Roadside Car Surveys: Methodological Constraints and Solutions for Estimating Parrot Abundances across the World
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Areas and Field Work
2.2. Roadside Surveys
2.3. Distance Sampling Modeling
2.4. Traits of Parrot Species
2.5. Statistical Analyses
3. Results
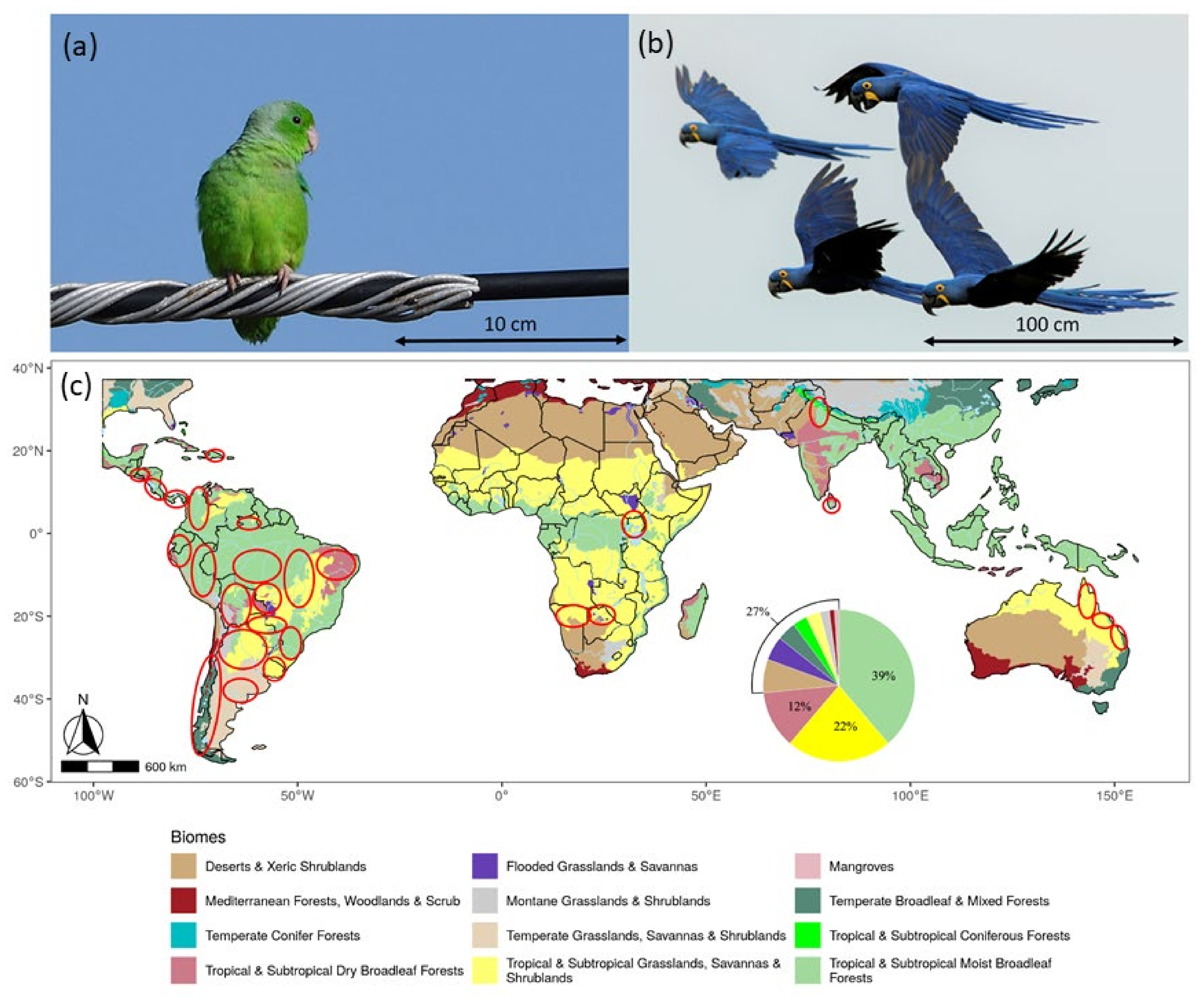
3.1. Distribution of Surveys and Species Recorded
3.2. Traits of the Species Recorded
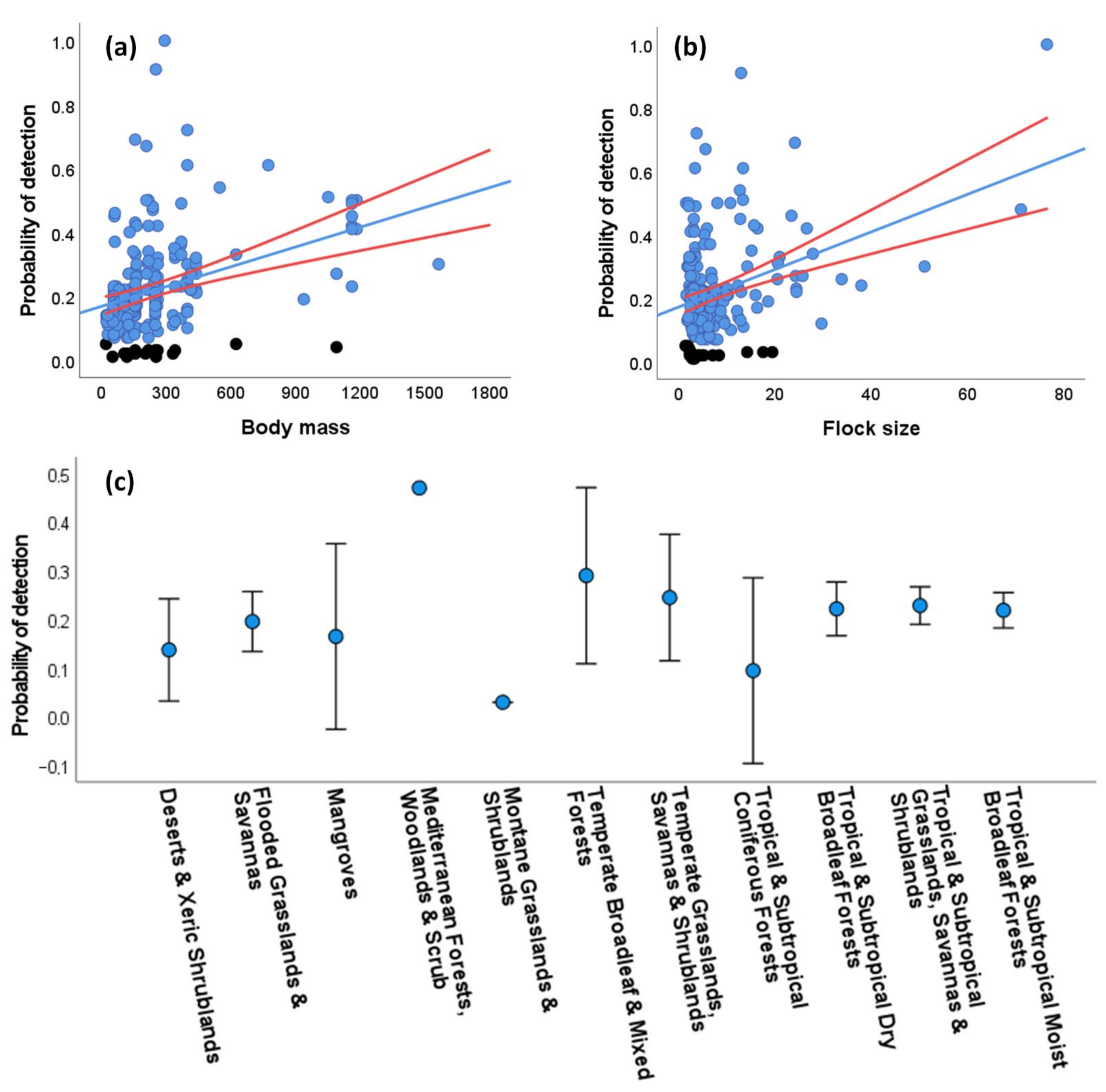
3.3. Sources of Variation in the Detectability of Species
3.3.1. Aural and Visual Encounter Rates
3.3.2. Distance-Dependent Detectability
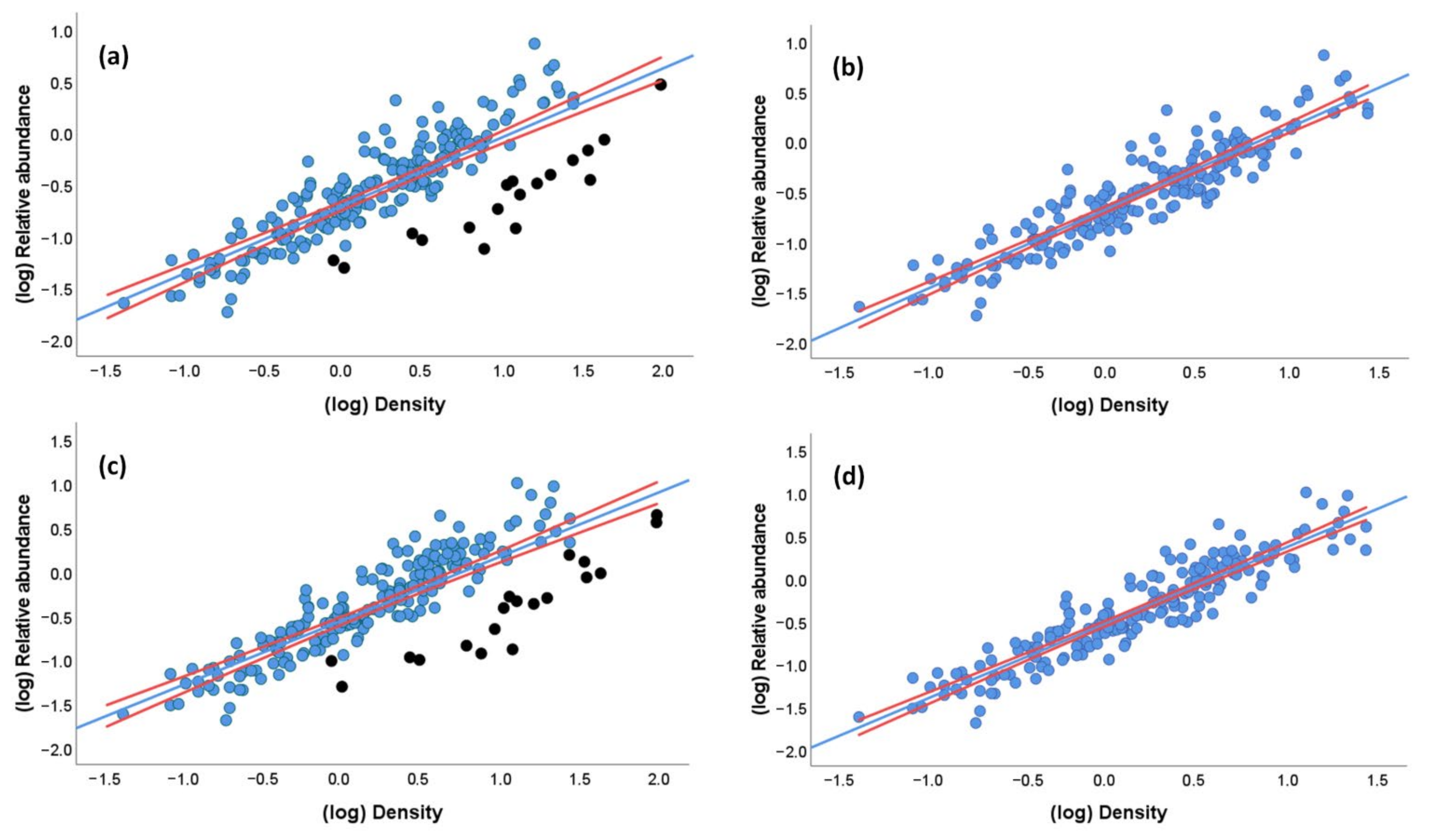
3.4. Relationships between Densities and Relative Abundances
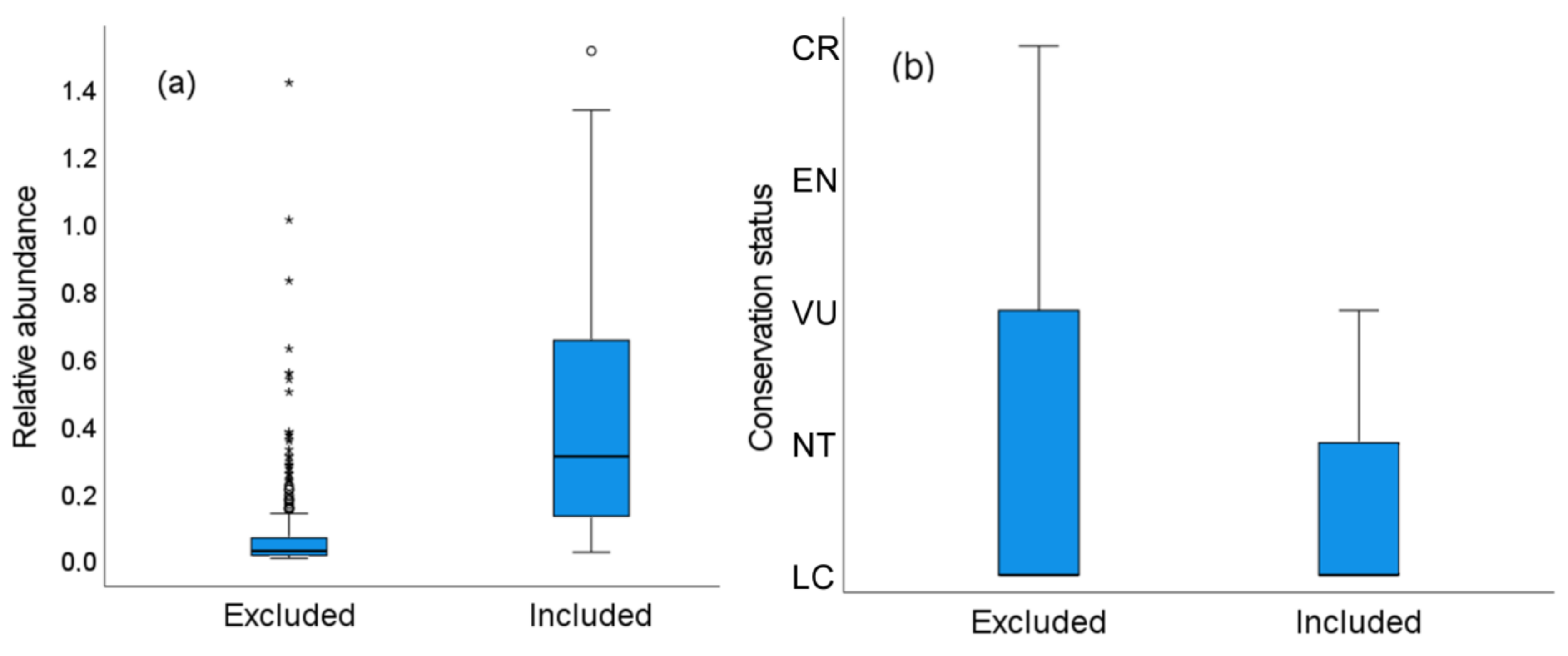
3.5. Characteristics of the Species and Surveys Lost When Using Distance Sampling
4. Discussion
4.1. Roadside Parrot Surveys: Caveats, Solutions, and Prospects
4.2. Do We Need to Account for Parrot Detectability?
4.3. Pros and Cons of Distance Sampling
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Olah, G.; Butchart, S.H.M.; Symes, A.; Guzmán, I.M.; Cunningham, R.; Brightsmith, D.; Heinsohn, R. Ecological and socio-economic factors affecting extinction risk in parrots. Biodivers. Conserv. 2016, 25, 205–223. [Google Scholar] [CrossRef]
- McClure, C.J.W.; Rolek, B.W. Relative Conservation Status of Bird Orders With Special Attention to Raptors. Front. Ecol. Evol. 2020, 8, 420. [Google Scholar] [CrossRef]
- IUCN. The IUCN Red List of Threatened Species. Available online: https://www.iucnredlist.org (accessed on 17 January 2021).
