The Hesperornithiformes: A Review of the Diversity, Distribution, and Ecology of the Earliest Diving Birds
Abstract
:1. Introduction
2. General Anatomy






3. Taxonomy
3.1. Enaliornithidae
3.2. Baptornithidae
3.3. Brodavidae

3.4. The Hesperornithidae
3.5. Taxa Outside of Recognized Families
3.5.1. Pasquiaornis
3.5.2. Chupkaornis
3.5.3. Fumicollis
3.5.4. Potamornis
3.6. Taxonomic Challenges
4. Phylogeny of the Hesperornithiformes
5. Evolutionary Trends
5.1. Flightlessness
5.2. Foot-Propelled Diving
5.3. Gigantism
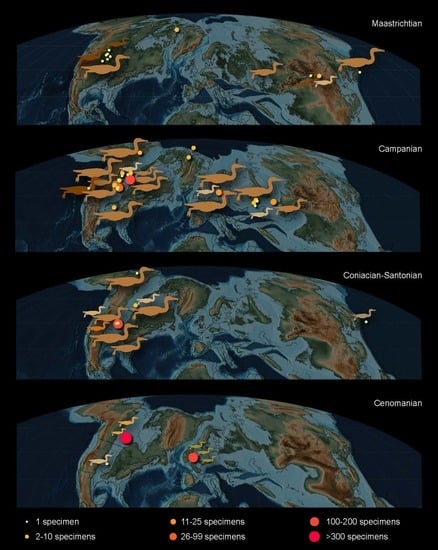
6. Paleoecology
7. Summary and Future Directions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Marsh, O.C. Odontornithes: A Monograph on the Extinct Toothed Birds of North America; United States Government Printing Office: Washington, DC, USA, 1880; 312p.
- Marsh, O.C. Discovery of a remarkable fossil bird. Am. J. Sci. Ser. 1872, 3, 56–57. [Google Scholar] [CrossRef]
- Marsh, O.C. ART. XLVIII.—Preliminary Description of Hesperornis regalis, with Notices of Four Other New Species of Cretaceous Birds. Am. J. Sci. Arts 1872, 3, 360. [Google Scholar] [CrossRef]
- Marsh, O.C. Fossil birds from the Cretaceous of North America. Am. J. Sci. Arts 1872, 5, 229–231. [Google Scholar]
- Marsh, O.C. On a new subclass of fossil birds. Am. J. Sci. Arts 1873, 5, 161. [Google Scholar]
- Marsh, O.C. On the Odontornithes, or birds with teeth. Am. J. Sci. Arts 1873, 10, 403. [Google Scholar]
- Marsh, O.C. Notice of new Odontornithes. Am. J. Sci. Arts 1876, 11, 509–511. [Google Scholar] [CrossRef]
- Seeley, H.G. On the British Fossil Cretaceous Birds. Q. J. Geol. Soc. 1876, 32, 496–512. [Google Scholar] [CrossRef]
- Furbinger, M. Untersuchungen Zur Morphologie und Systematik der Vögel, Zugleich ein Beitrag Zur ANATOMIE der Stütz- und Bewegungsorgan; T. van Holkema: Amsterdam, The Netherlands, 1888. [Google Scholar]
- Lydekker, R. Catalogue of the Fossil Birds in the British Museum (Natural History) United Kingdom, Order of the Trustees; Longmans & Co.: London, UK, 1891. [Google Scholar]
- Wetmore, A. The fossil birds of the AOU Check-list. Condor 1930, 32, 12–14. [Google Scholar] [CrossRef]
- Storer, R.W. Evolution in the Diving Birds. In Proceedings of the XII International Ornithological Congress; Tilgmannin Kirjapaino: Helsinki, Finland, 1958; Volume 2, pp. 694–707. [Google Scholar]
- Martin, L.D.; Tate, J. The Skeleton of Baptornis advenus (Aves: Hesperornithiformes). Smithson. Contrib. Paleobiol. 1976, 27, 35–66. [Google Scholar]
- Cumbaa, S.L.; Schröder-Adams, C.; Day, R.G.; Phillips, A.J. Cenomanian Bonebed Faunas from the Northeastern Margin, Western Interior Seaway, Canada. In Late Cretaceous Vertebrates from the Western Interior. New Mexico Museum of Natural History and Science Bulletin 35; Lucas, S., Sullivan, R., Eds.; Kansas Academy of Science: Kansas, MO, USA, 2006; pp. 139–155. [Google Scholar]
- Sanchez, J. Late Cretaceous (Cenomanian) Hesperornithiformes from the Pasquia Hills, Saskatchewan, Canada. Master’s Thesis, Carleton University, Ottawa, ON, Canada, 2010. [Google Scholar]
