Zoantharia (Cnidaria: Hexacorallia) of the Dutch Caribbean and One New Species of Parazoanthus
Abstract
:1. Introduction
2. Materials and Methods
2.1. Specimens Analyzed
2.2. Specimen Identification
2.3. Cnidae Analyses
2.4. DNA Extraction, Polymerase Chain Reaction (PCR) Amplification, and Sequencing
2.5. Phylogenetic Analyses and Species Delimitations
3. Results
3.1. Diversity in the Dutch Caribbean
3.2. Specimens and Species
3.2.1. Parazoanthidae sp. (Figure 2)
3.2.2. Antipathozoanthus aff. macaronesicus Ocaña & Brito, 2003 [59] (Figure 3)
3.2.3. Bergia catenularis Duchassaing de Fonbressin & Michelotti, 1860 [23] (Figure 4)
3.2.4. Bergia cf. cutressi (West, 1979) [26] (Figure 5)
3.2.5. Bergia puertoricense (West, 1979) [26] (Figure 6)
3.2.6. Parazoanthus swiftii (Duchassaing de Fonbressin & Michelotti, 1860) [23] (Figure 7)
3.2.7. Parazoanthidae? sp. (Figure 8)
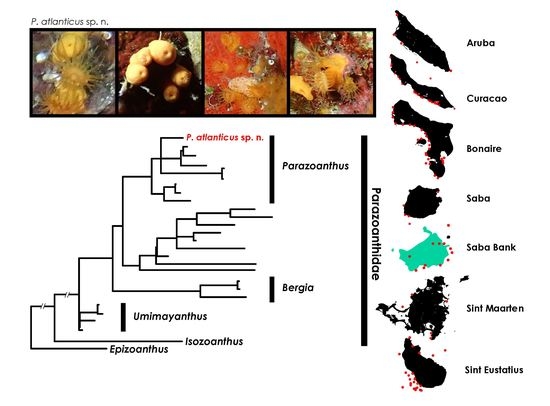
3.2.8. Parazoanthus atlanticus sp. n. (Figure 9)
3.2.9. Umimayanthus parasiticus (Duchassaing de Fonbressin & Michelotti, 1860) [23] (Figure 12)
3.2.10. Umimayanthus sp. (Figure 13)
3.2.11. Epizoanthus sp. (Figure 14)
3.2.12. Hydrozoanthus antumbrosus (Swain, 2009) [62] (Figure 15)
3.2.13. Hydrozoanthus tunicans (Duerden, 1900) [68] (Figure 16)
3.2.14. (a) Palythoa caribaeorum (Duchassaing de Fonbressin & Michelotti, 1860) [23] (Figure 17a,c)
- 14(b)
- Palythoa caracasiana Pax, 1924 [150] (Figure 17b)
- 14(c)
- Palythoa horstii Pax, 1924 [150]
- 14(d)
- Palythoa mammillosa (Ellis & Solander, 1786) [165] (Figure 17d)
3.2.15. Palythoa grandiflora (Verrill, 1900) [92] (Figure 18)
3.2.16. Palythoa grandis (Verrill, 1900) [92] (Figure 19)
3.2.17. Palythoa variabilis (Duerden, 1898) [76] (Figure 20)
3.2.18. Palythoa sp. (Figure 21)
3.2.19. Zoanthus pulchellus (Duchassaing de Fonbressin & Michelotti, 1860) [23] (Figure 22)
3.2.20. Zoanthus aff. pulchellus (Duchassaing de Fonbressin & Michelotti, 1860) [23]
3.2.21. Zoanthus sociatus (Ellis, 1768) [185] (Figure 23)
3.2.22. Zoanthus solanderi Le Sueur, 1817 [192] (Figure 24)
3.2.23. Zoanthus sp. (Figure 25)
3.2.24. Isaurus tuberculatus Gray, 1828 [197] (Figure 26)
3.2.25. Zoantharian Species Distribution across the Dutch Caribbean
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| NA | information not available. |
| RMNH | Rijksmuseum van Natuurlijke Historie (at Naturalis Biodiversity Center), Leiden, Netherlands |
| ZMA | Zoological Museum Amsterdam (at Naturalis Biodiversity Center), Leiden, Netherlands |
| NSMT | National Science Museum, Tsukuba, Ibaraki, Japan |
| MISE | Molecular Invertebrate Systematics and Ecology Laboratory, University of the Ryukyus, Nishihara, Okinawa, Japan |
| Por | Porifera collection |
| Coel | Coelenterata collection |
| Sta. LUY | Station number Luymes Expedition [47] |
| Sta. EUX | Station number Marine Biodiversity Expedition to Sint Eustatius, 2015 [48] |
| coll. BWH | collector: Bert W. Hoeksema |
| coll. JCH | collector: J.C. (Koos) den Hartog |
| coll. JDR | collector: James D. Reimer |
| coll. JGH | collectoer: Jaaziel E. Garcia-Hernandez |
| coll. PWH | collector: P. Wagenaar Hummelinck |
References
- Santos, M.E.A.; Kitahara, M.V.; Lindner, A.; Reimer, J.D. Overview of the order Zoantharia (Cnidaria: Anthozoa) in Brazil. Mar. Biodivers. 2016, 46, 547–559. [Google Scholar] [CrossRef]
- Fautin, D.G.; Daly, M. Actiniaria, Corallimorpharia, and Zoanthidea (Cnidaria: Anthozoa) of the Gulf of Mexico. In The Gulf of Mexico—Origins, Waters, and Biota; Felder, D.L., Camp, D.K., Eds.; Texas A&M University Press: College Station, TX, USA, 2009; pp. 349–357. [Google Scholar]
- Humann, P.; DeLoach, N.; Wilk, L. Reef Creature Identification: Florida, Caribbean, Bahamas, 3rd ed.; New World Publications: Jacksonville, FL, USA, 2013. [Google Scholar]
- Reimer, J.D.; Hirose, M.; Wirtz, P. Zoanthids of the Cape Verde Islands and their symbionts: Previously unexamined diversity in the Northeastern Atlantic. Contrib. Zool. 2010, 79, 147–163. [Google Scholar] [CrossRef] [Green Version]
- Santos, M.E.A.; Wirtz, P.; Montenegro, J.; Kise, H.; López, C.; Brown, J.; Reimer, J.D. Diversity of Saint Helena Island and zoogeography of zoantharians in the Atlantic Ocean: Jigsaw falling into place. System. Biodivers. 2019, 17, 165–178. [Google Scholar] [CrossRef]
- Cairns, S.D.; den Hartog, J.C.; Arneson, C.; Rützler, K. Class Anthozoa (Corals, Anemones). In Marine Fauna and Flora of Bermuda: A Systematic Guide to the Identification of Marine Organisms; Sterrer, W., Ed.; Wiley: New York, NY, USA, 1986. [Google Scholar]
- Häussermann, V. Ordnung Zoantharia (=Zoanthinaria, Zoanthidae) (Krustenanemonen). In Das Mittelmeer—Fauna, Flora, Ökologie. Band II/1: Bestimmungsführer Prokaryota, Protista, Fungi, Plantae, Animalia (bis Nemertea); Hofrichter, R., Ed.; Spektrum Akademischer Verlag: Heidelberg, Germany, 2003. [Google Scholar]
- López, C.; Reimer, J.D.; Brito, A.; Simón, D.; Clemente, S.; Hernández, M. Diversity of zoantharian species and their symbionts from the Macaronesian and Cape Verde ecoregions demonstrates their widespread distribution in the Atlantic Ocean. Coral Reefs 2019, 38, 269–283. [Google Scholar] [CrossRef]
- Reimer, J.D.; Lorion, J.; Irei, Y.; Hoeksema, B.W.; Wirtz, P. Ascension Island shallow-water Zoantharia (Hexacorallia: Cnidaria) and their zooxanthellae (Symbiodinium). J. Mar. Biol. Assoc. UK 2017, 97, 695–703. [Google Scholar] [CrossRef] [Green Version]
- Carreiro-Silva, M.; Braga-Henriques, A.; Sampaio, I.; de Matos, V.; Porteiro, F.M.; Ocaña, O. Isozoanthus primnoidus, a new species of zoanthid (Cnidaria: Zoantharia) associated with the gorgonian Callogorgia verticillata (Cnidaria: Alcyonacea). ICES J. Mar. Sci. 2011, 68, 408–415. [Google Scholar] [CrossRef]
- Carreiro-Silva, M.; Ocaña, O.; Stanković, D.; Sampaio, Í.; Porteiro, F.M.; Fabri, M.-C.; Stefanni, S. Zoantharians (Hexacorallia: Zoantharia) associated with cold-water corals in the Azores Region: New species and associations in the deep sea. Front. Mar. Sci. 2017, 4, 88. [Google Scholar] [CrossRef]
- Hoeksema, B.W.; Reimer, J.D.; Vonk, R. Editorial: Biodiversity of Caribbean coral reefs (with a focus on the Dutch Caribbean). Mar. Biodivers. 2017, 47, 1–10. [Google Scholar] [CrossRef]
- Van der Horst, C.J. Bijdragen tot de kennis der fauna van Curaçao. Narrative of the voyage and short description of localities. Bijdr. Dierk. 1924, 23, 1–12, pls. 1–2. [Google Scholar]
- Wagenaar Hummelinck, P. Description of new localities. Stud. Fauna Curaçao Caribb. Isl. 1953, 4, 1–108, pls. 1–8. [Google Scholar]
- Wagenaar Hummelinck, P. Marine localities. Stud. Fauna Curaçao Caribb. Isl. 1977, 51, 1–68, pls. 1–55. [Google Scholar]
- Van den Hoek, C.; Cortel-Breeman, A.M.; Wanders, J.B.W. Algal zonation in the fringing coral reef of Curaçao, Netherlands Antilles, in relation to zonation of corals and gorgonians. Aquat. Bot. 1975, 1, 269–308. [Google Scholar] [CrossRef]
- Wanders, J.B.W. The role of benthic algae in the shallow reef of Curaçao (Netherlands Antilles). I: Primary productivity in the coral reef. Aquat. Bot. 1976, 2, 235–270. [Google Scholar] [CrossRef]
- Nagelkerken, I.; Nagelkerken, W.P. Loss of coral cover and biodiversity on shallow Acropora and Millepora reefs after 31 years on Curaçao, Netherlands Antilles. Bull. Mar. Sci. 2004, 74, 213–223. [Google Scholar]