- Marsden, S.J.; Royle, K. Abundance and abundance change in the world’s parrots. Ibis 2015, 157, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Berkunsky, I.; Quillfeldt, P.; Brightsmith, D.J.; Abbud, M.C.; Aguilar, J.M.R.E.; Alemán-Zelaya, U.; Aramburú, R.M.; Arce Arias, A.; Balas McNab, R.; Balsby, T.J.S.; et al. Current threats faced by Neotropical parrot populations. Biol. Conserv. 2017, 214, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Martin, R.O.; Perrin, M.R.; Boyes, R.S.; Abebe, Y.D.; Annorbah, N.D.; Asamoah, A.; Bizimana, D.; Bobo, K.S.; Bunbury, N.; Brouwer, J.; et al. Research and conser-vation of the larger parrots of Africa and Madagascar: A review of knowledge gaps and opportunities. Ostrich 2014, 85, 205–233. [Google Scholar] [CrossRef]
- Vergara-Tabares, D.L.; Cordier, J.M.; Landi, M.A.; Olah, G.; Nori, J. Global trends of habitat destruction and consequences for parrot conservation. Glob. Chang. Biol. 2020, 26, 4251–4262. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.M.; Hiraldo, F.; Romero, M.A.; Tella, J.L. When does agriculture enter into conflict with wildlife? A global assess-ment of parrot–agriculture conflicts and their conservation effects. Divers. Distrib. 2021, 27, 4–17. [Google Scholar] [CrossRef]
- Sánchez-Mercado, A.; Ferrer-Paris, J.; Rodríguez, J.; Tella, J.L. A Literature Synthesis of Actions to Tackle Illegal Parrot Trade. Diversity 2021, 13, 191. [Google Scholar] [CrossRef]
- Dénes, F.V.; Tella, J.L.; Beissinger, S.R. Revisiting methods for estimating parrot abundance and population size. Emu Austral. Ornithol. 2017, 118, 67–79. [Google Scholar] [CrossRef] [Green Version]
- Casagrande, D.G.; Beissinger, S.R. Evaluation of Four Methods for Estimating Parrot Population Size. Condor 1997, 99, 445–457. [Google Scholar] [CrossRef]