- Tokaryk, T.T.; Harington, C.R. Baptornis sp. (Aves: Hesperornithiformes) from the Judith River Formation (Campanian) of Saskatchewan, Canada. J. Paleontol. 1992, 66, 1010–1012. [Google Scholar] [CrossRef]
- Lambrecht, K. Handbuch der Palaeomithologie; Gebruder Borntraeger: Berlin, Germany, 1933; 1022p. [Google Scholar]
- Brodkorb, P. Origin and Evolution of Birds; Farner, D.S., King, J.R., Eds.; Avian Biology: New York, NY, USA; Academic Press: London, UK, 1971; Volume 1. [Google Scholar]
- Cracraft, J. Phylogenetic Relationships and Monophyly of Loons, Grebes, and Hesperornithiform Birds, with Comments on the Early History of Birds. Syst. Zool. 1982, 31, 22. [Google Scholar] [CrossRef]
- Hackett, S.J.; Kimball, R.T.; Reddy, S.; Bowie, R.C.; Braun, E.L.; Braun, M.J.; Chojnowski, J.L.; Cox, W.A.; Han, K.; Harshman, J.; et al. A phylogenomic study of birds reveals their evolutionary history. Science 2008, 320, 1763–1768. [Google Scholar] [CrossRef]
- Jetz, W.; Thomas, G.H.; Joy, J.B.; Hartmann, K.; Mooers, A.O. The Global Diversity of Birds in Space and Time. Nature 2012, 491, 444–448. [Google Scholar] [CrossRef]
- Bell, A.; Wu, Y.-H.; Chiappe, L.M. Morphometric Comparison of the Hesperornithiformes and Modern Diving Birds. Palaeogeogr. Palaeoclimatol. Palaeoecol. 2019, 513, 196–207. [Google Scholar] [CrossRef]
- Bell, A.; Chiappe, L.M. A Species-Level Phylogeny of the Cretaceous Hesperornithiformes (Aves: Ornithuromorpha): Implications for Body Size Evolution amongst the Earliest Diving Birds. J. Syst. Palaeontol. 2016, 14, 239–251. [Google Scholar] [CrossRef]
- Tanaka, T.; Kobayashi, Y.; Kurihara, K.; Fiorillo, A.R.; Kano, M. The Oldest Asian Hesperornithiform from the Upper Cretaceous of Japan and the Phylogenetic Reassessment of Hesperornithiformes. J. Syst. Palaeontol. 2017, 16, 689–709. [Google Scholar] [CrossRef]
- Bell, A.; Chiappe, L. Anatomy of Parahesperornis: Evolutionary Mosaicism in the Cretaceous Hesperornithiformes (Aves). Life 2020, 10, 62. [Google Scholar] [CrossRef] [PubMed]
- O’Connor, J.K. The trophic habits of early birds. Palaeogeo. Palaeoclim. Palaeoeco. 2019, 513, 178–195. [Google Scholar] [CrossRef]
- O’Connor, J.K.; Wang, M.; Hu, H. A new ornithuromorph (Aves) with an elongate rostrum from the Jehol Biota, and the early evolution of rostralization in birds. J. Syst. Palaeontol. 2016, 14, 939–948. [Google Scholar] [CrossRef]
- Wu, Y.H.; Chiappe, L.M.; Bottjer, D.J.; Nava, W.; Martinelli, A.G. Dental replacement in Mesozoic birds: Evidence from newly discovered Brazilian enantiornithines. Sci. Rep. 2021, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Scotese, C.R. Atlas of Earth History; PALEOMAP Project: Arlington, TX, USA, 2001; Volume 1. [Google Scholar]
- Dumont, M.; Tafforeau, P.; Bertin, T.; Bhullar, B.-A.; Field, D.; Schulp, A.; Strilisky, B.; Thivichon-Prince, B.; Viriot, L.; Louchart, A. Synchrotron Imaging of Dentition Provides Insights into the Biology of Hesperornis and Ichthyornis, the “Last” Toothed Birds. BMC Evol. Biol. 2016, 16, 178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Howgate, M.E. The teeth of Archaeopteryx and a reinterpretation of the Eichstätt specimen. Zoo. J. Linn. Soc. 1984, 82, 159–175. [Google Scholar] [CrossRef]