- Bak, R.P.M. Ecological aspects of the distribution of reef corals in the Netherlands Antilles. Bijdr. Dierk. 1975, 45, 181–190. [Google Scholar] [CrossRef] [Green Version]
- Van Duyl, F.C. Atlas of the Living Reefs of Curaçao and Bonaire (Netherlands Antilles); Foundation for Scientific Research in Surinam and the Netherlands Antilles: Utrecht, The Netherlands, 1985. [Google Scholar]
- Reimer, J.D. Zoantharia of St. Eustatius. In Marine Biodiversity Survey of St. Eustatius, Dutch Caribbean; Hoeksema, B.W., Ed.; Naturalis and ANEMOON Foundation: Leiden, The Netherlands, 2016; pp. 43–45. [Google Scholar]
- Garcia-Hernandez, J.E.; Reimer, J.D.; Hoeksema, B.W. Sponges hosting the Zoantharia-associated crab Platypodiella spectabilis at St. Eustatius, Dutch Caribbean. Coral Reefs 2016, 35, 209. [Google Scholar] [CrossRef] [Green Version]
- Duchassaing de Fonbressin, P.; Michelotti, J. Supplément au mémoire sur les Coralliaires des Antilles. Memorie della Reale Accademia delle Scienze di Torino; Imprimerie Royale: Turin, Italy, 1860. [Google Scholar]
- Reimer, J.D.; Wee, H.B.; García-Hernández, J.E.; Hoeksema, B.W. Zoantharia (Anthozoa: Hexacorallia) abundance and associations with Porifera and Hydrozoa across a depth gradient on the west coast of Curaçao. System. Biodivers. 2018, 16, 820–830. [Google Scholar] [CrossRef]
- Pax, F. Studien an westindischen Actinien. Zool. Jahrb. Supp. 1910, 11, 157–330. [Google Scholar]
- West, D.A. Symbiotic zoanthids (Anthozoa: Cnidaria) of Puerto Rico. Bull. Mar. Sci. 1979, 29, 253–271. [Google Scholar]
- Burnett, W.J.; Benzie, J.A.H.; Beardmore, J.A.; Ryland, J.S. Zoanthids (Anthozoa, Hexacorallia) from the Great Barrier Reef and Torres Strait, Australia: Systematics, evolution and a key to species. Coral Reefs 1997, 16, 55–68. [Google Scholar] [CrossRef] [Green Version]
- Reimer, J.D.; Ono, S.; Fujiwara, Y.; Takishita, K.; Tsukahara, J. Reconsidering Zoanthus spp. diversity: Molecular evidence of conspecifity within four previously presumed species. Zool. Sci. 2004, 21, 517–525. [Google Scholar] [CrossRef] [PubMed]
- Swain, T.D.; Wulff, J.L. Diversity and specificity of Caribbean sponge–zoanthid symbioses: A foundation for understanding the adaptive significance of symbioses and generating hypotheses about higher-order systematics. Biol. J. Linn. Soc. 2007, 92, 695–711. [Google Scholar] [CrossRef]
- Rasband, W.S. ImageJ: Image processing and analysis in Java. ASCL 2012, 1, 6013. [Google Scholar]
- England, K.W. Nematocysts of sea anemones (Actiniaria, Ceriantharia and Corallimorpharia: Cnidaria): Nomenclature. Hydrobiologia 1991, 216–217, 691–697. [Google Scholar] [CrossRef]
- Ryland, J.S.; Lancaster, J.E. A review of zoanthid nematocyst types and their population structure. Hydrobiologia 2004, 530–531, 179–187. [Google Scholar]
- Schmidt, H. On evolution in the Anthozoa. In Proceedings of the 2nd International Coral Reef Symposium, Marco Polo (Ship), 22 June–2 July 1973; Cameron, A.M., Campbell, B.M., Cribb, A.B., Endean, R., Jell, J.S., Jones, O.A., Mather, P., Talbot, F.H., Eds.; International Coral Reef Society: Brisbane, Australia, 1974; pp. 533–560. [Google Scholar]
- Hidaka, M.; Miyazaki, I.; Yamazato, K. Nematocysts characteristic of the sweeper tentacles of the coral Galaxea fascicularis (Linnaeus). Galaxea 1987, 6, 195–207. [Google Scholar]
- Hidaka, M. Use of nematocyst morphology for taxonomy of some related species of scleractinian corals. Galaxea 1992, 11, 21–28. [Google Scholar]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotech. 1994, 3, 294–299. [Google Scholar]
- Sinniger, F.; Montoya-Burgos, J.I.; Chevaldonné, P.; Pawlowski, J. Phylogeny of the order Zoantharia (Anthozoa, Hexacorallia) based on the mitochondrial ribosomal genes. Mar. Biol. 2005, 147, 1121–1128. [Google Scholar] [CrossRef]
- Reimer, J.D.; Takishita, K.; Ono, S.; Tsukahara, J.; Maruyama, T. Molecular evidence suggesting interspecific hybridization in Zoanthus spp. (Anthozoa: Hexacorallia). Zool. Sci. 2007, 24, 346–359. [Google Scholar] [CrossRef]
- Kearse, M.; Moir, R.; Wilson, A.; Stones-Havas, S.; Cheung, M.; Sturrock, S.; Buxton, S.; Cooper, A.; Markowitz, S.; Duran, C.; et al. Geneious Basic: An integrated and extendable desktop software platform for the organization and analysis of sequence data. Bioinformatics 2012, 28, 1647–1649. [Google Scholar] [CrossRef] [PubMed]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Edgar, R.C. MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nuc. Acids Res. 2004, 32, 1792–1797. [Google Scholar] [CrossRef] [Green Version]
- Milne, I.; Lindner, D.; Bayer, M.; Husmeier, D.; McGuire, G.; Marshall, D.F.; Wright, F. TOPALi v2: A rich graphical interface for evolutionary analyses of multiple alignments on HPC clusters and multi-core desktops. Bioinformatics 2009, 25, 126–127. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.R.; Huelsenbeck, J.P. MRBAYES: Bayesian inference of phylogeny. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Avise, J.C.; Ball, R.M., Jr. Principles of genealogical concordance in species concepts and biological taxonomy. In Oxford Surveys in Evolutionary Biology; Futuyama, D., Antonovics, J., Eds.; Oxford University Press: Oxford, UK, 1990; Volume 7, pp. 45–67. [Google Scholar]
- Sites, J.W., Jr.; Marshal, J.C. Operational criteria for delimiting species. Ann. Rev. Ecol. Evol. System. 2004, 35, 199–227. [Google Scholar] [CrossRef] [Green Version]
- Van der Land, J. The Saba Bank—A large atoll in the Northeastern Caribbean. FAO Fish. Rep. 1977, 200, 469–481. [Google Scholar]
- Hoeksema, B.W. Marine Biodiversity Survey of St. Eustatius, Dutch Caribbean, 2015; Naturalis Biodiversity Center: Leiden, The Netherlands; ANEMOON Foundation: Leiden, The Netherlands, 2016. [Google Scholar]
- Rafinesque, C.S. Analyse de la Nature, ou Tableau de L’univers et des Corps Organisés; Selbstverl: Palerme, Italy, 1815. [Google Scholar]
- Haddon, A.C.; Shackleton, A.M. A revision of the British Actiniae. Part II. The Zoantheae. In Reports on the zoological collections made in the Torres Straits by A.C. Haddon, 1888–1889. Sci. Trans. Roy. Dublin Soc. 1891, 4, 609–660. [Google Scholar]
- Delage, Y.; Hérouard, E. Zoanthidés–Zoanthidae. In Traité de Zoologie Concrète. Tome II—2me Partie; Les Coelentérés: Paris, France, 1901. [Google Scholar]
- Swain, T.D.; Swain, L.M. Molecular parataxonomy as taxon description: Examples from recently named Zoanthidea (Cnidaria: Anthozoa) with revision based on serial histology of microanatomy. Zootaxa 2014, 3796, 81–107. [Google Scholar] [CrossRef] [Green Version]
- Swain, T.D.; Schellinger, J.L.; Strimaitis, A.M.; Reuter, K.E. Evolution of anthozoan polyp retraction mechanisms: Convergent functional morphology and evolutionary allometry of the marginal musculature in order Zoanthidea (Cnidaria: Anthozoa: Hexacorallia). BMC Evol. Biol. 2015, 15, 123. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carlgren, O. Zoantharia. Dan. Ingolf-Exped. 1913, 5, 1–64. [Google Scholar]
- Carlgren, O. Ceriantharia und Zoantharia der deutschen Tiefsee-Expedition. Zoantharia. In Wissenschaftliche Ergebnisse der Deutschen Tiefsee-Expedition auf dem Dampfer “Valdivia” 1898–1899; Gustav Fischer: Jena, Germany, 1923; Volume 19, pp. 252–337. [Google Scholar]