- Marsden, S.J. Estimation of parrot and hornbill densities using a point count distance sampling method. Ibis 2008, 141, 327–390. [Google Scholar] [CrossRef]
- Legault, A.; Theuerkauf, J.; Baby, E.; Moutin, L.; Rouys, S.; Saoumoé, M.; Verfaille, L.; Barré, N.; Chartendrault, V.; Gula, R. Standardising distance sampling surveys of parrots in New Caledonia. J. Ornithol. 2013, 154, 19–33. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Zapata, J.A.; Calvo, J.F. Raptor distribution in relation to landscape composition in semi-arid Mediterranean habitats. J. Appl. Ecol. 1999, 36, 254–262. [Google Scholar] [CrossRef]
- Carrete, M.; Tella, J.L.; Blanco, G.; Bertellotti, M. Effects of habitat degradation on the abundance, richness and diversity of raptors across Neotropical biomes. Biol. Conserv. 2009, 142, 2002–2011. [Google Scholar] [CrossRef]
- Dénes, F.V.; Solymos, P.; Lele, S.; Silveira, L.F.; Beissinger, S.R. Biome-scale signatures of land-use change on raptor abun-dance: Insights from single-visit detection-based models. J. Appl. Ecol. 2017, 54, 1268–1278. [Google Scholar] [CrossRef] [Green Version]
- Tella, J.; Rojas, A.; Carrete, M.; Hiraldo, F. Simple assessments of age and spatial population structure can aid conservation of poorly known species. Biol. Conserv. 2013, 167, 425–434. [Google Scholar] [CrossRef]
- Luna, Á.; Romero-Vidal, P.; Hiraldo, F.; Tella, J.L. Cities may save some threatened species but not their ecological functions. PeerJ 2018, 6, e4908. [Google Scholar] [CrossRef]
- Tella, J.L.; Dénes, F.; Zulian, V.; Prestes, N.P.; Martínez, J.; Blanco, G.; Hiraldo, F. Endangered plant-parrot mutualisms: Seed tolerance to predation makes parrots pervasive dispersers of the Parana pine. Sci. Rep. 2016, 6, 31709. [Google Scholar] [CrossRef]