- Clarke, J.A. Morphology, Phylogenetic Taxonomy, and Systematics of Ichthyornis and Apatornis (Avialae: Ornithurae). Bull. Am. Mus. Nat. Hist. 2004, 286, 1–179. [Google Scholar] [CrossRef]
- Clarke, J.A.; Zhou, Z.; Zhang, F. Insight into the evolution of avian flight from a new clade of Early Cretaceous ornithurines from China and the morphology of Yixianornis Grabaui. J. Anat. 2006, 208, 287–308. [Google Scholar] [CrossRef]
- O’Connor, J.K.; Chiappe, L.M. A revision of enantiornithine (Aves: Ornithothoraces) skull morphology. J. Syst. Palaeo. 2011, 9, 135–157. [Google Scholar] [CrossRef]
- Sander, P.M. The microstructure of reptilian tooth enamel: Terminology, function, and phylogeny. F. Pfeil 1999, 38, 1–102. [Google Scholar]
- Martin, L.D.; Stewart, J.D. Implantation and replacement of bird teeth. Smiths. Cont. Paleobio. 1999, 89, 295–300. [Google Scholar]
- Elzanowski, A.; Galton, P. Braincase of Enaliornis, an Early Cretaceous Bird from England. J. Vertebr. Paleontol. 1991, 11, 90–107. [Google Scholar] [CrossRef]
- Ribak, G.; Weihs, D.; Arad, Z. Consequences of Buoyancy to the Maneuvering Capabilities of a Foot-Propelled Aquatic Predator, the Great Cormorant (Phalacrocorax carbo sinensis). J. Exp. Biol. 2008, 211, 3009–3019. [Google Scholar] [CrossRef] [Green Version]
- Lovvorn, J.R. Mechanics of Underwater Swimming in Foot-Propelled Diving Birds. Proc. Int. Ornithol. Congr. 1991, 20, 1868–1874. [Google Scholar]
- Wang, M.; O’Connor, J.K.; Pan, Y.; Zhou, Z. A bizarre Early Cretaceous enantiornithine bird with unique crural feathers and an ornithuromorph plough-shaped pygostyle. Nat. Commun. 2017, 8, 14141. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machalski, M. The Cenomanian ammonite Schloenbachia varians (J. Sowerby, 1817) from the Cambridge Greensand of eastern England: Possible sedimentological and taphonomic implications. Cret. Res. 2018, 87, 120–125. [Google Scholar] [CrossRef]
- Lyell, C. Manual of Elementary Geology, Supplement to the 5th Edn, 3rd ed.; Murray: London, UK, 1859. [Google Scholar]
- Storer, R.W. [Review of] P. Brodkorb, Catalogue of Fossil Birds, Part 1. Auk 1965, 82, 657–658. [Google Scholar] [CrossRef]
- Galton, P.M.; Martin, L.D. Postcranial Anatomy and Systematics of Enaliornis SEELEY, 1876, a Foot-Propelled Diving Bird (Aves: Ornithurae: Hesperornithiformes) from the Early Cretaceous of England. Rev. Paleobiol. 2002, 21, 489–538. [Google Scholar]
- Brodkorb, P. Catalogue of Fossil Birds. Bull. Fla. St. Mus. Bio. Sci. 1963, 7, 179–293. [Google Scholar]
- Kurochkin, E.N. Mesozoic Birds of Mongolia and the Former USSR. In The Age of Dinosaurs in Russia and Mongolia; Benton, M., Shishkin, M., Unwin, D., Kurochkin, E., Eds.; Cambridge University Press: Cambridge, UK, 2000; pp. 544–559. [Google Scholar]
- Tokaryk, T.T.; Cumbaa, S.L.; Storer, J.E. Early Late Cretaceous Birds from Saskatchewan, Canada: The Oldest Diverse Avifauna Known from North America. J. Vertebr. Paleontol. 1997, 17, 172–176. [Google Scholar] [CrossRef]
- Everhart, M.J.; Bell, A. A Hesperornithiform Limb Bone from the Basal Greenhorn Formation (Late Cretaceous; Middle Cenomanian) of North Central Kansas. J. Vertebr. Paleontol. 2009, 29, 952–956. [Google Scholar] [CrossRef]
