichthyol. 2007, 23, 547–554. [Google Scholar] [CrossRef]
- Milano, D.; Barriga, J.P. Reproductive aspects of Galaxias platei (Pisces, Galaxiidae) in a deep lake in North Patagonia. Mar. Freshw. Res. 2018, 69, 1379–1388. [Google Scholar] [CrossRef]
- Laurenson, L.J.B.; French, R.P.; Jones, P.; Ierodiaconou, D.; Gray, S.; Versace, V.L.; Rattray, A.; Brown, S.; Monk, J. Aspects of the biology of Galaxias maculatus. J. Fish Biol. 2012, 81, 1085–1100. [Google Scholar] [CrossRef] [PubMed]
- McDowall, R.M. Galaxias maculatus (Jenyns), the New Zealand Whitebait. N. Z. Mar. Dept. Fish. Res. Bull. 1968, 2, 1–84. [Google Scholar]
- Peredo, S.; Sobarzo, C. Actividad gonádica estacional de Galaxias maculatus (Jenyns, 1842) en el Río Cautín, IX Región, Chile. Bol. Soc. Biol. Concep. 1994, 65, 65–70. [Google Scholar]
- Barriga, J.P.; Battini, M.A.; Macchi, P.J.; Milano, D.; Cussac, V.E. Spatial and temporal distribution of landlocked Galaxias maculatus and Galaxias platei (Pisces: Galaxiidae) in a Lake in the South American Andes. N. Z. J. Mar. Freshw. Res. 2002, 36, 345–359. [Google Scholar] [CrossRef]
- Boy, C.C.; Pérez, A.F.; Lattuca, M.E.; Calvo, J.; Morriconi, E. Reproductive biology of Galaxias maculatus (Jenyns 1842) in the Río Ovando, a high-latitude environment in southernmost Patagonia, Argentina. J. Appl. Ichthyol. 2009, 25, 661–668. [Google Scholar] [CrossRef]
- Vigliano, P.H.; Beauchamp, D.A.; Milano, D.; Macchi, P.J.; Alonso, M.F.; García Asorey, M.I.; Denegri, M.A.; Ciancio, J.E.; Lippolt, G.; Rechencq, M.; et al. Quantifying predation on galaxiids and other native organisms by introduced rainbow trout in an ultraoligotrophic lake in northern Patagonia, Argentina: A bioenergetics modeling approach. Trans. Am. Fish. Soc. 2009, 138, 1405–1419. [Google Scholar] [CrossRef]
- Milano, D.; Lozada, M.; Zagarese, H.E. Predator-induced reaction patterns of landlocked Galaxias maculatus to visual and chemical cues. Aquat. Ecol. 2010, 44, 741–748. [Google Scholar] [CrossRef]
- Cussac, V.E.; Fernández, D.A.; Gómez, S.E.; López, H.L. Fishes of Southern South America: A story driven by temperature. Fish Physiol. Biochem. 2009, 35, 29–42. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Magnuson, J.J. History and heroes: The thermal niche of fishes and long-term lake ice dynamics. J. Fish Biol. 2010, 77, 1731–1744. [Google Scholar] [CrossRef] [PubMed]
- Servicio Meteorológico Nacional. Available online: https://www.smn.gob.ar/clima/tendencias (accessed on 16 April 2020).
- Secretaría de Ambiente y Desarrollo Sustentable de la Nación. Tercera Comunicación Nacional de la República Argentina a la Convención Marco de las Naciones Unidas Sobre el Cambio Climático. 2015. Available online: https://www.argentina.gob.ar/ambiente/sustentabilidad/cambioclimatico/comunicacionnacional/tercera (accessed on 24 March 2020).
- Pascual, M.A.; Cussac, V.; Dyer, B.; Soto, D.; Vigliano, P.; Ortubay, S.; Macchi, P. Freshwater fishes of Patagonia in the 21st century after a hundred years of human settlement, species introductions, and environmental change. Aquat. Ecosyst. Health Manag. 2007, 10, 212–227. [Google Scholar] [CrossRef]
- Macchi, P.J.; Cussac, V.E.; Alonso, M.F.; Denegri, M.A. Predation relationships between introduced salmonids and the native fish fauna in lakes and reservoirs in Northern Patagonia. Ecol. Freshw. Fish 1999, 8, 227–236. [Google Scholar] [CrossRef]
- Cussac, V.E.; Becker, L.A.; Aigo, J.; Conte-Grand, C.; Blasetti, G.; Cordero, P.; Crichigno, S.; Nabaes, D. Abundance of native fishes, wild introduced salmonids, and escaped farmed rainbow trout in a Patagonian reservoir. Lakes Reserv. Res. Manag. 2014, 19, 74–85. [Google Scholar] [CrossRef]
- Nabaes Jodar, D.N.; Becker, L.A.; Cordero, P.; Blasetti, G.; Cussac, V.E. Native and exotic fishes in a Patagonian reservoir with rainbow trout cage culture: Spatial and trophic resource use. Knowl. Manag. Aquat. Ecosyst. 2017, 418, 33. [Google Scholar] [CrossRef] [Green Version]
- Nabaes Jodar, D.N.; Cussac, V.E.; Becker, L.A. Into the wild: Escaped farmed rainbow trout show a dispersal-associated diet shift towards natural prey. Hydrobiologia 2020, 847, 105–120. [Google Scholar] [CrossRef]