- Baños-Villalba, A.; Blanco, G.; Díaz-Luque, J.A.; Dénes, F.; Hiraldo, F.; Tella, J.L. Seed dispersal by macaws shapes the landscape of an Amazonian ecosystem. Sci. Rep. 2017, 7, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Blanco, G.; Hiraldo, F.; Rojas, A.; Dénes, F.; Tella, J.L. Parrots as key multilinkers in ecosystem structure and functioning. Ecol. Evol. 2015, 5, 4141–4160. [Google Scholar] [CrossRef] [Green Version]
- Romero-Vidal, P.; Hiraldo, F.; Rosseto, F.; Blanco, G.; Carrete, M.; Tella, J.L. Opportunistic or Non-Random Wildlife Crime? Attractiveness rather than Abundance in the Wild Leads to Selective Parrot Poaching. Diversity 2020, 12, 314. [Google Scholar] [CrossRef]
- Lee, A.T.K.; Marsden, S.J. The Influence of Habitat, Season, and Detectability on Abundance Estimates across an Amazonian Parrot Assemblage. Biotropica 2012, 44, 537–544. [Google Scholar] [CrossRef]
- Reuleaux, A.; A Siregar, B.; Collar, N.J.; Panggur, M.R.; Mardiastuti, A.; Jones, M.J.; Marsden, S.J. Protected by dragons: Density surface modeling confirms large population of the critically endangered Yellow-crested Cockatoo on Komodo Island. Condor 2020, 122, 042. [Google Scholar] [CrossRef]
- Lopes, D.C.; Martin, R.O.; Henriques, M.; Monteiro, H.; Cardoso, P.; Tchantchalam, Q.; Pires, A.J.; Regalla, A.; Catry, P. Combining local knowledge and field surveys to determine status and threats to Timneh Parrots Psittacus timneh in Guinea-Bissau. Bird Conserv. Int. 2019, 29, 400–412. [Google Scholar] [CrossRef]