- Reiswig, H.M.; Dohrmann, M. Three new species of glass sponges (Porifera: Hexactinellida) from the West Indies, and molecular phylogenetics of Euretidae and Auloplacidae (Sceptrulophora). Zool. J. Linn. Soc. 2014, 171, 233–253. [Google Scholar] [CrossRef]
- Van Soest, R.W.M.; Meesters, E.H.W.G.; Becking, L.E. Deep-water sponges (Porifera) from Bonaire and Klein Curaçao, Southern Caribbean. Zootaxa 2014, 3878, 401–443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sinniger, F.; Reimer, J.D.; Pawlowski, J. The Parazoanthidae (Hexacorallia: Zoantharia) DNA taxonomy: Description of two new genera. Mar. Biodivers. 2010, 40, 57–70. [Google Scholar] [CrossRef]
- Ocaña, O.; Brito, A. A review of Gerardiidae (Anthozoa: Zoantharia) from the Macaronesian islands and the Mediterranean Sea with the description of a new species. Rev. Acad. Canaria Cien. 2003, 15, 159–189. [Google Scholar]
- Kise, H.; Fujii, T.; Masucci, G.D.; Biondi, P.; Reimer, J.D. Three new species and the molecular phylogeny of Antipathozoanthus from the Indo-Pacific Ocean (Anthozoa, Hexacorallia, Zoantharia). ZooKeys 2017, 725, 97–122. [Google Scholar] [CrossRef] [Green Version]
- Reimer, J.D.; Fujii, T. Four new species and one new genus of zoanthids (Cnidaria, Hexacorallia) from the Galapagos Islands. ZooKeys 2010, 42, 1–36. [Google Scholar] [CrossRef]
- Swain, T.D. Isozoanthus antumbrosus, a new species of zoanthid (Cnidaria: Anthozoa: Zoanthidea) symbiotic with Hydrozoa from the Caribbean, with a key to hydroid and sponge-symbiotic zoanthid species. Zootaxa 2009, 2051, 41–48. [Google Scholar] [CrossRef]
- Duerden, J.E. West Indian sponge incrusting actinians. Bull. Am. Mus. Nat. Hist. 1903, 19, 495–503, pls. 44–47. [Google Scholar]
- Lewis, T.B.; Finelli, C.M. Epizoic zoanthids reduce pumping in two Caribbean vase sponges. Coral Reefs 2015, 34, 291–300. [Google Scholar] [CrossRef]
- Crocker, L.A.; Reiswig, H.M. Host specificity in sponge-encrusting Zoanthidea (Anthozoa: Zoantharia) of Barbados, West Indies. Mar. Biol. 1981, 65, 231–236. [Google Scholar] [CrossRef]
- Swain, T.D. Phylogeny-based species delimitations and the evolution of host associations in symbiotic zoanthids (Anthozoa, Zoanthidea) of the wider Caribbean region. Zool. J. Linn. Soc. 2009, 156, 223–238. [Google Scholar] [CrossRef] [Green Version]
- Montenegro, J.; Acosta, A. Habitat preference of Zoantharia genera depends on host sponge morphology. Universitas Scientiarum 2010, 15, 110–121. [Google Scholar] [CrossRef]
- Fontaine, A. The colonial sea-anemones of Jamaica. Nat. Hist. Notes Nat. Hist. Soc. Jamaica 1954, 66, 107–109. [Google Scholar]
- Sammarco, P.W.; Porter, S.A.; Genazzio, M.; Sinclair, J. Success in competition for space in two invasive coral species in the western Atlantic—Tubastraea micranthus and T. coccinea. PLoS ONE 2015, 10, e0144581. [Google Scholar] [CrossRef] [PubMed]
- González-Muñoz, R.; Simões, N.; Guerra-Castro, E.J.; Hernández-Ortíz, C.; Carrasquel, G.; Mendez, E.; Lira, C.; Rada, M.; Hernández, I.; Pauls, S.M. Sea anemones (Cnidaria: Actiniaria, Corallimorpharia, Ceriantharia, Zoanthidea) from marine shallow-water environments in Venezuela: New records and an updated inventory. Mar. Biodivers. Rec. 2016, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Montenegro, J.; Low, M.E.Y.; Reimer, J.D. The resurrection of the genus Bergia (Anthozoa, Zoantharia, Parazoanthidae). Syst. Biodivers. 2016, 14, 63–73. [Google Scholar] [CrossRef]
- Gray, J.E. Notes on Zoanthinae, with the descriptions of some new genera. Proc. Zool. Soc. Lond. 1867, 1, 233–240. [Google Scholar]
- Irving, R.A. A preliminary investigation of the sublittoral habitats and communities of Ascension Island. Prog. Underw. Sci. 1989, 13, 65–78. [Google Scholar]
- Diez, Y.L.; Campos-Castro, A. Soft corals (Anthozoa: Corallimorpharia, Actinaria and Zoantharia) from southeastern of Cuba, and its distribution in Marine Protected Areas. Rev. Investig. Mar. 2016, 36, 80–93. [Google Scholar]
- Williams, E.H., Jr.; Clavijo, I.; Kimmel, J.J.; Colin, P.L.; Carela, C.D.; Bardales, A.T.; Armstrong, R.H.; Williams, L.B.; Boulon, R.H.; Garcia, J.R. A checklist of marine plants and animals of the south coast of the Dominican Republic. Carib. J. Sci. 1983, 19, 39–53. [Google Scholar]
- Duerden, J.E. Jamaican Actiniaria. Part, I.-Zoantheae. Sci. Trans. Roy. Dublin Soc. 1898, 6, 329–385. [Google Scholar]
- Duerden, J.E. Jamaican Actiniaria. Part II. Stichodactylinae and Zoantheae. Sci. Trans. Roy. Dublin Soc. 1900, 7, 133–200. [Google Scholar]
- Cubit, J.D.; Williams, S. The invertebrates of Galeta Reef (Caribbean Panama): A species list and bibliography. Atoll Res. Bull. 1983, 269, 1–45. [Google Scholar] [CrossRef]
- Norman, A.M. Shetland final dredging report—Part II. On the Crustacea, Tunicata, Polyzoa, Echinodermata, Actinozoa, Hydrozoa, and Porifera. In Proceedings of the Thirty-Eighth Meeting of the British Association for the Advancement of Science, Norwich, UK, 19–26 August 1868; John Murray: London, UK, 1869; pp. 247–336. [Google Scholar]
- Carlgren, O. Actiniaria and Zoantharia. In Further Zoological Results of the Swedish Antartic Expedition 1901-1903 under the Direction of Dr. Otto Nordnskjold, 1st ed.; Odhner, T., Ed.; Norstedt and Soner: Stockholm, Sweden, 1927; pp. 93–95. [Google Scholar]
- Pax, F. Aktinien der Aru-Inseln; Senckenbergischen Naturforschenden Gesellschaft: Frankfurt, Germany, 1911; pp. 297–304. [Google Scholar]
- McMurrich, J.P. The Actiniae of the Plate Collection. Zool. Jahrb. Supp. 1904, 6, 215–306. [Google Scholar]
- Carlgren, O. Actiniaria und Zoantharia von Juan Fernandez und der Osterinsel. In The Natural History of Juan Fernandez and Easter Island; Skottsberg, C., Ed.; Almquist & Wiksells Boktryckeri: Upsalla, Sweden, 1922; Volume 3, Pt 2, pp. 145–160. [Google Scholar]
- Cutress, C.E. Chapter 7, Corallimorpharia, Actiniaria and Zoanthidea. In Memoirs of the National Museum of Victoria 32; Gill, E.D., Ed.; National Museum of Victoria: Melbourne, Australia, 1971; pp. 89–90. [Google Scholar]
- Schmidt, O. Die Spongien des Adriatischen Meeres, 1st ed.; Wilhelm Engelmann: Leipzig, Germany, 1862; pp. 1–88. [Google Scholar]
- Duerden, J.E. A new species of Parazoanthus. In Records Albany Museum 2; Albany Museum: Grahamstown, South Africa, 1907; p. 80. [Google Scholar]
- Montenegro, J.; Sinniger, F.; Reimer, J.D. Unexpected diversity and new species in the sponge-Parazoanthidae association in southern Japan. Mol. Phylogenet. Evol. 2015, 89, 73–90. [Google Scholar] [CrossRef]
- Kobuk, D.R.; Van Soest, R.W. Cavity-dwelling sponges in a southern Caribbean coral reef and their paleontological implications. Bull. Mar. Sci. 1989, 44, 1207–1235. [Google Scholar]
- Ryland, J.S. Reproduction in Zoanthidea (Anthozoa: Hexacorallia). Invertebr. Reprod. Dev. 1997, 31, 177–188. [Google Scholar] [CrossRef]
- Swain, T.D. Context-dependent effects of symbiosis: Zoanthidea colonization generally improves Demospongiae condition in native habitats. Mar. Biol. 2012, 159, 1429–1438. [Google Scholar] [CrossRef]
- Ryland, J.S.; Westphalen, D. The reproductive biology of Parazoanthus parasiticus (Hexacorallia: Zoanthidea) in Bermuda. Hydrobiologia 2004, 530/531, 411–419. [Google Scholar] [CrossRef]
- Verrill, A.E. Additions to the Anthozoa and Hydrozoa of the Bermudas. Trans. Conn. Acad. Arts Sci. 1900, 10, 551–572. [Google Scholar] [CrossRef]
