Relation of Biospeckle Activity with Quality Attributes of Apples
Abstract
: Biospeckle is nondestructive optical technique based on the analysis of variations of laser light scattered from biological samples. Biospeckle activity reflects the state of the investigated object. In this study the relation of biospeckle activity (BA) with firmness, soluble solids content (SSC), titratable acidity (TA) and starch content (SC) during the shelf life of seven apple cultivars was studied. The results showed that the quality attributes change significantly during storage. Significant and pronounced positive correlation between BA and SC was found. This result shows that degradation of starch granules, which could be stimulated to vibration by intracellular cyclosis, causes a lesser number of laser light scattering centers and results in smaller apparent biospeckle activity.1. Introduction
Postharvest softening of apple [Malus domestica (Borkh.)] fruit is a serious problem for growers [1]. Hence, there is need to evaluate apple quality at different stages of pre- and post-harvest technology in order to provide product of the best quality to consumers [2]. Recently, a few interesting optical techniques and devices have been developed and successfully used for nondestructive evaluation of fruit and vegetables: Vis/NIR spectrophotometry [3], time-resolved reflectance spectroscopy [4], hyperspectral backscattering imaging [5,6], laser-induced light backscattering [7,8] or chlorophyll fluorescence [9–11]. Nikolai et al. [12] have reviewed most of the above techniques, collectively naming them NIR spectroscopy, and have shown their feasibility and areas where more research is still needed.
Biospeckle is another optical technique, but less known, that was introduced for nondestructive evaluation of biological materials about fifteen years ago [13,14]. In the method, coherent laser light illuminates an object of interest. The backscattered light interferes and a speckle pattern is created in an observation plane. If the sample does not show activity, the speckle pattern is stable in time. However in the case of biological samples, the speckle pattern consists of two components: the static one from stationary elements of the tissue and the variable one from moving particles of the tissue. The variable in time speckle pattern is characteristic for biological tissue and has been called as the biospeckle (see AVI supplementary material which presents biospeckle of apple) [13,14]. If material is transparent, as in the case of biological tissues, biospeckle activity provides more complex information from the bulk of an object. Bragga et al. [15] have summarized that processes related with movement of the scattering centers in the tissue, such as cytoplasmic streaming, organelle movement, cell growth and division during fruits maturation and biochemical reactions, that are responsible for a certain biospeckle activity. Brownian motions should be considered as a source of biospeckle activity too [14]. The knowledge about biospeckle in relation to fruit and vegetables is still limited. Especially, there is lack of robust calibration for certain pre- and postharvest problems. In general, it has been shown that biospeckle activity changes with an age or with some surface properties, for example an infection of a biological object. On the other hand there is lack of consistent biological interpretation of the phenomena.
So far, attempts to apply biospeckle methods in biological studies include measurements of blood flow in blood vessels [16], viability of seeds [17,18], activity of parasites in living tissues [19,20], analysis of maturation and bruising of fruits and vegetables [13,21,22]. These studies showed that decaying of a tissue conditions caused by age, illness/infection or damage, relates with lower biospeckle activity. Monitoring of apples during shelf life showed that the decrease in firmness correlates with decrease of biospeckle activity [23,24]. Surprisingly, up to now no complex comparison of biospeckle activity with other basic quality attribute for apples has been done. Hence the goal of this study was to check whether biospeckle activity correlates with the basic quality attributes: firmness, soluble solids content, titratable acidity and starch content of stored apple under shelf life conditions.
2. Material and Methods
2.1. Apples and Storage
Apple [Malus domestica (Borkh.)] cultivars: ‘Elstar’, ‘Free Redstar’, ‘Gala’, ‘Gold Milenium’, ‘Melfree’, ‘Rajka’ and ‘Szampion’, purchased from the orchard of the Research Institute of Pomology and Floriculture in Skierniewice, Poland, were used for the experiments. Fruits were harvested at the optimum window for the each cultivar and then stored under a normal atmosphere at a temperature of 2 °C for approximately two months prior to the experiments which were performed on all cultivars simultaneously. For each cultivar, about 36 apples of uniform size and free from visible blemishes were selected for the experiments. Then, apples were conditioned at room temperature for one day before a shelf life program consisting of 1, 3, and 6 days of storage. At each of the three days of the experiments, one-third of the apples was tested. First biospeckle activity was evaluated and then firmness was measured on the same spot on the apple equator. Then apples of a given cultivar were mixed together and the pulp was used for the other destructive tests: starch content (SC), soluble solids content (SSC) and titratable acidity (TA). These tests were performed at days 1 and 6 of the experiments.