- Zambrano, L.; Martínez-Meyer, E.; Menezes, N.; Townsend Peterson, A. Invasive potential of common carp (Cyprinus carpio) and Nile tilapia (Oreochromis niloticus) in American freshwater systems. Can. J. Fish. Aquat. Sci. 2006, 63, 1903–1910. [Google Scholar] [CrossRef] [Green Version]
- Aigo, J. Interacción Entre Peces Nativos y Salmónidos en Patagonia: Su Vulnerabilidad Frente al Cambio Climático. Ph.D. Thesis, Universidad Nacional del Comahue, Bariloche, Argentina, 2010. [Google Scholar]
- Alvear, P.; Rechencq, M.; Macchi, P.J.; Alonso, M.F.; Lippolt, G.E.; Denegri, M.A.; Navone, G.; Zattara, E.; García Asorey, M.I.; Vigliano, P.H. Composición, distribución y relaciones tróficas de la ictiofauna del río Negro, Patagonia Argentina. Ecol. Austral 2007, 17, 231–246. [Google Scholar]
- Tagliaferro, M.; Quiroga, A.; Pascual, M. Spatial pattern and habitat requirements of Galaxias maculatus in the last un-interrupted large river of Patagonia: A baseline for management. Environ. Nat. Resour. Res. 2014, 4. [Google Scholar] [CrossRef]
- Moorman, M.C.; Eggleston, D.B.; Anderson, C.B.; Mansilla, A.; Szejner, P. Implications of beaver Castor canadensis and trout introductions on native fish in the Cape Horn Biosphere Reserve, Chile. Trans. Am. Fish. Soc. 2009, 138, 306–313. [Google Scholar] [CrossRef]
- Ferriz, R.A.; Salas Aramburu, W.; Gómez, S.E.; Menni, R.C. Morphological differences in Galaxias maculatus (Jenyns, 1842) population, an osmeriform fish from Southern Argentina. Bioikos 2001, 15, 83–89. [Google Scholar]
- Ondarza, P.M.; Miglioranza, K.S.B.; Gonzalez, M.; Shimabukuro, V.M.; Aizpún, J.E.; Moreno, V.J. Organochlorine compounds in common carp (Cyprinus carpio) from Patagonia Argentina. J. Braz. Soc. Ecotoxicol. 2010, 5, 41–47. [Google Scholar] [CrossRef]
- Ondarza, P.M.; Gonzalez, M.; Fillmann, G.; Miglioranza, K.S.B. Increasing levels of persistent organic pollutants in rainbow trout (Oncorhynchus mykiss) following a mega-flooding episode in the Negro River basin, Argentinean Patagonia. Sci. Total Environ. 2012, 419, 233–239. [Google Scholar] [CrossRef] [PubMed]
- Ondarza, P.M.; Gonzalez, M.; Fillmann, G.; Miglioranza, K.S.B. PBDEs, PCBs and organochlorine pesticides distribution in edible fish from Negro River basin, Argentinean Patagonia. Chemosphere 2014, 94, 135–142. [Google Scholar] [CrossRef] [PubMed]
- Martínez, O.A.; Kutschker, A. The ‘Rodados Patagónicos’ (Patagonian shingle formation) of eastern Patagonia: Environmental conditions of gravel sedimentation. Biol. J. Linn. Soc. 2011, 103, 336–345. [Google Scholar] [CrossRef] [Green Version]
- Aragón, E.; Goin, F.J.; Aguilera, Y.E.; Woodburne, M.O.; Carlini, A.A.; Roggiero, M.F. Palaeogeography and palaeoenvironments of northern Patagonia from the Late Cretaceous to the Miocene: The Palaeogene Andean gap and the rise of the North Patagonian High Plateau. Biol. J. Linn. Soc. 2011, 103, 305–315. [Google Scholar] [CrossRef]
- Benito, G.; Thorndycraft, V.R. Catastrophic glacial-lake outburst flooding of the Patagonian Ice Sheet. Earth-Sci. Rev. 2020, 200, 102996. [Google Scholar] [CrossRef]


© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cussac, V.E.; Barrantes, M.E.; Boy, C.C.; Górski, K.; Habit, E.; Lattuca, M.E.; Rojo, J.H. New Insights into the Distribution, Physiology and Life Histories of South American Galaxiid Fishes, and Potential Threats to This Unique Fauna. Diversity 2020, 12, 178. https://0-doi-org.brum.beds.ac.uk/10.3390/d12050178
Cussac VE, Barrantes ME, Boy CC, Górski K, Habit E, Lattuca ME, Rojo JH. New Insights into the Distribution, Physiology and Life Histories of South American Galaxiid Fishes, and Potential Threats to This Unique Fauna. Diversity. 2020; 12(5):178. https://0-doi-org.brum.beds.ac.uk/10.3390/d12050178
Chicago/Turabian StyleCussac, Víctor Enrique, María Eugenia Barrantes, Claudia Clementina Boy, Konrad Górski, Evelyn Habit, María Eugenia Lattuca, and Javier Hernán Rojo. 2020. "New Insights into the Distribution, Physiology and Life Histories of South American Galaxiid Fishes, and Potential Threats to This Unique Fauna" Diversity 12, no. 5: 178. https://0-doi-org.brum.beds.ac.uk/10.3390/d12050178