- Verrill, A.E. The Bermuda Islands: Part, V. An account of the coral reefs (characteristic life of the Bermuda coral reefs). Trans. Conn. Acad. Arts Sci. 1907, 12, 280–296. [Google Scholar]
- De la Cruz-Francisco, V.; González-González, M.; Morales-Quijano, I. Inventario taxonómico de Hydrozoa (Orden: Anthoathecata) y Anthozoa (Subclases: Hexacorallia y Octocorallia) del Arrecife Enmedio, Sistema Arrecifal Lobos-Tuxpan. CICIMAR Oceánides 2016, 31, 23–34. [Google Scholar]
- Hill, A.; Wagner, A.; Hill, M. Hox and paraHox genes from the anthozoan Parazoanthus parasiticus. Mol. Phylogenet. Evol. 2003, 28, 529–535. [Google Scholar] [CrossRef]
- Hill, M.S. Sponges harbor genetically identical populations of the zoanthid Parazoanthus parasiticus. Bull. Mar. Sci. 1998, 63, 513–521. [Google Scholar]
- Hertwig, R. Report on the Actiniaria dredged by H.M.S. Challenger during the years 1873–1876. In Reports on the Scientific Results of the Exploring Voyage of H.M.S. Challenger during the Years 1873–1876; Neill: Edinburgh, UK, 1882; pp. 1–134. [Google Scholar]
- Lamouroux, J.V. Histoire des Polypiers Coralligenes Flexibles, Vulgairement Nommés Zoophytes; F. Poisson: Caen, France, 1816. [Google Scholar]
- Finney, J.C.; Pettay, D.T.; Sampayo, E.M.; Warner, M.E.; Oxenford, H.A.; LaJeunesse, T.C. The relative significance of host–habitat, depth, and geography on the ecology, endemism, and speciation of coral endosymbionts in the genus Symbiodinium. Microb. Ecol. 2010, 60, 250–263. [Google Scholar] [CrossRef]
- Acosta, A. Disease in zoanthids: Dynamics in space and time. Hydrobiologia 2001, 460, 113–130. [Google Scholar] [CrossRef]
- Acosta, A.; Sammarco, P.W.; Duarte, L.F. Asexual reproduction in a zoanthid by fragmentation: The role of exogenous factors. Bull. Mar. Sci. 2001, 68, 363–381. [Google Scholar]
- Acosta, A.; Sammarco, P.W.; Duarte, L.F. New fission processes in the zoanthid Palythoa caribaeorum: Description and quantitative aspects. Bull. Mar. Sci. 2005, 76, 1–26. [Google Scholar]
- Acosta, A.; González, A.M. Fission in the Zoantharia Palythoa caribaeorum (Duchassaing and Michelotii, 1860) populations: A latitudinal comparison. Boletín de Investig. Mar. Costeras INVEMAR 2007, 36, 151–165. [Google Scholar] [CrossRef]
- Almeida, J.G.L.; Maia, A.I.; Wilke, D.; Silveira, E.R.; Braz-Filho, R.; La Clair, J.J.; Costa-Lotufo, L.; Pessoa, O.D.L. Palyosulfonoceramides A and B: Unique sulfonylated ceramides from the Brazilian zoanthids Palythoa caribaeorum and Protopalyhtoa variabilis. Mar. Drugs 2012, 10, 2846–2860. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amaral, F.D.; Hudson, M.M.; da Silveira, F.L.; Migotto, A.E.; Pinto, S.M.; Longo, L. Cnidarians of Saint Peter and St. Paul Archipelago, Northeast Brazil. In Proceedings of the 9th International Coral Reef Symposium, Bali, Indonesia, 23–27 October 2000; Moosa, M.K., Soemodihardjo, S., Soegiarto, A., Romimohtarto, K., Nontji, A., Soekarno, S., Eds.; International Coral Reef Society: Bali, Indonesia, 2000; pp. 567–572. [Google Scholar]
- Amaral, F.M.D.; Ramos, C.A.C.; Leão, Z.; Kikuchi, R.K.P.; Lima, K.K.M.; Longo, L.L.; Cordeiro, R.T.S.; Lira, S.M.A.; Vasconcelos, S.L. Checklist and morphometry of benthic cnidarians from the Fernando de Noronha Archipelago, Brazil. Cah. Biol. Mar. 2009, 50, 277–290. [Google Scholar]
- Azevedo, C.A.A.; Carneiro, M.A.A.; Oliveira, S.R.; Marinho-Soriano, E. Macrolgae as an indicator of the environmental health of the Pirangi reefs, Rio Grande do Norte, Brazil. Rev. Brasil. Farmacog. 2011, 21, 323–328. [Google Scholar] [CrossRef] [Green Version]
- Barreira, C.; Echeverría, C.A.; de Oliveira Pires, D.; Fonseca, C.G. Distribuição do bentos (Cnidaria e Echinodermata) em costões rochosos da Baía de Ilha Grande, Rio de Janeiro, Brasil. Oecol. Brasil 1999, 7, 179–193. [Google Scholar]
- Boscolo, H.K.; Silveira, F.L. Reproductive biology of Palythoa caribaeorum and Protopalythoa variabilis (Cnidaria, Anthozoa, Zoanthidea) from the southeastern coast of Brazil. Braz. J. Biol. 2005, 65, 29–41. [Google Scholar] [CrossRef] [Green Version]
- Bouzon, J.L.; Brandini, F.P.; Rocha, R.M. Biodiversity of sessile fauna on rocky shores of coastal islands in Santa Catarina, Southern Brazil. Mar. Sci. 2012, 2, 39–47. [Google Scholar] [CrossRef]
- Castro, C.B.; Segal, B.; Negrão, F.; Calderon, E.N. Four-year monthly sediment deposition on turbid southwestern Atlantic coral reefs, with a comparison of benthic assemblages. Braz. J. Oceanog. 2012, 60, 49–63. [Google Scholar] [CrossRef] [Green Version]
- Chimetto, L.A.; Brocchi, M.; Thompson, C.C.; Martins, R.C.R.; Ramos, H.R.; Thompson, F.L. Vibrios dominate as culturable nitrogen-fixing bacteria of the Brazilian coral Mussismilia hispida. System. App. Microb. 2008, 31, 312–319. [Google Scholar] [CrossRef]
- Chimetto, L.A.; Cleenwerck, I.; Thompson, C.C.; Brocchi, M.; Willems, A.; De Vos, P.; Thompson, F.L. Photobacterium jeanii sp. nov., isolated from corals and zoanthids. Int. J. System. Evol. Microb. 2010, 60, 2843–2848. [Google Scholar] [CrossRef]
- Chimetto, L.A.; Cleenwerck, I.; Moreira, A.P.B.; Brocchi, M.; Willems, A.; De Vos, P.; Thompson, F.L. Vibrio variabilis sp. nov. and Vibrio maritimus sp. nov., isolated from Palythoa caribaeorum. Int. J. System. Evol. Microb. 2011, 61, 3009–3015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Correia, M.D.; Sovierzoski, H.H. Macrobenthic diversity reaction to human impacts on Maceió coral reefs, Alagoas, Brazil. In Proceedings of the 11th International Coral Reef Symposium, Fort Lauderdale, FL, USA, 7–11 July 2008; Cunning, J.R., Thurmond, J.E., Smith, G.W., Weil, E., Ritchie, K.B., Eds.; International Coral Reef Society: Davie, FL, USA, 2008; pp. 1083–1087. [Google Scholar]
- Costa, D.L.; Gomes, P.B.; Santos, A.M.; Valença, N.S.; Vieira, N.A.; Pérez, C.D. Morphological plasticity in the reef zoanthid Palythoa caribaeorum as an adaptive strategy. Ann. Zool. Fenn. 2011, 48, 349–358. [Google Scholar] [CrossRef]
- Da Silveira, F.L.; Morandini, A.C. Checklist dos Cnidaria do estado de São Paulo, Brasil. Biota Neotropica 2011, 11 (Suppl. S1), 445–454. [Google Scholar] [CrossRef] [Green Version]
- De Andrade Melo, L.F.; da Camara, C.A.G.; de Albuquerque Modesto, J.C.; Pérez, C.D. Toxicity against Artemia salina of the zoanthid Palythoa caribaeorum (Cnidaria: Anthozoa) used in folk medicine on the coast of Pernambuco, Brazil. Biotemas 2012, 25, 145–151. [Google Scholar]
- de Barros, M.M.L.; Castro, C.B.; Pires, D.O.; Segal, B. Coexistence of reef organisms in the Abrolhos Archipelago, Brazil. Rev. Biol. Trop. 2000, 48, 741–747. [Google Scholar]
- De Santana, E.F.C.; Alves, A.L.; Santos, A.D.M.; Maria Da Gloria, G.S.; Perez, C.D.; Gomes, P.B. Trophic ecology of the zoanthid Palythoa caribaeorum (Cnidaria: Anthozoa) on tropical reefs. J. Mar. Biol. Assoc. UK 2015, 95, 301–309. [Google Scholar] [CrossRef]
- Echeverría, C.; Pires, D.; Medeiros, M.; Castro, C. Cnidarians of the Atol das Rocas, Brazil. In Proceedings of the 8th International Coral Reef Symposium, Panama, Panama, 24–29 June 1996; Lessios, H.A., Macintyre, I.G., Eds.; International Coral Reef Society: Panama, Panama, 1997; pp. 443–446. [Google Scholar]