- Grilli, P.G.; Soave, G.E.; Arellano, M.L.; Masello, J.F. Abundancia relativa del Loro Barranquero (Cyanoliseus patagonus) en la provincia de Buenos Aires y zonas limítrofes de La Pampa y Río Negro, Argentina. Hornero 2012, 27, 63–71. [Google Scholar]
- Salinas-Melgoza, A.; Salinas-Melgoza, V.; Wright, T.F. Behavioral plasticity of a threatened parrot in human-modified land-scapes. Biol. Conserv. 2013, 159, 303–312. [Google Scholar] [CrossRef]
- Figueira, L.; Tella, J.L.; de Camargo, U.M.; Ferraz, G. Autonomous sound monitoring shows higher use of Amazon old growth than secondary forest by parrots. Biol. Conserv. 2015, 184, 27–35. [Google Scholar] [CrossRef] [Green Version]
- Flores-López, E.; Montero-Castro, J.C.; Monterrubio-Rico, T.C.; Ibarra-Manríquez, G.; López-Toledo, L.; Bonilla-Ruz, C. Diffe-rential Use of Forest Patches by the Military Macaw Ara militaris (Psittacidae) in Coastal Tropical Forests of Jalisco, Mexico. Ardeola 2020, 67, 423–432. [Google Scholar] [CrossRef]
- Wirminghaus, J.; Downs, C.T.; Perrin, M.; Symes, C. Abundance and activity patterns of the Cape parrot ( Poicephalus robustus ) in two afromontane forests in South Africa. Afr. Zool. 2001, 36, 71–77. [Google Scholar] [CrossRef]
- Salinas-Melgoza, A.; Renton, K. Seasonal variation in activity patterns of juvenile Lilac-crowned parrots in tropical dry forest. Wilson Bull. 2006, 117, 291–295. [Google Scholar] [CrossRef]
- Montesinos-Navarro, A.; Hiraldo, F.; Tella, J.L.; Blanco, G. Network structure embracing mutualism–antagonism continuums increases community robustness. Nat. Ecol. Evol. 2017, 1, 1661–1669. [Google Scholar] [CrossRef]