2.2. Biospeckle
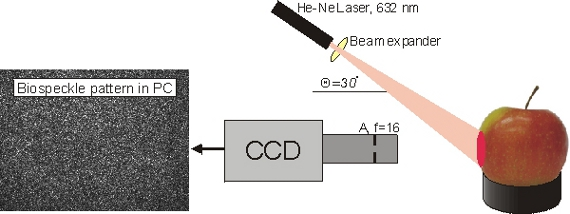
The biospeckle measurement device was similar to that which was previously used by Zdunek et al. [23,24] (Figure 1). The system consisted of a low power He-Ne laser (RBM R. Braumann GMBH, 632 nm, 0.98 mW), with a microscope objective 10X/0,24 (PZO, Poland) as the beam expander to illuminate the sample. Biospeckle were recorded by a CCD camera (Monochrome FireWire Astronomy Camera DMK 21AF04.AS, The Imaging Source Europe GmbH, Bremen, Germany) with a 25 mm defocused objective and a 20 mm extension ring. The camera-object distance was 37 mm and the laser-object one 180 mm. The incident angle was θ ≈ 30 degrees. A stack of uncompressed images (BMP, 8 bits) was recorded during 14 s at a rate of 15 fps. The image size was 640 × 480 pixels, which corresponded to a 32 mm2 observation area. Image exposure time of the CCD camera was 1/250 s. Gain and brightness of the CCD camera were optimized experimentally, in order to avoid overexposed pixels on an image histogram. The image acquisition settings were kept unchanged during the whole experiment.
Biospeckle activity was evaluated using the correlation coefficient Ckτ, where k = 0, 1, 2 … and τ = 1/15 s. Ckτ was calculated as the correlation coefficient of data matrix of the first frame (k = 0) with the data matrixes of the following frames (at kτ) from the bitmaps of biospeckle. In this study, C14 was analyzed only as the correlation coefficient between the first frame kτ = 0 and the frame at kτ = 14 s. Then, biospeckle activity BA = 1-C14 value was determined. Correlation coefficient Ckτ was calculated using the Matlab® R2010a software.
2.3. Firmness
Firmness (N) was measured with a contact acoustic emission detector (CAED, Institute of Agrophysics PAS, Lublin, Poland) [25,26]. The device has a force sensor of 200 N capacity with accuracy of 0.1% full scale. CAED punctures the fruit with a 11.1 mm diameter probe with a dome-shaped tip with a radius of curvature of 8.73 mm which is inserted 8 mm into the apple at a speed of approximately 20 mm/min. The experiment was performed on peeled apples.
2.4. Starch Content
Starch content (SC, %) of apple pulps was determined by the Ewers polarimetric method (ISO 10520:1997) adjusted to low-starch materials. Apple pulp (about 30 g) was weighed in a volumetric flask, suspended in 1.124% HCl (50 mL) and boiled for 15 min. After boiling deionised water (10 mL) was added to the starch suspension which was then cooled. Solutions of 15% K4Fe(CN)6 (2 mL) and 30% (CH3COO)2Zn (2 mL) were added to cold suspension, vigorously mixed and topped up with water to 100 mL. The mixture was then filtered, placed in a 200 mm polarimetric tube and the angle of rotation of polarized light was measured.
2.5. Soluble Solids Content
Soluble solids content (SSC, Brix) was determined using a digital refractometer (PAL-BX/RI, Atago Co. Ltd., Tokyo, Japan). Apple pulp filtrate was poured onto a prism of the refractometer and soluble solid content was immediately measured. The measurement was performed five times for each sample.
2.6. Titratable Acidity
Titratable acidity (TA, g/100g) was determined according to Polish Standard PN-90/A-75101/04. Apple pulp (approximately 40 g) was weighed with 0.001 g accuracy. Deionised water (100 g) was added to the volumetric flask with the pulp. The suspension was heated to boiling and then cooled. Deionised water was added up to 250 mL. After pulp (15 mL) suspension was filtered and water extract (50 mL) were titrated to pH 8.1 with 0.1 M NaOH. The results were calculated as malic acid and expressed as g/100 g apple fresh weight.