- Zelenkov, N.V.; Panteleyev, A.V.; Yarkov, A.A. New Finds of Hesperornithids in the European Russia, with Comments on the Systematics of Eurasian Hesperornithidae. Paleontol. J. 2017, 51, 547–555. [Google Scholar] [CrossRef]
- Martin, L.D.; Kurochkin, E.N.; Tokaryk, T.T. A New Evolutionary Lineage of Diving Birds from the Late Cretaceous of North America and Asia. Palaeoworld 2012, 21, 59–63. [Google Scholar] [CrossRef]
- Martin, J.; Cordes-Person, A. A New Species of the Diving Bird Baptornis (Ornithurae: Hesperornithiformes) from the Lower Pierre Shale Group (Upper Cretaceous) of Southwestern South Dakota. Geol. Soc. Am. Spec. Pap. 2007, 427, 227–237. [Google Scholar]
- Nessov, L.A.; Prizemlin, B. A Large Advanced Flightless Marine Bird of the Order Hesperornithiformes of the Late Senonian of Turgai Strait-the First Finding of the Group in the USSR. Tr. Zool. Inst. SSSR 1991, 239, 85–107. [Google Scholar]
- Hou, L.-I. New Hesperornithid (Aves) from the Canadian Arctic. Gu Ji Zhui Dong Wu Xue Bao 1999, 37, 231–237. [Google Scholar]
- Nessov, L.A.; Yarkov., A.A. Hesperornis in Russia, Russk. Ornitol. Zh 1993, 2, 37–54. [Google Scholar]
- Martin, L.D.; Lim, J.-D. New Information on the Hesperornithiform Radiation. In Proceedings of the 5th Symposium of the Society of Avian Paleontology and Evolution, Beijing, China, 1–4 June 2000; Science Press: Beijing, China, 2002. [Google Scholar]
- Aotsuka, K.; Sato, T. Hesperornithiformes (Aves: Ornithurae) from the Upper Cretaceous Pierre Shale, Southern Manitoba, Canada. Cretac. Res. 2016, 63, 154–169. [Google Scholar] [CrossRef]
- Gill, J.; Cobban, W. Regional unconformity in late cretaceous, Wyoming. Am. Geol. Surv. Prof. Pap. 1966, 550-B, B20–B27. [Google Scholar]
- Bell, A.; Chiappe, L.M. Identification of a New Hesperornithiform from the Cretaceous Niobrara Chalk and Implications for Ecologic Diversity among Early Diving Birds. PLoS ONE 2015, 10, e0141690. [Google Scholar] [CrossRef] [Green Version]
- Lucas, F.A. Notes on the Osteology and Relationship of the Fossil Birds of the Genera Hesperornis, Hargeria, Baptornis, and Diatryma. Proc. U. S. Natl. Mus. 1903, 26, 545–556. [Google Scholar] [CrossRef]
- Elzanowski, A.; Paul, G.S.; Stidham, T.A. An Avian Quadrate from the Late Cretaceous Lance Formation of Wyoming. J. Vertebr. Paleontol. 2000, 20, 712–719. [Google Scholar] [CrossRef]
- Rees, J.; Lindgren, J. Aquatic Birds from the Upper Cretaceous (Lower Campanian) of Sweden and the Biology and Distribution of Hesperornithiforms: Cretaceous Aquatic Birds. Palaeontology 2005, 48, 1321–1329. [Google Scholar] [CrossRef]
- Shufeldt, R.W. The Fossil Remains of a Species of Hesperornis Found in Montana. Auk 1915, 32, 290–294. [Google Scholar] [CrossRef]
- Martin, L.D. A New Hesperornithid and the Relationships of the Mesozoic Birds. Trans. Kans. Acad. Sci. 1984, 87, 141–150. [Google Scholar] [CrossRef]
- Olson, S.L. Neogaeornis wetzeli Lambrecht, a cretaceous loon from Chile (Aves: Gaviidae). J. Vertebr. Paleontol. 1992, 12, 122–124. [Google Scholar] [CrossRef]