- Sebastián-González, E.; Hiraldo, F.; Blanco, G.; Hernández-Brito, D.; Romero-Vidal, P.; Carrete, M.; Gómez-Llanos, E.; Pacífico, E.C.; Díaz-Luque, J.A.; Dénes, F.V.; et al. The extent, frequency and ecological functions of food wasting by parrots. Sci. Rep. 2019, 9, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Hernández-Brito, D.; Romero-Vidal, P.; Hiraldo, F.; Blanco, G.; Díaz-Luque, J.A.; Barbosa, J.M.; Symes, C.T.; White, T.H.; Pacífico, E.C.; Sebastián-González, E.; et al. Epizoochory in parrots as an overlooked yet widespread plant–animal mutualism. Plants 2021, 10, 760. [Google Scholar] [CrossRef]
- Blanco, G.; Romero-Vidal, P.; Carrete, M.; Chamorro, D.; Bravo, C.; Hiraldo, F.; Tella, J. Burrowing Parrots Cyanoliseus patagonus as Long-Distance Seed Dispersers of Keystone Algarrobos, Genus Prosopis, in the Monte Desert. Diversity 2021, 13, 204. [Google Scholar] [CrossRef]
- Buckland, S.T.; Anderson, D.R.; Burnham, K.P.; Laake, J.L.; Borchers, D.L.; Thomas, L. Introduction to Distance Sampling: Estimating Abundance of Biological Populations; Oxford University Press: Oxford, UK, 2001. [Google Scholar]
- Buckland, S.T. Point transect surveys for songbirds: Robust methodologies. Auk 2006, 123, 345–357. [Google Scholar] [CrossRef]
- Thomas, L.; Buckland, S.T.; Rexstad, E.; Laake, J.L.; Strindberg, S.; Hedley, S.L.; Bishop, J.R.B.; Marques, T.A.; Burnham, K.P. Distance software: Design and analysis of distance sampling surveys for estimating population size. J. Appl. Ecol. 2010, 47, 5–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miller, D.L.; Rexstad, E.; Thomas, L.; Marshall, L.; Laake, J.L. Distance Sampling in R. J. Stat. Softw. 2019, 89, 1–28. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018; Available online: http://www.R-project.org/ (accessed on 6 July 2019).