2.7. Statistical Analysis
Statistical analysis was performed using Unscrambler X 10.0.1 (Camo Process SA, Trondheim, Norway) and Statistica 9.0 (StatSoft, Inc., Tulsa, OK, USA). The mean values and standard deviation (SD) were determined from 12 replicates at each day in the case of biospeckle activity and firmness measurements (for each individual apple). In the case of SC, SSC and TA, three replicates were measured from pulp of 12 apples at day 1 and 6. Principal component analysis PCA on normalized data to their standard deviations was performed and matrix of Pearson’s correlation coefficients (R) was calculated to evaluate the relations between variables. A shelf life effect for individual cultivars was investigated with one-way ANOVA followed by the post hoc Tukey’s Honestly Significant Difference (HSD) test. Additionally, shelf life, cultivar and shelf life*cultivar effects were investigated with a two-way ANOVA.
3. Results
Figure 2(a) presents the PCA plot of scores grouped according to shelf life days. PC1 and PC2 together explain 70% of the samples’ variance. Samples show clear separation according to shelf life storage from right to left on PC1.
Table 1 presents the mean values of apples maturity indexes. Firmness, titratable acidity TA and starch content SC decreased, whereas soluble solids content SSC increased during shelf life. The changes are significant (p < 0.05) with the exception of two cases: SSC for ‘Melfree’ and ‘Rajka’ (p > 0.05). These results are typical for apples during maturation and postharvest ripening and confirm many revious observations [1,27–29]. These quality attributes are used extensively for prediction of the harvest window or to monitor quality during storage however the main disadvantage is their destructive character. Table 1 shows that biospeckle activity BA decreases during storage similarly to previous results [23,24]. The change of BA is gradual and significant after 6 days of shelf life, with exception of cv. ‘Melfree’ which did not change. After 3 days of shelf life significant change was observed only in a few cases. Similarly, firmness significantly decreased after 6 days in most cases and rarely after 3 days of storage. It implies that BA could be used for nondestructive evaluation of apples quality with accuracy comparable to other techniques.
Figure 2(b) presents loadings of each variable projected on PC1 and PC2. Each variable explains more than 50% of the displayed components. Loading of biospeckle activity BA has the same sign and lies close to starch content SC loading along PC1. It means that sample grouping in Figure 2(a) along PC1 relates with SC and BA. The rest of variables, particularly SSC and TA do not correlate with SC and BA and they both lie along PC2. Table 2 presents the matrix of Pearsons’ correlation coefficients calculated for the whole data set presented in Table 1.
Correlation between BA and SC is significant (R = 0.849, p < 0.01), whereas the rest of the mutual correlations are very weak and not significant (p > 0.05). It must be emphasized that in this study firmness neither correlated significantly with quality attributes of apples nor with biospeckle activity, despite the significant single-cultivar shelf life effect for the each variable and the same trend with other variables (Table 1). This lack of correlation could be the result of mixing of different cultivars for correlation matrix calculation which was shown by ANOVA as the cultivar effect (Table 1).
On one hand, firmness is a mechanical parameter and may not obviously correlate directly with sweetness or acidity of fruits. On the other hand, even if firmness during storage follows change of SSC, TA or SC, the dynamic may be different for cultivars. Thus in this case, common model for many cultivars for SSC, TA or SC prediction with firmness rather does not make sense.
From this point of view the significant correlation between BA and SC for the mixed data set for seven cultivars is very interesting. Figure 3 shows that the relation BA vs. SC is linear; higher starch content relates with higher biospeckle activity. The points for particular cultivars are randomly located along the linear regression line. The linear trend is clearly visible for apples just removed from cold storage (D1 in Figure 3). It means that biospeckle activity is indeed related with apparent starch content and individual cultivar related properties do not influence on the relationship in this case.