- Bell, A.K. Evolution and Ecology of Mesozoic Birds: A Case Study of the Derived Hesperornithiformes and the Use of Morphometric Data in Quantifying Avian Paleoecology. Master’s Thesis, The University of Southern California, Los Angeles, CA, USA, 2013. [Google Scholar]
- Chiappe, L.M. The first 85 million years of avian evolution. Nature 1995, 378, 349–355. [Google Scholar] [CrossRef]
- Chiappe, L.M.; Jorge, O.C. Neuquenornis volans, a new Late Cretaceous bird (Enantiornithes: Avisauridae) from Patagonia, Argentina. J. Vert. Paleont. 1994, 14, 230–246. [Google Scholar] [CrossRef]
- Padian, K.; Chiappe, L.M. The origin and early evolution of birds. Bio. Rev. 1998, 73, 1–42. [Google Scholar] [CrossRef]
- Liu, D.; Chiappe, L.M.; Zhang, Y.; Bell, A.; Meng, Q.; Ji, Q.; Wang, X. An advanced, new long-legged bird from the Early Cretaceous of the Jehol Group (northeastern China): Insights into the temporal divergence of modern birds. Zootaxa 2014, 3884, 253–266. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, J.K.; Zhou, Z. A redescription of Chaoyangia beishanensis (Aves) and a comprehensive phylogeny of Mesozoic birds. J. Syst. Palaeont. 2013, 11, 889–906. [Google Scholar] [CrossRef]
- O'Connor, J.K.; Chiappe, L.M.; Bell, A. Pre-modern birds: Avian divergences in the Mesozoic. In Living Dinosaurs: The Evolutionary History of Modern Birds; John Wiley & Sons: Hoboken, NJ, USA, 2011; pp. 39–114. [Google Scholar]
- Pittman, M.; O’Connor, J.; Tse, E.; Makovicky, P.; Field, D.J.; Ma, W.; Turner, A.H.; Norell, M.A.; Pei, R.; Xu, X. The Fossil Record of Mesozoic and Paleocene Pennaraptorans. Bull. Am. Mus. Nat. Hist. 2020, 440, 37–95. [Google Scholar]
- Wang, M.; O’Connor, J.K.; Zhou, Z. A new robust enantiornithine bird from the Lower Cretaceous of China with scansorial adaptations. J. Vert. Paleont. 2014, 34, 657–671. [Google Scholar] [CrossRef]
- Fluteau, F.; Ramstein, G.; Besse, J.; Giraud, R.; Masse, J.P. Impacts of palaeogeography and sea level change on Mid-Cretaceous climate. Palaeogeog. Palaeoclim. Palaeoeco. 2007, 247, 357–381. [Google Scholar] [CrossRef]
- Gradzinski, R.; Kielan-Jaworowska, Z.; Maryanska, T. Upper Cretaceous Djadokhta, Barun Goyot and Nemegt formations of Mongolia, including remarks on previous subdivisions. Acta Geol. Pol. 1977, 27, 281–326. [Google Scholar]
- Breithaupt, B. Paleontology and paleoecology of the Lance Formation (Maastrichtian), east flank of Rock Springs Uplift, Sweetwater County, Wyoming. Rocky Mt. Geol. 1982, 21, 123–151. [Google Scholar]
- Kupsch, W. Frenchman formation of eastern Cypress Hills, Saskatchewan, Canada. GSA Bull. 1957, 68, 413–420. [Google Scholar] [CrossRef]
- Ogunyomi, O.; Hills, L. Depositional environments, foremost formation (Late Cretaceous), Milk river area, southern Alberta. Bull. Can. Petrol. Geol. 1977, 25, 929–968. [Google Scholar]
- Wood, J.; Thomas, R.; Vasser, J. Fluvial processes and vertebrate taphonomy: The upper cretaceous Judith River formation, south-central dinosaur Provincial Park, Alberta, Canada. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1988, 66, 127–143. [Google Scholar] [CrossRef]