- Burgio, K.R.; Davis, K.E.; Dreiss, L.M.; Cisneros, L.M.; Klingbeil, B.; Presley, S.J.; Willig, M.R. Phylogenetic supertree and functional trait database for all extant parrots. Data Brief. 2019, 24, 103882. [Google Scholar] [CrossRef]
- Jovani, R.; Tella, J.L. Parasite prevalence and sample size: Misconceptions and solutions. Trends Parasitol. 2006, 22, 214–218. [Google Scholar] [CrossRef]
- Bibby, C.J.; Burgess, N.D.; Hill, D.A. Bird Census Techniques; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Mammides, C.; Kounnamas, C.; Goodale, E.; Kadis, C. Do unpaved, low-traffic roads affect bird communities? Acta Oecologica 2016, 71, 14–21. [Google Scholar] [CrossRef]
- Cooke, S.; Balmford, A.; Donald, P.F.; E Newson, S.; Johnston, A. Roads as a contributor to landscape-scale variation in bird communities. Nat. Commun. 2020, 7, 1–10. [Google Scholar] [CrossRef]
- Meunier, F.D.; Verheyden, C.; Jouventin, P. Use of roadsides by diurnal raptors in agricultural landscapes. Biol. Conserv. 2000, 92, 291–298. [Google Scholar] [CrossRef] [Green Version]
- Blanco, G.; Bravo, C.; Chamorro, D.; Lovas-Kiss, A.; Hiraldo, F.; Tella, J.L. Herb endozoochory by cockatoos: Is ‘foliage the fruit”? Austral. Ecol. 2020, 45, 122–126. [Google Scholar] [CrossRef]
- Hernández-Brito, D.; Carrete, M.; Blanco, G.; Romero-Vidal, P.; Senar, J.C.; Mori, E.; White, T.H., Jr.; Luna, A.; Tella, J.L. The Role of Monk Parakeets as Nest-Site Facilitators in Their Native and Invaded Areas. Biology 2021. (under review). [Google Scholar]
- Darras, K.; Batáry, P.; Furnas, B.J.; Grass, I.; Mulyani, Y.A.; Tscharntke, T. Autonomous sound recording outperforms human observation for sampling birds: A systematic map and user guide. Ecol. Appl. 2019, 29, e01954. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tella, J.; Canale, A.; Carrete, M.; Petracci, P.; Zalba, S.M. Anthropogenic Nesting Sites Allow Urban Breeding in Burrowing Parrots Cyanoliseus patagonus. Ardeola 2014, 61, 311–321. [Google Scholar] [CrossRef] [Green Version]
- Carrete, M.; Tella, J.L. Individual consistency in flight initiation distances in burrowing owls: A new hypothesis on disturb-ance-induced habitat selection. Biol. Lett. 2010, 6, 167–170. [Google Scholar] [CrossRef] [Green Version]
- Carrete, M.; Tella, J.L. Inter-individual variability in fear of humans and relative brain size of the species are related to con-temporary urban invasion in birds. PLoS ONE 2011, 6, e18859. [Google Scholar] [CrossRef] [Green Version]
- Buckland, S.; Marsden, S.J.; Green, R.E. Estimating bird abundance: Making methods work. Bird Conserv. Int. 2008, 18, S91–S108. [Google Scholar] [CrossRef] [Green Version]
- Keller, C.M.; Scallan, J.T. Potential roadside biases due to habitat changes along breeding bird survey routes. Condor 1999, 101, 50–57. [Google Scholar]
- Romero-Vidal, P.; Barbosa, J.M.; Blanco, G.; Hiraldo, F.; Carrete, M.; Tella, J.L. Deforestation or overharvesting? Pet-keeping cultural burden rather than habitat transformation causes selective parrot defaunation in Costa Rica. 2021. (in preparation) [Google Scholar]
- Gilardi, J.D.; Munn, C.A. Patterns of Activity, Flocking, and Habitat Use in Parrots of the Peruvian Amazon. Condor 1998, 100, 641–653. [Google Scholar] [CrossRef]
- Española, C.P.; Collar, N.J.; Marsden, S.J. Are populations of large-bodied avian frugivores on Luzon, Philippines, facing imminent collapse? Anim. Conserv. 2013, 16, 467–479. [Google Scholar] [CrossRef]
- Rivera-Milán, F.F.; Simal, F.; Bertuol, P.; Boomer, G.S. Population monitoring and modelling of yellow-shouldered parrot on Bonaire, Caribbean Netherlands. Wildl. Biol. 2018, 2018, wlb.00384. [Google Scholar] [CrossRef] [Green Version]
- Dénes, F.V.; Silveira, L.F.; Beissinger, S.R. Estimating abundance of unmarked animal populations: Accounting for imperfect detection and other sources of zero inflation. Methods Ecol. Evol. 2015, 6, 543–556. [Google Scholar] [CrossRef]