4. Discussion
Chloroplasts, other organelles and intracellular particles move together with cytoskeleton in plant cells. The cytoplasmic movement (cyclosis) is needed to maintain optimum conditions for tissue life. Cyclosis causes transports nutrients, enzymes, and larger particles within cells, enhances the exchange of materials between organelles, as well as between cells. In some plant cells the movement is rotary and limited to the peripheral parts of the cell next to the cell wall. This movement may be increased by light, and is dependent on temperature and pH, for example. Laser light is elastically scattered (according to Rayleigh when the target diameter is much smaller than the light wavelength or Mie when the target is similar or larger than the light wavelength) on each boundary, like intracellular membranes, organelles and other particles. If any object due to cyclosis is moving, the scattering causes an unstable biospeckle pattern. From this point of view, the biospeckle activity is a function of particles’ activity (mobility) and vitality of a tissue. Apples, like many other fruit crops, accumulate starch at early stages of maturation and progressively degrade starch to increase sweetness during ripening [30–32]. Starch granules are formed in amyloplasts within cells and have a size from 1 μm to 100 μm. For apples the average size of about 2 to 12 μm was found [31,32]. Large particles within cells obviously affect optical properties of the tissue. Laser light of 632 nm is scattered on starch granules which are bigger than 2 μm according to Mie’s theory. Starch does not move around together with organelles however the cyclosis presumably cause some vibrations of the granules. Thus, apart other moving organelles, starch granules would give many additional non-stationary scattering centers. In result, more starch particles means higher apparent biospeckle activity (Figure 3). Probably some other ripening-related processes like cell wall polysaccharides depolymerisation or malic acid enzymatic degradation would alter laser light scattering inside apple tissue if any particles are actuated in cells, according to hypothesis that even Brownian motion could be the source of non-stationary laser light scattering. However this should be studied more in the future to give consistent evidence.
In this study, chlorophyll content was not measured. There is evidence that less chlorophyll content causes higher apparent biospeckle activity [33] due to light absorption by this pigment and in consequence shallower light penetration through a tissue. In apples, postharvest storage is associated with chlorophyll degradation, hence it gives an opposite effect to the starch effect on BA, i.e., presumably the effects compensate each other. It suggests that if apples used in this experiment contained any chlorophyll, a real starch effect on biospeckle activity would be even more pronounced than this shown in Figure 3.
5. Conclusions
This study showed that biospeckle activity for apples depends significantly on starch content and the relation is general for various cultivars, which is not true in the case of firmness, soluble solids content and titratable acidity. Postharvest ripening of fruits is a very complex phenomenon and some processes significantly change the physical composition of the tissue. At present one can summarize this study and previous observations that starch degradation decreases the biospeckle activity of apples whereas chlorophyll degradation causes its increase. Another issue, which has not been studied yet, is what happens with molecules after postharvest pectin or starch degradation: do they contribute to biospeckle activity? Postharvest water evaporation while free air storage presumably would decrease particle mobility, but there is no clear evidence yet to prove this. In summary, it seems that biospeckle method is very promising for nondestructive monitoring of ripening processes however more specific research should be done to obtain a robust calibration due to competitive effects of starch and chlorophyll degradations on biospeckle activity.
Acknowledgments
The study was supported under the project NR 12 0137 10 founded by The National Centre for Research and Development (NCBiR).
References
- Johnston, JW; Hewett, EW; Hertog, MLATM. Postharvest softening of apple (Malus domestica) fruit: A review. New Zeal. J. Crop Hort. Sci 2002, 30, 145–160. [Google Scholar]
- Abbott, JA. Quality measurement of fruits and vegetables. Postharvest Biol. Technol 1999, 15, 207–225. [Google Scholar]
- Zude-Sasse, Z; Truppel, I; Herold, B. An approach to non-destructive apple fruit chlorophyll determination. Postharvest Biol. Technol 2002, 25, 123–133. [Google Scholar]
- Zerbini, PE; Grassi, M; Cubeddu, R; Pifferi, A; Torricelli, A. Time-resolved reflectance spectroscopy can detect internal defects in pears. Acta Horticul 2003, 599, 359–365. [Google Scholar]
- Peng, Y; Lu, R. Analysis of spatially resolved hyperspectral scattering images for assessing apple fruit firmness and soluble solids content. Postharvest Biol. Technol 2008, 48, 52–62. [Google Scholar]
- Firtha, F; Fekete, A; Kaszab, T; Gillay, B; Nogula-Nagy, M; Kovács, Z; Kantor, DB. Methods for improving image quality and reducing data load of NIR hyperspectral images. Sensors 2008, 8, 3287–3298. [Google Scholar]
- Qing, Z; Ji, B; Zude, M. Predicting soluble solid content and firmness in apple fruit by means of laser light backscattering image analysis. J. Food Eng 2007, 82, 58–67. [Google Scholar]
- Baranyai, L; Zude, M. Analysis of laser light propagation in kiwifruit using backscattering imaging and Monte Carlo simulation. Comput. Electron Agr 2009, 69, 33–39. [Google Scholar]
- Herppich, WB. Application Potential of Chlorophyll Fluorescence Imaging Analysis in Horticultural Research. Proceedings of the 6th International Symposium: Fruit, Nut and Vegetable Production Engineering, Potsdam, Germany, 11–19 September 2001; pp. 609–614.