- Eberth, D.A. Stratigraphy and sedimentology of vertebrate microfossil sites in the uppermost Judith River formation (Campanian), Dinosaur Provincial Park, Alberta, Canada. Palaeogeogr. Palaeoclimatol. Palaeoecol. 1990, 78, 1–36. [Google Scholar] [CrossRef]
- Johnson, K.R.; Nichols, D.J.; Hartman, J.H. Hell Creek formation: A 2001 synthesis. In The Hell Creek Formation and the Cretaceous-Tertiary Boundary in the Northern Great Plains: An Integrated Continental Record of the End of the Cretaceous; Geological Society of America Special Paper, 361; Hartman, J.H., Johnson, K.R., Nichols, D.J., Eds.; Geological Society of America: Boulder, CO, USA, 2002; pp. 503–510. [Google Scholar]
- Bardack, D. Fossil vertebrates from the marine Cretaceous of Manitoba. Can. J. Earth Sci. 1968, 5, 145–153. [Google Scholar] [CrossRef]
- Reifenstuhl, R.R. Gilead sandstone, northeastern Brooks Range, Alaska: An Albian to Cenomanian marine clastic succession. In Short Notes on Alaskan Geology; Professional Report; Reger, R.D., Ed.; Alaska Division of Geological & Geophysical Surveys: Fairbanks, AK, USA, 1991; Volume 111, pp. 69–76. [Google Scholar]
- Barlow, L.; Kauffman, E. Depositional cycles in the Niobrara formation, Colorado front range. In The Society of Economic Paleontologists and Mineralogists (SEPM) Fine-Grained Deposits and Biofacies of the Cretaceous Western Interior Seaway: Evidence of Cyclic Sedimentary Processes (FG4); AAPG: Tulsa, OK, USA, 1985. [Google Scholar]
- Russell, D. Cretaceous vertebrates from the Anderson River, N.W.T. Can. J. Earth Sci. 1967, 4, 21–43. [Google Scholar] [CrossRef]
- Panteleyev, A.V.; Popov, E.V.; Averianov, A.O. New record of Hesperornis rossicus (Aves, Hesperornithiformes) in the campanian of Saratov Province, Russia. Paleontol. Res. 2004, 8, 115–122. [Google Scholar] [CrossRef] [Green Version]
- Hills, L.; Strong, W. Multivariate analysis of late cretaceous Kanguk formation (Arctic Canada) Palynomorph assemblages to identify nearshore to distal marine groupings. Bull. Can. Petrol. Geol. 2007, 55, 160–172. [Google Scholar] [CrossRef]
- Shultz, L.G.; Tourtelot, H.A.; Gill, J.R.; Boerngen, J.G. Composition and properties of the Pierre Shale and equivalent rocks, northern Great Plains region. Geol. Surv. Prof. Pap. 1980, 1064-B, 123. [Google Scholar]
- Hilton, R. Dinosaurs and Other Mesozoic Reptiles of California; University of California Press: Berkeley, CA, USA, 2003. [Google Scholar]
- Bell, A.; Irwin, K.J.; Davis, L.C. Hesperornithiform birds from the Late Cretaceous (Campanian) of Arkansas, USA. Trans. Kans. Acad. Sci. 2015, 118, 219–229. [Google Scholar] [CrossRef]
- Tanaka, T.; Kobayashi, Y.; Ikuno, K.; Ikeda, T.; Saegusa, H. A marine hesperornithiform (Avialae: Ornithuromorpha) from the Maastrichtian of Japan: Implications for the paleoecological diversity of the earliest diving birds in the end of the Cretaceous. Cretac. Res. 2020, 113, 104492. [Google Scholar] [CrossRef]
- Dyke, G.; Malakhov, D.; Chiappe, L.M. A re-analysis of the marine bird Asiahesperornis from northern Kazakhstan. Cretac. Res. 2006, 27, 947–953. [Google Scholar] [CrossRef]
- Marsh, O.C. A New Cretaceous Bird Allied to Hesperornis. Am. J. Sci. 1893, 45, 81–82. [Google Scholar] [CrossRef]