- Francini-Filho, R.B.; Ferreira, C.M.; Coni, E.O.C.; De Moura, R.L.; Kaufman, L. Foraging activity of roving herbivorous reef fish (Acanthuridae and Scaridae) in eastern Brazil: Influence of resource availability and interference competition. J. Mar. Biol. Assoc. UK 2010, 90, 481–492. [Google Scholar] [CrossRef] [Green Version]
- Francini-Filho, R.B.; de Moura, R.L. Predation on the toxic zoanthid Palythoa caribaeorum by reef fishes in the Abrolhos Bank, eastern Brazil. Braz. J. Oceanog. 2010, 58, 77–79. [Google Scholar] [CrossRef] [Green Version]
- Kelecom, A.; Solé-Cava, A.M. Comparative study of zoanthid sterols the genus Palythoa (Hexacorallia, Zoanthidea). Comp. Biochem. Physiol. Part B Comp. Biochem. 1982, 72, 677–682. [Google Scholar] [CrossRef]
- Longo, G.O.; Krajewski, J.P.; Segal, B.; Floeter, S.R. First record of predation on reproductive Palythoa caribaeorum (Anthozoa: Sphenopidae): Insights on the trade-off between chemical defences and nutritional value. Mar. Biodivers. Rec. 2012, 5, 1–3. [Google Scholar] [CrossRef] [Green Version]
- MacCord, F.S.; Duarte, L.F.L. Dispersion in populations of Tropiometra carinata (Crinoidea: Comatulida) in the Sao Sebastiao Channel, Sao Paulo State, Brazil. Est. Coast. Shelf Sci. 2002, 54, 219–225. [Google Scholar] [CrossRef]
- Martinez, A.S.; Mendes, L.F.; Leite, T.S. Spatial distribution of epibenthic molluscs on a sandstone reef in the northeast of Brazil. Braz. J. Biol. 2012, 72, 287–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendonça-Neto, J.P.; Ferreira, C.E.L.; Chaves, L.C.T.; Pereira, R.C. Influence of Palythoa caribaeorum (Anthozoa, Cnidaria) zonation on site-attached reef fishes. An. Acad. Bras. Ciênc. 2008, 80, 495–513. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mendonça-Neto, J.P.; da Gama, B.A.P. The native Palythoa caribaeorum overgrows on invasive species in the intertidal zone. Coral Reefs 2008, 28, 497. [Google Scholar] [CrossRef] [Green Version]
- Migotto, A.E.; Silveira, F.L.; Schlenz, E.; Castro, C.B.; Marques, A.C. Lista dos Cnidaria registrados na costa Brasileira. Invertebrados marinhos registrados no litoral Brasileiro; University of São Paulo: São Paulo, Brazil, 1998; pp. 1–59. [Google Scholar]
- Oigman-Pszczol, S.S.; Figueiredo, M.A.d.O.; Creed, J.C. Distribution of benthic communities on the tropical rocky subtidal of Armação dos Búzios, southeastern Brazil. Mar. Ecol. 2004, 25, 173–190. [Google Scholar] [CrossRef]
- Pérez, C.D.; Vila-Nova, D.A.; Santos, A.M. Associated community with the zoanthid Palythoa caribaeorum (Duchassaing & Michelotti, 1860) (Cnidaria, Anthozoa) from littoral of Pernambuco, Brazil. Hydrobiologia 2005, 548, 207–215. [Google Scholar] [CrossRef]
- Rabelo, E.F.d.O.; Soares, M.; Matthews-Cascon, H. Competitive interactions among zoanthids (Cnidaria: Zoanthidae) in an intertidal zone of Northeastern Brazil. Braz. J. Oceanog. 2013, 61, 35–42. [Google Scholar] [CrossRef] [Green Version]
- Rabelo, E.F.; Rocha, L.L.; Colares, G.B.; Bomfim, T.A.; Nogueira, V.L.R.; Katzenberger, M.; Matthews-Cascon, H.; Melo, V.M.M. Symbiodinium diversity associated with zoanthids (Cnidaria: Hexacorallia) in Northeastern Brazil. Symbiosis 2015, 64, 105–113. [Google Scholar] [CrossRef]
- Sebens, K.P. Autotrophic and heterotrophic nutrition of coral reef zoanthids. In Proceedings of the 3rd International Coral Reef Symposium, Miami, FL, USA, May 1977; Taylor, D.L., Ed.; International Coral Reef Society: Miami, FL, USA, 1977; pp. 397–406. [Google Scholar]
- Segal, B.; Castro, C.B. Coral community structure and sedimentation at different distances from the coast of the Abrolhos Bank, Brazil. Braz. J. Oceanog. 2011, 59, 119–129. [Google Scholar] [CrossRef]
- Soares, C.L.S.; Pérez, C.D.; Maia, M.B.S.; Silva, R.S.; Melo, L.F.A. Avaliação da atividade antiinflamatória e analgésica do extrato bruto hidroalcoólico do zoantídeo Palythoa caribaeorum (Duchassaing & Michelotti, 1860). Braz. J. Pharmacog. 2006, 16, 463–468. [Google Scholar]
- Soares, M.O.; Rabelo, E.F.; Mathews-Cascon, H. Intertidal anthozoans from the coast of Ceará, Brazil. Rev. Bras. Biociênc. 2011, 9, 437–443. [Google Scholar]
- Souza, D.S.L.; Grossi-de-Sa, M.M.F.; Silva, L.P.; Franco, O.L.; Gomes-Junior, J.E.; Oliveira, G.R.; Rocha, T.L.; Magalhaes, C.P.; Marra, B.M.; Grossi-de-Sa, M.M.F. Identification of a novel β-N-acetylhexosaminidase (Pcb-NAHA1) from marine zoanthid Palythoa caribaeorum (Cnidaria, Anthozoa, Zoanthidea). Prot. Express. Purif. 2008, 58, 61–69. [Google Scholar] [CrossRef]
- Stampar, S.N.; da Silva, P.F.; Osmar Jr, J.L. Predation on the zoanthid Palythoa caribaeorum (Anthozoa, Cnidaria) by a Hawksbill turtle (Eretmochelys imbricata) in Southeastern Brazil. Mar. Turtle Newsl. 2007, 1, 3–5. [Google Scholar]
- Villaça, R.; Pitombo, F.B. Benthic communities of shallow-water reefs of Abrolhos, Brazil. Rev. Bras. Oceanogr. 1997, 45, 35–43. [Google Scholar] [CrossRef] [Green Version]
- Barquin-Diez, J.; Gonzalez-Lorenzo, G.; Martin-Garcia, L.; Candelaria Gil-Rodriguez, M.; Brito-Hernandez, A. Spatial distribution of benthic subtidal communities of shallow waters of the Canary Islands. I: Soft bottom communities of Tenerife coast. Vieraea 2005, 33, 435–448. [Google Scholar]
- Morri, C.; Bianchi, C.N. Cnidarian zonation at Ilha do Sal (Arquipelago de Cabo Verde). Beitr. Paläont. 1995, 20, 41–49. [Google Scholar]
- Morri, C.; Cattaeno-Vietti, R.; Sartoni, G.; Bianchi, C.N. Shallow epibenthic communities of Ilha do Sal (Cape Verde Archipelago, eastern Atlantic). Arquipelago 2000, 2, 157–165. [Google Scholar]
- Gleibs, S.; Mebs, D.; Werding, B. Studies on the origin and distribution of palytoxin in a Caribbean coral reef. Toxicon 1995, 33, 1531–1537. [Google Scholar] [CrossRef]
- Cortés, J. Biodiversidad marina de Costa Rica: Filo Cnidaria. Rev. Biol. Trop. 1997, 44, 323–334. [Google Scholar]
- Cortés, J.; Murillo, M.M.; Guzmán, H.M.; Acuña, J. Pérdida de zooxantelas y muerte de corales y otros organismos arrecifales en el Caribe y Pacífico de Costa Rica. Rev. Biol. Trop. 1984, 32, 227–231. [Google Scholar]
- Duerden, J.E. Report on the actinians of Puerto Rico. U. S. Fish. Comm. Bull. 1902, 20, 321–354. [Google Scholar]
- Varela, C.; Guitart, B.; Ortiz, M.; Lalana, R. Los zoantideos (Cnidaria, Anthozoa, Zoanthiniaria), de la región occidental de Cuba. Rev. Investig. Mar. 2002, 23, 179–184. [Google Scholar]
- Pax, F. Actiniarien, Zoantharien und Ceriantharien von Curaçao. Bijdr. Dierk. 1924, 23, 93–121. [Google Scholar]
- Goreau, T.F. The ecology of Jamaican coral reefs I. Species composition and zonation. Ecology 1959, 40, 67–90. [Google Scholar] [CrossRef]
- Goreau, T.F. Mass expulsion of zooxanthellae from Jamaican reef communities after Hurricane Flora. Science 1964, 145, 383–386. [Google Scholar] [CrossRef]
- Karlson, R.H. Alternative competitive strategies in a periodically disturbed habitat. Bull. Mar. Sci. 1980, 30, 894–900. [Google Scholar]
- Banaszak, A.T.; Santos, M.G.B.; LaJeunesse, T.C.; Lesser, M.P. The distribution of mycosporine-like amino acids (MAAs) and the phylogenetic identity of symbiotic dinoflagellates in cnidarian hosts from the Mexican Caribbean. J. Exp. Mar. Biol. Ecol. 2006, 337, 131–146. [Google Scholar] [CrossRef]
- LaJeunesse, T. Diversity and community structure of symbiotic dinoflagellates from Caribbean coral reefs. Mar. Biol. 2002, 141, 387–400. [Google Scholar] [CrossRef]