- Herppich, WB; Linke, M; Landahl, S; Gzik, A. Preharvest and postharvest responses of radish to reduced water supply during growth. Acta Horticult 2001, 553, 89–90. [Google Scholar]
- Bauriegel, E; Giebel, A; Herppich, WB. Hyperspectral and chlorophyll fluorescence imaging to analyse the impact of Fusarium culmorum on the photosynthetic integrity of infected wheat ears. Sensors 2011, 11, 3765–3779. [Google Scholar]
- Nicolai, BM; Beullens, K; Bobelyn, E; Peirs, A; Saeys, W; Theron, KI; Lammertyna, J. Nondestructive measurement of fruit and vegetable quality by means of NIR spectroscopy: A review. Postharvest Biol. Technol 2007, 46, 99–118. [Google Scholar]
- Xu, Z; Joenathan, C; Khorana, BM. Temporal and spatial properties of the time-varying speckles of botanical specimens. Opt. Eng 1995, 34, 1487–1502. [Google Scholar]
- Zhao, Y; Wang, J; Wu, X; Williams, FW; Schmidt, RJ. Point-wise and whole-field laser speckle intensity fluctuation measurements applied to botanical specimens. Opt. Lasers Eng 1997, 28, 443–456. [Google Scholar]
- Braga, RA; Dupuy, L; Pasqual, M; Cardosos, RR. Live biospeckle laser imaging of root tissues. Eur. Biophys. J 2009, 38, 679–686. [Google Scholar]
- Briers, JD; Fercher, AF. Retinal blood-flow visualization by means of laser speckle photography. Investig. Ophthalmol. Vis. Sci 1982, 22, 255–259. [Google Scholar]
- Braga, RA; DalFabbro, IM; Borem, FM; Rabelo, G; Arizaga, R; Rabal, HJ; Trivi, M. Assessment of seed viability by laser speckle techniques. Biosyst. Eng 2003, 86, 287–294. [Google Scholar]
- Sendra, GH; Arizaga, R; Rabal, HJ; Trivi, M. Decomposition of biospeckle images in temporary spectral bands. Opt. Lett 2005, 30, 1641–1643. [Google Scholar]
- Pomarico, JA; DiRocco, HO; Alvarez, L; Lanusse, C; Mottier, L; Saumell, C; Arizaga, R; Rabal, H; Trivi, M. Speckle interferometry applied to pharmacodynamic studies: Evaluation of parasite motility. Eur. Biophys. J 2004, 33, 694–699. [Google Scholar]
- Braga, RA; Rabelo, GF; Granato, LR; Santos, EF; Machado, JC; Arizaga, R; Rabal, HJ; Trivi, M. Detection of fungi in beans by the laser biospeckle technique. Biosyst. Eng 2005, 91, 465–469. [Google Scholar]
- Pajuelo, M; Baldwin, G; Rabal, H; Cap, N; Arizaga, R; Trivi, M. Bio-speckle assessment of bruising in fruits. Opt. Lasers Eng 2003, 40, 13–24. [Google Scholar]
- Rabelo, GF; Braga, RA, Jr; Fabbro, IMD; Arizaga, R; Rabal, HJ; Trivi, MR. Laser speckle techniques applied to study quality of fruits. Revista Brasileira de Engenharia Agrícola e Ambiental 2005, 9, 570–575. [Google Scholar]
- Zdunek, A; Muravsky, L; Frankevych, L; Konstankiewicz, K. New nondestructive method based on spatial-temporal speckle correlation technique for evaluation of apples quality during shelf-life. Int. Agrophy 2007, 21, 305–310. [Google Scholar]