- Hinić-Frlog, S.; Motani, R. Relationship between osteology and aquatic locomotion in birds: Determining modes of locomotion in extinct Ornithurae. J. Evol. Bio. 2010, 23, 372–385. [Google Scholar] [CrossRef]
- Galantsev, V.P. Adaptational changes in the venous system of diving mammals. Can. J. Zool. 1991, 69, 414–419. [Google Scholar] [CrossRef]
- Girgis, S. Anatomical and functional adaptations in the venous system of a diving reptile, Trionyx triunquis. Proc. Zool. Soc. Lond. 1962, 138, 355–377. [Google Scholar] [CrossRef]
- Jessen, C. Selective brain cooling in mammals and birds. Jpn. J. Physiol. 2001, 51, 291–301. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinsamy, A.; Martin, L.D.; Dobson, P. Bone microstructure of the diving Hesperornis and the voltant Ichthyornis from the Niobrara Chalk of western Kansas. Cretac. Res. 1998, 19, 225–235. [Google Scholar] [CrossRef]
- Marsh, O.C. ART. XII. Characters of the Odontornithes, with notice of a new allied genus. Am. J. Sci. Arts 1877, 14, 79–84. [Google Scholar]
- Houde, P. Histological evidence for the systematic position of Hesperornis (Odontornithes: Hesperornithiformes). Auk 1987, 104, 125–129. [Google Scholar] [CrossRef]
- Wilson, L.E.; Chin, K.; Cumbaa, S.; Dyke, G. A High Latitude Hesperornithiform (Aves) from Devon Island: Palaeobiogeography and Size Distribution of North American Hesperornithiforms. J. Syst. Palaeontol. 2011, 9, 9–23. [Google Scholar] [CrossRef]
- Carpenter, K. Vertebrate biostratigraphy of the Smoky Hill Chalk (Niobrara Formation) and the Sharon Springs Member (Pierre Shale). In High-Resolution Approaches in Stratigraphic Paleontology; Springer: Dordrecht, The Netherlands, 2008; pp. 421–437. [Google Scholar]
- Petalas, C.; Lazarus, T.; Layoie, R.A.; Elliott, K.H.; Guigueno, M.F. Foraging niche partitioning in sympatric seabird populations. Sci. Res. 2021, 11, 2493. [Google Scholar] [CrossRef] [PubMed]





| Class | Family | Genus | Species |
|---|---|---|---|
| Hesperornithiformes | Enaliornithidae | Enaliornis | barretti |
| sedgewicki | |||
| seeleyi | |||
| Baptornithidae | Baptornis | advenus | |
| varneri | |||
| Judinornis | nogontsavensis | ||
| Parascaniornis | stensoei | ||
| Brodavidae | Brodavis | americanus | |
| baileyi | |||
| mongoliensis | |||
| varneriv | |||
| Hesperornithidae | Asiahesperornis | bazhanoviv | |
| Canadaga | arctica | ||
| Coniornis | altus | ||
| Hargeria | gracilis | ||
| Hesperornis | altus | ||
| bairdi | |||
| chowi | |||
| crassipes | |||
| gracilis | |||
| macdonaldi | |||
| mengeli | |||
| montana | |||
| regalis | |||
| rossicus | |||
| Lestornis | crassipes | ||
| Parahesperornis | alexi | ||
| NA | Pasquiaornis | hardei | |
| tankei | |||
| NA | Chupkaornis | keraorum | |
| NA | Fumicollis | hoffmani | |
| NA | Potamornis | skutchi |
| Continental | ||
|---|---|---|
| Mesa Verde Formation (Teapot Sandstone) [57] | Hesperornis regalis | Campanian |
| Hesperornis sp. | ||
| Nemegt Formation [75] | Brodavis mongoliensis | Maastrichtian |
| Judinornis nogontsavensis | ||
| Hesperornithidae indet. | ||
| Lance Formation [76] | Potamornis skutchi | Late Maastrichtian |