- Fadlallah, Y.H.; Karlson, R.H.; Sebens, K.P. A comparative study of sexual reproduction in three species of Panamanian zoanthids (Coelenterata: Anthozoa). Bull. Mar. Sci. 1984, 35, 80–89. [Google Scholar]
- Sebens, K.P. Intertidal distribution of zoanthids on the Caribbean coast of Panama: Effects of predation and desiccation. Bull. Mar. Sci. 1982, 32, 316–335. [Google Scholar]
- Edmunds, P.J. Patterns in the distribution of juvenile corals and coral reef community structure in St. John, US Virgin Islands. Mar. Ecol. Prog. Ser. 2000, 202, 113–124. [Google Scholar] [CrossRef] [Green Version]
- Haywick, D.W.; Mueller, E.M. Sediment retention in encrusting Palythoa spp.—A biological twist to a geological process. Coral Reefs 1997, 16, 39–46. [Google Scholar] [CrossRef]
- Mueller, E.; Haywick, D.W. Sediment assimilation and calcification by the Western Atlantic reef zoanthid, Palythoa caribaeorum. Bull. L’Institut Oceanogr. Monaco 1995, 14, 89–100. [Google Scholar]
- Reimer, J.D.; Foord, C.; Irei, Y. Species diversity of shallow water zoanthids (Cnidaria: Anthozoa: Hexacorallia) in Florida. J. Mar. Biol. 2012, 2012, 856079. [Google Scholar] [CrossRef] [Green Version]
- Bastidas, C.; Bone, D. Competitive strategies between Palythoa caribaeorum and Zoanthus sociatus (Cnidaria: Anthozoa) at a reef flat environment in Venezuela. Bull. Mar. Sci. 1996, 59, 543–555. [Google Scholar]
- Núñez, J.G.; Ariza, L.A.; Jiménez, M. Evaluación de la estructura de las comunidades coralinas en la franja sublitoral de la zona costera sur del Golfo de Cariaco, Venezuela. Parte I: Eje turpialito-quetepe. Bol. Inst. Oceanogr. Venezuela 2011, 50, 149–159. [Google Scholar]
- Ong, C.W.; Reimer, J.D.; Todd, P.A. Morphologically plastic responses to shading in the zoanthids Zoanthus sansibaricus and Palythoa tuberculosa. Mar. Biol. 2013, 160, 1053–1064. [Google Scholar] [CrossRef]
- Ellis, J.; Solander, D. The Natural History of Many Curious and Uncommon Zoophytes Collected from Various Parts of the Globe; Benjamin White and Son: London, UK, 1786. [Google Scholar]
- Dana, J.D. Zoophytes. In United States Exploring Expedition during the Years 1838, 1839, 1840, 1841, 1842; Dougal, W.H., Stuart, F.D., Wilkes, C., Eds.; C. Sherman: Philadelphia, PA, USA, 1846; pp. 7–113. [Google Scholar]
- Milne Edwards, H. Histoire Naturelle des Coralliaires ou Polypes Proprement Dits; Librairie encyclopédique de Roret: Paris, France, 1857; Volume 1. [Google Scholar]
- Rodríguez-Viera, L.; Rodríguez-Casariego, J.; Pérez-García, J.A.; Olivera, Y.; Perera-Pérez, O. Invertebrados marinos de la zona central del golfo de Ana María, Cuba. Rev. Investig. Mar. 2012, 32, 30–38. [Google Scholar]
- Haddon, A.C.; Shackleton, A.M. Actiniae: I. Zoantheae. In Reports on the Zoological Collections Made in the Torres Straits by Professor, A.C. Haddon, 1888–1889. Sci. Trans. Roy. Dublin Soc. ser. 2 1891, 4, 658–673, pls. 61–64. [Google Scholar]
- Winston, J.E. Diversity and distribution of bryozoans in the Pelican Cays, Belize, Central America. Atoll Res. Bull. 2007, 546, 1–24. [Google Scholar] [CrossRef] [Green Version]
- Schoenberg, D.A.; Trench, R.K. Genetic variation in Symbiodinium (=Gymnodinium) microadriaticum Freudenthal, and specificity in its symbiosis with marine invertebrates. I. Isoenzyme and soluble protein patterns of axenic cultures of Symbiodinium microadriaticum. Proc. Roy. Soc. B 1980, 207, 405–427. [Google Scholar] [CrossRef]
- Costa, D.L.; Santos, A.M.; da Silva, A.F.; Padilha, R.M.; Nogueira, V.O.; Wanderlei, E.B.; Bélanger, D.; Gomes, P.B.; Pérez, C.D. Biological impacts of the port complex of Suape on benthic reef communities (Pernambuco–Brazil). J. Coast. Res. 2014, 30, 362–370. [Google Scholar] [CrossRef]
- Cruz, I.C.S.; de Kikuchi, R.K.P.; Longo, L.L.; Creed, J.C. Evidence of a phase shift to Epizoanthus gabrieli Carlgreen, 1951 (Order Zoanthidea) and loss of coral cover on reefs in the Southwest Atlantic. Mar. Ecol. 2015, 36, 318–325. [Google Scholar] [CrossRef]
- Cruz, I.C.S.; Loiola, M.; Albuquerque, T.; Reis, R.; José de Anchieta, C.C.; Reimer, J.D.; Mizuyama, M.; Kikuchi, R.K.P.; Creed, J.C. Effect of phase shift from corals to Zoantharia on reef fish assemblages. PLoS ONE 2015, 10, e0116944. [Google Scholar] [CrossRef]
- Kelmo, F.; Attrill, M.J.; Jones, M.B. Effects of the 1997–1998 El Niño on the cnidarian community of a high turbidity coral reef system (northern Bahia, Brazil). Coral Reefs 2003, 22, 541–550. [Google Scholar] [CrossRef]
- Metri, R.; Rocha, R.M. Bancos de algas calcárias, um ecossistema rico a ser preservado. Natureza & Conservação 2008, 8, 8–17. [Google Scholar]
- Soares, M.O.; de Souza, L.P. Osmorregulação no zoantídeo tropical Protopalythoa variabilis (Cnidaria: Anthozoa). Acta Sci. Biol. Sci. 2013, 35, 123–127. [Google Scholar] [CrossRef] [Green Version]
- Wilke, D.V.; Jimenez, P.C.; Araújo, R.M.; Pessoa, O.D.L.; Silveira, E.R.; Pessoa, C.; Moraes, M.O.; Lopes, N.P.; Costa-Lotufo, L.V. A new cytotoxic 2-amino-n-alkyl-carboxylic acid mixture obtained from the zoanthid Protopalythoa variabilis collected at Paracuru beach, Ceará State, Brazil. Planta Med. 2008, 74, 1060. [Google Scholar] [CrossRef]
- Wilke, D.V.; Jimenez, P.C.; Pessoa, C.; de Moraes, M.O.; Araújo, R.M.; da Silva, W.M.B.; Silveira, E.R.; Pessoa, O.D.L.; Braz-Filho, R.; Lopes, N.P. Cytotoxic lipidic α-amino acids from the zoanthid Protopalythoa variabilis from the northeastern coast of Brazil. J. Braz. Chem. Soc. 2009, 20, 1455–1459. [Google Scholar] [CrossRef]
- Diop, M.; Leug-Tack, D.; Braekman, J.C.; Kornprobst, J.M. Sterol composition of four Zoathidae members of the genus Palythoa from the Cape Verde Peninsula. Biochem. Syst. Ecol. 1986, 14, 151–154. [Google Scholar] [CrossRef]
- Karlson, R.H. Disturbance and monopolization of a spatial resource by Zoanthus sociatus (Coelenterata, Anthozoa). Bull. Mar. Sci. 1983, 33, 118–131. [Google Scholar]
- Koehl, M.A. Water flow and the morphology of zoanthid colonies. In Proceedings of the 3rd International Coral Reef Symposium, Miami, FL, USA, May 1977; Taylor, D.L., Ed.; International Coral Reef Society: Miami, FL, USA, 1977; pp. 437–444. [Google Scholar]
- Lamarck, J.B.P. Système des Animaux sans Vertèbres; Self-Published: Paris, France, 1801. [Google Scholar]
- Villar, R.M.; Gil-Longo, J.; Daranas, A.H.; Souto, M.L.; Fernandez, J.J.; Peixinho, S.; Barral, M.A.; Santafe, G.; Rodríguez, J.; Jiménez, C. Evaluation of the effects of several zoanthamine-type alkaloids on the aggregation of human platelets. Bioorg. Med. Chem. 2003, 11, 2301–2306. [Google Scholar] [CrossRef]
- Ellis, J. An account of the Actinia Sociata, or clustered animal-flower, lately found on the sea-coasts of the new-ceded islands: In a letter from John Ellis, Esquire, F.R.S. to the Right Honourable the Earl of Hillsborough, F.R.S. Phil. Trans. 1768, 57, 428–437. [Google Scholar]
- McMurrich, J.P. Notes on some Actinians from the Bahama Islands, collected by the late Dr. J.I. Northrop. Ann. NY Acad. Sci. 1896, IX, 181–194. [Google Scholar] [CrossRef]
- McMurrich, J.P. Report on the Actiniaria collected by the Bahama Expedition of the State University of Iowa, 1893. Bull. Lab. Nat. Hist. State Univ. Iowa 1898, 4, 225–249, pls. 1–3. [Google Scholar]