- Zdunek, A; Frankevych, L; Konstankiewicz, K; Ranachowski, Z. Comparison of puncture test, acoustic emission and spatial-temporal speckle correlation technique as methods for apple quality evaluation. Acta Agrophys 2008, 11, 303–315. [Google Scholar]
- Zdunek, A; Cybulska, J; Konopacka, D; Rutkowski, K. New contact acoustic emission detector for texture evaluation of apples. J. Food Eng 2010, 99, 83–91. [Google Scholar]
- Zdunek, A; Cybulska, J; Konopacka, D; Rutkowski, K. Inter-laboratory analysis of sensory texture of stored apples. Int. Agrophy 2011, 25, 67–75. [Google Scholar]
- Brummell, DA; Dal Cin, V; Crisosto, CH; Labavitch, JM. Cell wall metabolism during maturation, ripening and senescence of peach fruit. J. Exp. Bot 2004, 55, 2029–2039. [Google Scholar]
- Rutkowski, KP; Michalczuk, B; Konopacki, P. Nondestructive determination of ‘Golden Delicious’ apple quality and harvest maturity. J. Fruit Ornam. Plant Res 2008, 16, 39–52. [Google Scholar]
- Konopacka, D; Plocharski, WJ. Effect of storage conditions on the relationship between apple firmness and texture acceptability. Postharvest Biol. Technol 2004, 32, 205–211. [Google Scholar]
- Warrington, IJ; Fulton, TA; Halligan, EA; de Silva, HN. Apple fruit growth and maturity affected by early season temperatures. J. Amer. Soc. Hort. Sci 1999, 124, 468–477. [Google Scholar]
- Ernst, MK; Matitschka, G; Chatterton, NJ; Harrison, PA. A quantitative histochemical procedure for measurement of starch in apple fruit. Histochem. J 1999, 31, 705–710. [Google Scholar]
- Stevenson, DG; Domoto, PA; Jane, J. Structures and functional properties of apple (Malus domestica Borkh) fruit starch. Carbohydr. Polym 2006, 63, 432–441. [Google Scholar]
- Romero, GG; Martinez, CC; Alanis, EE; Salazar, GA; Broglia, VG; Alvarez, L. Bio-speckle activity applied to the assessment of tomato fruit ripening. Biosyst. Eng 2009, 103, 116–119. [Google Scholar]



| Cultivar | Shelf life (days) | BA ± SD | Firmness (N) ± SD | TA (g/100 g) ± SD | SSC (°Brix) ± SD | SC (%) ± SD |
|---|---|---|---|---|---|---|
| Elstar | 1 | 0.589 ± 0.037a | 50.7 ± 5.9a | 0.701 ± 0.002a | 14.4 ± 0.4a | 37.1 ± 3.0a |
| 3 | 0.556 ± 0.049ab | 42.1 ± 5.3b | ||||
| 6 | 0.542 ± 0.034b | 40.3 ± 5.2b | 0.650 ± 0.003b | 15.5 ± 0.1a | 21.2 ± 1.0b | |
| F-value | 4.2* | 11.6* | 221.0* | 19.0* | 144.0* | |
| Free Redstar | 1 | 0.595 ± 0.052a | 66.9 ± 6.7a | 0.576 ± 0.004a | 12.7 ± 0.1a | 37.1 ± 1.1a |
| 3 | 0.557 ± 0.100ab | 64.7 ± 7.3a | ||||
| 6 | 0.512 ± 0.054b | 57.1 ± 6.3b | 0.502 ± 0.001b | 13.6 ± 0.1b | 14.1 ± 1.2b | |