- Zulian, V.; Müller, E.S.; Cockle, K.L.; Lesterhuis, A.; Júnior, R.T.; Prestes, N.P.; Martinez, J.; Kéry, M.; Ferraz, G. Addressing multiple sources of uncertainty in the estimation of global parrot abundance from roost counts: A case study with the Vina-ceous-breasted Parrot (Amazona vinacea). Biol. Conserv. 2020, 248, 108672. [Google Scholar] [CrossRef]
- Geary, M.; Brailsford, C.J.; Hough, L.I.; Baker, F.; Guerrero, S.; Leon, Y.M.; Collar, N.J.; Marsden, S.J. Street-level green spaces support a key urban population of the threatened Hispaniolan parakeet Psittacara chloropterus. Urban. Ecosyst. 2021, 1–8. [Google Scholar] [CrossRef]
- Barbosa, A.E.A.; Tella, J.L. How much does it cost to save a species from extinction? Costs and rewards of conserving the Lear’s macaw. R. Soc. Open Sci. 2019, 6, 190190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dupin, M.K.; Dahlin, C.R.; Wright, T.F. Range-Wide Population Assessment of the Endangered Yellow-Naped Amazon (Amazona auropalliata). Diversity 2020, 12, 377. [Google Scholar] [CrossRef]

| DS Yes | DS No | Justification | |
|---|---|---|---|
| Sampling effectiveness | |||
| Attraction effect | - | + | DS may inflate densities of parrots attracted by roadside resources |
| Avoidance effect | + | - | DS may account for the potential avoidance of highly transited roads |
| Aural-only encounters | - | + | Estimating distances for DS from non-visual encounters is challenging |
| Flying individuals | = | = | Including flying individuals should not affect results from roadside surveys |
| Uncommon species | - | + | Encounters of naturally scarce and threatened species are not sufficient for DS |
| Gregarious species | - | + | Encounters of common but highly gregarious species may not be sufficient for DS |
| Detectability | + | - | DS allows the correction of abundances for distance-dependent detectability and associated covariates |
| Methodological constraints | |||
| Survey length | - | + | DS requires longer surveys to obtain enough encounters for statistical modelling |
| Time invested | - | + | Time saved by not recording distances allows for longer surveys |
| Data analysis | - | + | DS requires statistical modeling instead of simple divisions |
| Output variables | |||
| Single-species abundance | + | - | More accurate estimates can be obtained through DS for common species |
| Multi-species abundances | - | + | DS excludes a high percentage of species |
| Occupancy | - | + | Only presence/absence data are required |
| Species richness | - | + | DS is not needed |
| Species diversity | - | + | DS excludes a high percentage of species |
| Density | + | - | DS allows for calculating densities for species with enough encounters |
| Population size | + | - | DS allows extrapolating densities to the species distribution and thus estimating population size |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tella, J.L.; Romero-Vidal, P.; Dénes, F.V.; Hiraldo, F.; Toledo, B.; Rossetto, F.; Blanco, G.; Hernández-Brito, D.; Pacífico, E.; Díaz-Luque, J.A.; et al. Roadside Car Surveys: Methodological Constraints and Solutions for Estimating Parrot Abundances across the World. Diversity 2021, 13, 300. https://0-doi-org.brum.beds.ac.uk/10.3390/d13070300
Tella JL, Romero-Vidal P, Dénes FV, Hiraldo F, Toledo B, Rossetto F, Blanco G, Hernández-Brito D, Pacífico E, Díaz-Luque JA, et al. Roadside Car Surveys: Methodological Constraints and Solutions for Estimating Parrot Abundances across the World. Diversity. 2021; 13(7):300. https://0-doi-org.brum.beds.ac.uk/10.3390/d13070300
Chicago/Turabian StyleTella, José L., Pedro Romero-Vidal, Francisco V. Dénes, Fernando Hiraldo, Bernardo Toledo, Federica Rossetto, Guillermo Blanco, Dailos Hernández-Brito, Erica Pacífico, José A. Díaz-Luque, and et al. 2021. "Roadside Car Surveys: Methodological Constraints and Solutions for Estimating Parrot Abundances across the World" Diversity 13, no. 7: 300. https://0-doi-org.brum.beds.ac.uk/10.3390/d13070300