- Laborel, J. Les peuplements de Madréporaires des côtes tropicales du Brésil. Ann. Univ. Abidjan 1970, 2, 1–260. [Google Scholar]
- De O. Pires, D.; Migotto, A.E.; Marques, A.C. Cnidários bentônicos do Arquipélago de Fernando de Noronha, Brasil. Boletim Museu Nac. Rio Jan. NS Zool. 1992, 354, 1–21. [Google Scholar]
- Rohlfs de Macedo, C.M.R.; Belém, M.J.C. The genus Zoanthus in Brazil. 1. Characterization and anatomical revision of Zoanthus sociatus (Cnidaria, Zoanthinaria, Zoanthidae). Iheringia 1994, 77, 135–144. [Google Scholar]
- Sarmento, F.; Correia, M.D. Description of ecological and external morphological parameters of Porifera at the Ponta Verde coral reef, Alagoas, Brazil. Rev. Bras. Zoociênc. 2002, 4, 215–226. [Google Scholar]
- Le Sueur, C.A. Observations on several species of the genus Actinia: Illustrated by figures. J. Acad. Nat. Sci. Phil. 1817, 1, 149–189. [Google Scholar]
- Karlson, R.H. Fission and the dynamics of genets and ramets in clonal cnidarian populations. In Coelenterate Biology: Recent Research on Cnidaria and Ctenophora, Developments in Hydrobiology; Williams, R.B., Cornelius, P.F.S., Hughes, R.G., Robson, E.A., Eds.; Springer: Dordrecht, Germany, 1991; pp. 235–240. [Google Scholar]
- Carlgren, O. Ostafrikanische Actinien, gesammelt von Herrn Dr. F. Stuhlmann 1898 und 1899, 1st ed.; Mitteilungen aus dem Naturhistorischen Museum in Hamburg: Hamburg, Germany, 1900; pp. 21–144. [Google Scholar]
- Kamezaki, M.; Higa, M.; Hirose, M.; Suda, S.; Reimer, J.D. Different zooxanthellae types in populations of the zoanthid Zoanthus sansibaricus along depth gradients in Okinawa, Japan. Mar. Biodivers. 2013, 43, 61–70. [Google Scholar] [CrossRef]
- Karlson, R.H. Size-dependent growth in two zoanthid species: A contrast in clonal strategies. Ecology 1988, 69, 1219–1232. [Google Scholar] [CrossRef]
- Gray, J.E. Spicilegia Zoologica: Original Figures and Short Systematic Descriptions of New and Unfigured Animals. Part 1; Treüttel, Würtz and Co.: London, UK, 1828. [Google Scholar]
- Brown, J.; Downes, K.; Mrowicki, R.J.; Nolan, E.L.; Richardson, A.J.; Swinnen, F.; Wirtz, P. New records of marine invertebrates from Ascension Island (Central Atlantic). Arquipélago 2016, 33, 71–79. [Google Scholar]
- Larson, K.S.; Larson, R.J. On the ecology of Isaurus duchassaingi (Andres) (Cnidaria: Zoanthidea) from South Water Cay, Belize. Smithson. Contrib. Mar. Sci. 1982, 12, 475–488. [Google Scholar]
- Grohman, P.A.; Peixinho, S. Isaurus tuberculatus (Cnidaria, Anthozoa, Zoanthidea), nova ocorrência para o Atlântico sudoeste Tropical. Nerítica 1995, 9, 19–22. [Google Scholar]
- Rabelo, E.F.; Matthews-Cascon, H. Influence of light on the feeding behaviour of Isaurus tuberculatus Gray, 1828 (Cnidaria: Zoanthidea) under laboratory conditions. Arquivos Ciênc. Mar. 2007, 40, 55–58. [Google Scholar]
- Riera, R.; Becerro, M.A.; Stuart-Smith, R.D.; Delgado, J.D.; Edgar, G.J. Out of sight, out of mind: Threats to the marine biodiversity of the Canary Islands (NE Atlantic Ocean). Mar. Pollut. Bull. 2014, 86, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Muirhead, A.; Ryland, J.S. A review of the genus Isaurus Gray, 1828 (Zoanthidea), including new records from Fiji. J. Nat. Hist. 1985, 19, 323–335. [Google Scholar] [CrossRef]
- Reimer, J.D.; Ono, S.; Tsukahara, J.; Iwase, F. Molecular characterization of the zoanthid genus Isaurus (Anthozoa: Hexacorallia) and associated zooxanthellae (Symbiodinium spp.) from Japan. Mar. Biol. 2008, 153, 351–363. [Google Scholar] [CrossRef]
- Cardinale, B.J.; Duffy, J.E.; Gonzalez, A.; Hooper, D.U.; Perrings, C.; Venail, P.; Narwani, A.; Mace, G.M.; Tilman, D.; Wardle, D.; et al. Biodiversity loss and its impact on humanity. Nature 2012, 489, 326. [Google Scholar] [CrossRef] [Green Version]
- Hughes, T.P. Catastrophes, phase shifts, and large-scale degradation of a Caribbean coral reef. Science 1994, 265, 1547–1551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burke, L.; Maidens, J. Reefs at Risk in the Caribbean; World Resources Institute (WRI): Washington, DC, USA, 2004. [Google Scholar]
- Mora, C. A clear human footprint in the coral reefs of the Caribbean. Proc. Roy. Soc. B Biol. Sci. 2008, 275, 767–773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miloslavich, P.; Díaz, J.M.; Klein, E.; Alvarado, J.J.; Díaz, C.; Gobin, J.; Escobar-Briones, E.; Cruz-Motta, J.J.; Weil, E.; Cortes, J.; et al. Marine biodiversity in the Caribbean: Regional estimates and distribution patterns. PLoS ONE 2010, 5, e11916. [Google Scholar] [CrossRef] [Green Version]
- Jackson, J.B.C.; Donovan, M.K.; Cramer, K.L.; Lam, V.V. Status and Trends of Caribbean Coral Reefs: 1970–2012; Global Coral Reef Monitoring Network, IUCN: Gland, Switzerland, 2014. [Google Scholar]
- Oliver, L.M.; Fisher, W.S.; Fore, L.; Smith, A.; Bradley, P. Assessing land use, sedimentation, and water quality stressors as predictors of coral reef condition in St. Thomas, U.S. Virgin Islands. Environ. Monitor. Assess. 2018, 190, 1–25. [Google Scholar] [CrossRef]
- Hoeksema, B.W.; van der Land, J.; van der Meij, S.E.T.; van Ofwegen, L.P.; Reijnen, B.T.; van Soest, R.W.M.; de Voogd, N.J. Unforeseen importance of historical collections as baselines to determine biotic change of coral reefs: The Saba Bank case. Mar. Ecol. 2011, 32, 135–141. [Google Scholar] [CrossRef]
- Rocha, L.A.; Aleixo, A.; Allen, G.; Almeda, F.; Baldwin, C.C.; Barclay, M.V.L.; Bates, J.M.; Bauer, A.M.; Benzoni, F.; Berns, C.M.; et al. Specimen collection: An essential tool. Science 2014, 344, 814–815. [Google Scholar] [CrossRef]
- Swain, T.D. Evolutionary transitions in symbioses: Dramatic reductions in bathymetric and geographic ranges of Zoanthidea coincide with loss of symbioses with invertebrates. Mol. Ecol. 2010, 19, 2587–2598. [Google Scholar] [CrossRef]
- Floeter, S.R.; Rocha, L.A.; Robertson, D.R.; Joyeux, J.C.; Smith-Vaniz, W.F.; Wirtz, P.; Edwards, A.J.; Barreiros, J.P.; Ferreira, C.E.L.; Gasparini, J.L.; et al. Atlantic reef fish biogeography and evolution. J. Biogeogr. 2008, 35, 22–47. [Google Scholar] [CrossRef] [Green Version]
- Veron, J.; Stafford-Smith, M.; DeVantier, L.; Turak, E. Overview of distribution patterns of zooxanthellate Scleractinia. Front. Mar. Sci. 2015, 1, 1–19. [Google Scholar] [CrossRef] [Green Version]
- Sinniger, F.; Ocaña, O.V.; Baco, A.R. Diversity of zoanthids (Anthozoa: Hexacorallia) on Hawaiian seamounts: Description of the Hawaiian gold coral and additional zoanthids. PLoS ONE 2013, 8, e52607. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reimer, J.D.; Kise, H.; Santos, M.E.; Lindsay, D.J.; Pyle, R.L.; Copus, J.M.; Bowen, B.W.; Nonaka, M.; Higashiji, T.; Benayahu, Y. Exploring the biodiversity of understudied benthic taxa at mesophotic and deeper depths: Examples from the order Zoantharia (Anthozoa: Hexacorallia). Front. Mar. Sci. 2019, 6, 305. [Google Scholar] [CrossRef] [Green Version]
- Reimer, J.D.; Poliseno, A.; Hoeksema, B.W. Shallow-water zoantharians (Cnidaria, Hexacorallia) from the Central Indo-Pacific. ZooKeys 2014, 444, 1–57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reimer, J.D.; Wee, H.B.; Ang, P.; Hoeksema, B.W. Zoantharia (Cnidaria: Anthozoa: Hexacorallia) of the South China Sea and Gulf of Thailand: A species list based on past reports and new photographic records. Raffles Bull. Zool. 2015, 63, 334–356. [Google Scholar]


