| Frenchman Formation [77] | Brodavis americanus | Late Maastrichtian |
| Transitional | ||
| Foremost Formation [78] | Hesperornis sp. | Campanian |
| Judith River Formation [79] | Baptornis sp. | Campanian |
| Hesperornis altus | ||
| Dinosaur Provincial Park Formation [80] | Baptornis sp. | Late Campanian |
| Hell Creek Formation [81] | Brodavis baileyi | Maastrichtian |
| Hesperornis sp. | ||
| Marine | ||
| Cambridge Greensand Member (West Melbury Chalk Formation) [44] | Enaliornis barretti | Early Cenomanian |
| Enaliornis seeleyi | ||
| Enaliornis sedgewicki | ||
| Belle Fouche Formation (formerly Ashville Formation) [14] | Pasquiaornis hardiei | Late Cenomanian |
| Pasquiaornis tankei | ||
| Greenhorn Formation [48] | Baptornis sp. | Cenomanian |
| Kashima Formation [24] | Chupkaornis keraorum | Coniacian to Santonian |
| Vermillion River Formation [82] | Hesperornis regalis | Coniacian to Santonian |
| Hesperornis sp. | ||
| Ignek Formation [83] | Hesperornis sp. | Late Coniacian to Campanian |
| Smoky Hill Chalk, Niobrara Formation [84] | Baptornis advenus | Late Coniacian to early Campanian |
| Hesperornis crassipes | ||
| Hesperornis gracilis | ||
| Hesperornis regalis | ||
| Hesperornis sp. | ||
| Fumicollis hoffmani | ||
| Parahesperornis alexi | ||
| Parahesperornis sp. | ||
| Smoking Hills Formation [85] | Hesperornis regalis | Middle Santonian to early late Campanian |
| Eginsaiskaya [49] | Baptornis advenus | Latest Santonian to Early Campanian |
| Asiahesperornis bazhanovi | ||
| Rybushka Formation [86] | Hesperornis rossicus | Early Campanian |
| Hesperornis sp. | ||
| Kristianstad Basin (unreported formation) [61] | Baptornis sp. | Latest early Campanian |
| Hesperornis rossicus | ||
| Hesperornis sp. | ||
| Kanguk Formation [87] | Canadaga arctica | Early to middle Campanian |
| Hesperornis sp. | ||
| Clagget Shale [57] | Hesperornis sp. | Campanian |
| Pierre Shale [88] | Baptornis advenus | Campanian |
| Brodavis varneri | ||
| Hesperornis bairdi | ||
| Hesperornis chowi | ||
| Hesperornis lumgairi | ||
| Hesperornis macdonaldi | ||
| Hesperornis mengeli | ||
| Hesperornis regalis | ||
| Hesperornis rossicus | ||
| Hesperornis sp. | ||
| Chico Formation [89] | Hesperornis sp. | Campanian |
| Ozan Formation [90] | Hesperornis sp. | Campanian |
| Kita-ama Formation [91] | Hesperornithiformes undet. | Early Maastrichtian |
| Zhuravlovskaya Svita [92] | Asiahesperornis bazhanovi | Maastrichtian |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bell, A.; Chiappe, L.M. The Hesperornithiformes: A Review of the Diversity, Distribution, and Ecology of the Earliest Diving Birds. Diversity 2022, 14, 267. https://0-doi-org.brum.beds.ac.uk/10.3390/d14040267
Bell A, Chiappe LM. The Hesperornithiformes: A Review of the Diversity, Distribution, and Ecology of the Earliest Diving Birds. Diversity. 2022; 14(4):267. https://0-doi-org.brum.beds.ac.uk/10.3390/d14040267
Chicago/Turabian StyleBell, Alyssa, and Luis M. Chiappe. 2022. "The Hesperornithiformes: A Review of the Diversity, Distribution, and Ecology of the Earliest Diving Birds" Diversity 14, no. 4: 267. https://0-doi-org.brum.beds.ac.uk/10.3390/d14040267