| F-value | 3.7* | 6.9* | 508.0* | 364.5* | 1,512.4* | |
| Gala | 1 | 0.614 ± 0.068a | 69.4 ± 7.6a | 0.276 ± 0.002a | 13.9 ± 0.3a | 37.5 ± 1.7a |
| 3 | 0.554 ± 0.047b | 70.6 ± 9.8a | ||||
| 6 | 0.516 ± 0.047b | 56.5 ± 7.4b | 0.258 ± 0.003b | 14.2 ± 0.1a | 25.4 ± 0.3b | |
| F-value | 9.8* | 10.7* | 908.8* | 2.7ns | 268.8* | |
| Gold Milenium | 1 | 0.613 ± 0.044a | 36.9 ± 3.2a | 0.411 ± 0.002a | 12.8 ± 0.1a | 46.1 ± 1.1a |
| 3 | 0.563 ± 0.050b | 33.3 ± 3.7a | ||||
| 6 | 0.579 ± 0.029b | 29.0 ± 3.8b | 0.355 ± 0.003b | 13.3 ± 0.1b | 16 .0 ± 0.6b | |
| F-value | 4.2* | 14.6* | 415.8* | 84.5* | 2,209.6* | |
| Melfree | 1 | 0.564 ± 0.064a | 49.6 ± 9.6a | 0.582 ± 0.002a | 13.2 ± 0.1a | 34.1 ± 0.6a |
| 3 | 0.537 ± 0.054a | 44.5 ± 12.2a | ||||
| 6 | 0.514 ± 0.045a | 34.7 ± 4.1b | 0.426 ± 0.001b | 13.9 ± 0.0b | 16 .0 ± 0.6b | |
| F-value | 2.3ns | 8.0* | 486.7* | 400.0* | 3,325.5* | |
| Rajka | 1 | 0.647 ± 0.096a | 52.4 ± 4.6a | 0.616 ± 0.005a | 14.7 ± 0.1a | 50.3 ± 0.6a |
| 3 | 0.577 ± 0.041ab | 50.7 ± 5.3a | ||||
| 6 | 0.539 ± 0.070b | 42.3 ± 4.5b | 0.410 ± 0.00b | 14.8 ± 0.1a | 16.2 ± 0.9b | |
| F-value | 6.4* | 15.2* | 1,287.6* | 1.0ns | 5,332.2* | |
| Szampion | 1 | 0.599 ± 0.060a | 46.3 ± 6.5a | 0.388 ± 0.000a | 12.9 ± 0.2a | 45.4 ± 1.7a |
| 3 | 0.557 ± 0.026ab | 38 .0± 3.5b | ||||
| 6 | 0.549 ± 0.046b | 30.4 ± 3.9b | 0.355 ± 0.004b | 13.5 ± 0.1b | 14.5 ± 0.9b | |
| F-value | 3.8* | 32.3* | 304.3* | 36.1* | 1,379* | |
| Effect | F-value of two-way ANOVA (day 3 excluded from analysis) | |||||
| Cultivar | 3.03* | 88.52* | 3858* | 132.0* | 23.09* | |
| Shelf life | 57.7* | 161.9* | 2705* | 133.5* | 2423* | |
| Shelf life*Cultivar | 1.50 | 1.50 | 256.9* | 6.8* | 44.35* | |
*means shelf life effect significant at p < 0.05. The same superscript letter means no significant difference.
| BA | Firmness | TA | SSC | |
|---|---|---|---|---|
| BA | --- | |||
| Firmness | 0.234 | --- | ||
| TA | 0.185 | 0.100 | --- | |
| SSC | −0.228 | −0.024 | 0.301 | --- |
| SC | 0.849** | 0.412 | 0.242 | −0.237 |
**means correlation is significant at p < 0.01.
© 2011 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/3.0/).
Share and Cite
Zdunek, A.; Cybulska, J. Relation of Biospeckle Activity with Quality Attributes of Apples. Sensors 2011, 11, 6317-6327. https://0-doi-org.brum.beds.ac.uk/10.3390/s110606317
Zdunek A, Cybulska J. Relation of Biospeckle Activity with Quality Attributes of Apples. Sensors. 2011; 11(6):6317-6327. https://0-doi-org.brum.beds.ac.uk/10.3390/s110606317
Chicago/Turabian StyleZdunek, Artur, and Justyna Cybulska. 2011. "Relation of Biospeckle Activity with Quality Attributes of Apples" Sensors 11, no. 6: 6317-6327. https://0-doi-org.brum.beds.ac.uk/10.3390/s110606317