| Index | Species | Range (m) | Southern Caribbean (m) | Eastern Caribbean (m) | ||
|---|---|---|---|---|---|---|
| Historic | Recent | Historic | Recent | |||
| 1 | Parazoanthidae sp. 1 | 140–248 | x | 140–248 | x | x |
| 2 | Antipathozoanthus aff. macaronesicus | 10 | 10 | x | x | x |
| 3 | Bergia catenularis | 6–50 | 6–36 | 10–38 | 20–50 | 12–26 |
| 4 | Bergia cf. cutressi * | 0–8 | 0–8 | x | x | x |
| 5 | Bergia puertoricense * | 10–55 | 10–55 | 10–38 | x | 13–37 |
| 6 | Parazoanthus swiftii | 10–40 | 10 | 19–40 | unknown | 14–29 |
| 7 | Parazoanthidae? sp. | unknown | unknown | x | x | x |
| 8 | Parazoanthus atlanticus | 10–34 | x | 10–34 | x | x |
| 9 | Umimayanthus parasiticus * | 1–44 | 1–40 | 15–38 | 16–44 | 3–34 |
| 10 | Umimayanthus sp. | 37 | x | 37 | x | x |
| 11 | Epizoanthus sp. | 980 | x | x | 980 | x |
| 12 | Hydrozoanthus antumbrosus * | 11–30 | x | 30 | x | 11–19 |
| 13 | Hydrozoanthus tunicans * | 2–30 | 2–4 | 30 | x | 14–19 |
| 14a | Palythoa caribaeorum | 0–35 | 0–2 | 0–14 | 0–35 | 2–29 |
| 14b | Palythoa caracasiana *† | unknown | unknown | x | x | x |
| 14c | Palythoa horstii *† | unknown | unknown | x | x | x |
| 14d | Palythoa mammilosa *† | 2 | unknown | x | 2 | x |
| 15 | Palythoa grandiflora | 1–6 | intertidal | x | 1–6 | x |
| 16 | Palythoa grandis | 11–64 | 18–64 | 11–12 | x | 13–18 |
| 17 | Palythoa variabilis | 0–37 | 0–24 | 37 | intertidal | 3 |
| 18 | Palythoa sp. | intertidal | intertidal | x | x | x |
| 19 | Zoanthus pulchellus | 0–24 | 0–24 | 1–11 | 0–20 | 15–16 |
| 20 | Zoanthus aff. pulchellus | 1 | x | 1 | x | x |
| 21 | Zoanthus sociatus | 0–24 | 0–3 | intertidal | 0–6 | 3–24 |
| 22 | Zoanthus solanderi | 0–21 | intertidal | 12–16 | 0–15 | 3–21 |
| 23 | Zoanthus sp. | intertidal | intertidal | x | intertidal | x |
| 24 | Isaurus tuberculatus | 0–15 | intertidal | x | 2 | 15 |
| Diversity recorded | 24 | 18 | 16 | 14 | 15 | |
| 22 | 17 | |||||
| Species/Specimens | COI-mtDNA | 16S r-DNA | ITS r-DNA |
|---|---|---|---|
| Antipathozoanthus macaronesicus | NA | HM130467 | EU591552 |
| Bergia catenularis | NA | EU828757 | EU418289 |
| Bergia catenularis (TOB37) | NA | NA | EU418292 |
| Bergia cutressi (1) | NA | EU828759 | EU418264 |
| Bergia cutressi (2) | NA | NA | EU418267 |
| Bergia puertoricense (1) | AB247351 | AY995933 | EU591584 |
| Bergia puertoricense (2) | NA | EU828758 | EU418312 |
| Bergia sp. Senegal | EF672656 | EF687820 | EU591582 |
| Bergia sp. 5 Sulawesi | EU591627 | AY995934 | NA |
| Bullagummizoanthus emilyacadiaarum | NA | KC218434 | NA |
| Corallizoanthus tsukaharai | NA | EU035625 | EU035621 |
| Epizoanthus arenaceus | AB247348 | AY995926 | EU591538 |
| Hurlizoanthus parrishi | NA | KC218433 | NA |
| Isozoanthus giganteus | NA | GQ464867 | GQ464896 |
| Kauluzoanthus kerbyi (SH12) | NA | KC218435 | NA |
| Kulamanamana haumeaae (SH2) | NA | KC218431 | NA |
| Mesozoanthus fossii | NA | EF687822 | EU591545 |
| Parazoanthid sp. 02_27 | NA | EU333760 | EU333810 |
| Parazoanthid sp. 3 Madagascar | EF672664 | EF687825 | EU591576 |
| Parazoanthid sp. Tasmania | EU591620 | EU591610 | NA |
| Parazoanthid sp. 3 Sulawesi | AB247354 | AY995937 | EU591575 |
| Parazoanthus aff. juanfernandezii (CA128) | NA | GQ464849 | GQ464878 |
| Parazoanthus aff. swiftii (PER241) | NA | GQ464853 | GQ464882 |
| Parazoanthus aff. swiftii (PER249) | NA | GQ464854 | GQ464883 |
| Parazoanthus anguicomus (1) | EF672660 | EF687827 | EU591574 |
| Parazoanthus anguicomus (2) | NA | GQ464851 | GQ464880 |
| Parazoanthus axinellae (1) | AB247355 | AF398921 | NA |
| Parazoanthus axinellae (2) | EF672659 | NA | EU591571 |
| Parazoanthus capensis (SA262) | NA | GQ464852 | GQ464881 |
| Parazoanthus darwini (1) | NA | EU333748 | EU333802 |
| Parazoanthus darwini (2) | NA | EU333751 | NA |
| Parazoanthus elongatus (Chile) | EF672661 | EF687829 | EU591565 |
| Parazoanthus elongatus (NZ) | EF672662 | EF687828 | EU591564 |
| Parazoanthus sp. 1401 | NA | HM130478 | NA |
| Parazoanthus sp. 269 | NA | HM130468 | NA |
| Parazoanthus sp. ‘hertwigi’ | KC218397 | NA | NA |
| Parazoanthus swiftii (1) | AB247350 | AY995936 | GQ848258 |
| Parazoanthus swiftii (2) | KJ794176 | EU828755 | EU418332 |
| Savalia savaglia | NA | HQ110948 | EU346888 |
| Umimayanthus chanpuru (16J) | KR092609 | KR092469 | KR092678 |
| Umimayanthus chanpuru (33J) | KR092594 | KR092504 | KR092680 |
| Umimayanthus miyabi (179TF) | KR092570 | KR092453 | KR092645 |
| Umimayanthus miyabi (70JR) | KR092573 | KR092454 | KR092646 |
| Umimayanthus nakama (363JR) | KR092577 | KR092458 | KR092644 |
| Umimayanthus nakama (3J) | KR092579 | KR092457 | KR092643 |
| Umimayanthus parasiticus (1) | EF672663 | AY995938 | GQ848263 |
| Umimayanthus parasiticus (2) | NA | EU828756 | EU418306 |
| Zibrowius ammophilus (SH15) | NA | KC218439 | NA |
| Parazoanthus atlanticus sp. n. (RMNH.COEL.42433) | NA | NA | MT103525 |
| Parazoanthus swiftii (MISE JDR170609-2-6) | MT102228 | MT103533 | MT103530 |
| Parazoanthus swiftii (MISE JDR170610-4-32) | MT102229 | MT103534 | MT103531 |
| Parazoanthus atlanticus sp. n. (MISE JDR170613-10-60) | MT102223 | MT103538 | MT103528 |
| Parazoanthus atlanticus sp. n. (MISE JDR170613-10-61) | MT102222 | MT103539 | MT103527 |
| Parazoanthus atlanticus sp. n. (NSMT-Co 1706) | MT102224 | MT103537 | MT103526 |
| Parazoanthus atlanticus sp. n. (NSMT-Co 1707) | MT102225 | MT103536 | MT103524 |
| Parazoanthus atlanticus sp. n. (MISE JDR170616-13-76) | MT102226 | MT103535 | MT103529 |
| Umimayanthus sp. (MISE JDR170619-20-94) | MT102227 | NA | NA |
| Sample ID: NSMT-Co 1706 | Length (Min-Max, Average) µm | Width (Min-Max, Average) µm | n | |
|---|---|---|---|---|
| Tentacles | Spirocysts | 13–29, 20 | 2–6, 3.5 | 222 |
| Holotrichs (L) | 32 | 15 | 1 | |
| Bastrichs and microbasic b-mastigophores | 14–23, 19.8 | 2–5, 4.3 | 24 | |
| Microbasic p-mastigophores | - | - | - | |
| Column | Spirocysts | - | - | - |
| Holotrichs (L) | 20–47, 29.2 | 11–15, 13.1 | 16 | |
| Bastrichs and microbasic b-mastigophores | - | - | - | |
| Microbasic p-mastigophores | - | - | - | |
| Pharynx | Spirocysts | 23 | 3 | 1 |
| Holotrichs (M) | 17 | 8 | 1 | |
| Bastrichs and microbasic b-mastigophores | 14–18, 16.1 | 2–4, 3.3 | 17 | |
| Microbasic p-mastigophores | - | - | - | |
| Filaments | Spirocysts | - | - | - |
| Holotrichs (L) | 25–31, 28.4 | 9–16, 13.5 | 32 | |
| Bastrichs and microbasic b-mastigophores | - | - | - | |
| Microbasic p-mastigophores | 12–20, 16 | 3–6, 4.8 | 10 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Montenegro, J.; Hoeksema, B.W.; Santos, M.E.A.; Kise, H.; Reimer, J.D. Zoantharia (Cnidaria: Hexacorallia) of the Dutch Caribbean and One New Species of Parazoanthus. Diversity 2020, 12, 190. https://0-doi-org.brum.beds.ac.uk/10.3390/d12050190
Montenegro J, Hoeksema BW, Santos MEA, Kise H, Reimer JD. Zoantharia (Cnidaria: Hexacorallia) of the Dutch Caribbean and One New Species of Parazoanthus. Diversity. 2020; 12(5):190. https://0-doi-org.brum.beds.ac.uk/10.3390/d12050190
Chicago/Turabian StyleMontenegro, Javier, Bert W. Hoeksema, Maria E. A. Santos, Hiroki Kise, and James Davis Reimer. 2020. "Zoantharia (Cnidaria: Hexacorallia) of the Dutch Caribbean and One New Species of Parazoanthus" Diversity 12, no. 5: 190. https://0-doi-org.brum.beds.ac.uk/10.3390/d12050190