Ammonia Gas Sensing Behavior of Tanninsulfonic Acid Doped Polyaniline-TiO2 Composite
Abstract
:1. Introduction
2. Experimental Section
2.1. Materials
2.2. Synthesis and Processing
2.3. Characterization
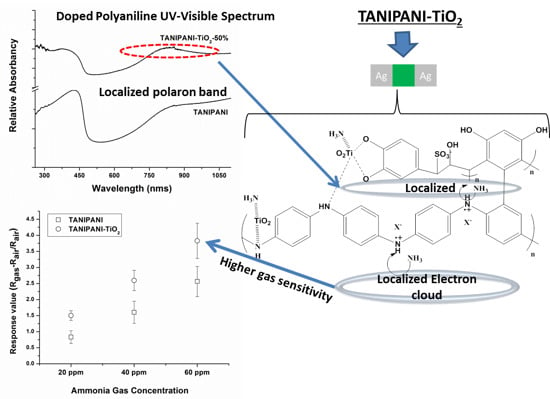
3. Results and Discussion






4. Ammonia Gas Sensing Application
4.1. Gas Sensor Fabrication

4.2. Gas Sensor Evaluation
4.3. Ammonia Gas Sensing Results and Discussion



| Samples | Response Value (20 ppm) | Response Value (40 ppm) | Response Value (60 ppm) | Reference |
|---|---|---|---|---|
| PANI/TiO2 | 0.12 | 0.29 | 0.375 | 30 |
| PANI/TiO2 | 1.67 (23ppm) | 2.33 (47 ppm) | N/A | 32 |
| PANI/TiO2 | 1.33 | 2.80 | N/A | 29 |
| PANI/TiO2 | 0.22 | 0.40 | 0.65 | 18 |
| TANIPANI/TiO2 | 1.50 | 2.59 | 3.82 | Current work |
| PANI | 0.49 | 0.74 | N/A | 32 |
| PANI | N/A | 0.83 | 0.88 | 17 |
| TANIPANI | 0.83 | 1.60 | 2.56 | Current work |
5. Conclusions
Author Contributions
Conflicts of Interest
References
- Faglia, G.; Baratto, C.; Comini, E.; Sberveglieri, G.; Zha, M.; Zappettini, A. Metal oxide nanocrystals for gas sensing. In Proceedings of the IEEE Sensors, Vienna, Austria, 24–27 October 2004; Volume 1, pp. 182–183.
- Fine, G.F.; Cavanagh, L.M.; Afonja, A.; Binions, R. Metal Oxide Semi-Conductor Gas Sensors in Environmental Monitoring. Sensors 2010, 10, 5469–5502. [Google Scholar] [CrossRef] [PubMed]
- Lu, H.F.; Li, F.; Liu, G.; Chen, Z.G.; Wang, D.W.; Fang, H.T.; Lu, G.Q.; Jiang, Z.H.; Cheng, H.M. Amorphous TiO2 nanotube arrays for low-temperature oxygen sensors. Nanotechnology 2008, 19. [Google Scholar] [CrossRef] [PubMed]
- Karunagaran, B.; Uthirakumar, P.; Chung, S.J.; Velumani, S.; Suh, E.K. TiO2 thin film gas sensor for monitoring ammonia. Mater. Charact. 2007, 58, 680–684. [Google Scholar] [CrossRef]
- Bai, H.; Shi, G. Gas Sensors Based on Conducting Polymers. Sensors 2007, 7, 267–307. [Google Scholar] [CrossRef]
- Bhadra, S.; Khastgir, D.; Singha, N.K.; Lee, J.H. Progress in preparation, processing and applications of polyaniline. Prog. Polym. Sci. 2009, 34, 783–810. [Google Scholar] [CrossRef]
- Tai, H.; Jiang, Y.; Xie, G.; Yu, J.; Zhao, M. Self-assembly of TiO2/polypyrrole nanocomposite ultrathin films and application for an NH3 gas sensor. Int. J. Environ. Anal. Chem. 2007, 87, 539–551. [Google Scholar] [CrossRef]
- Coltevieille, D.; le Méhauté, A.; Challioui, C.; Mirebeau, P.; Demay, J.N. Industrial applications of polyaniline. Synth. Met. 1999, 101, 703–704. [Google Scholar] [CrossRef]
- Fowler, J.D.; Virji, S.; Kaner, R.B.; Weiller, B.H. Hydrogen Detection by Polyaniline Nanofibers on Gold and Platinum Electrodes. J. Phys. Chem. C 2009, 113, 6444–6449. [Google Scholar] [CrossRef]
- Liu, M.-C.; Dai, C.-L.; Chan, C.-H.; Wu, C.-C. Manufacture of a Polyaniline Nanofiber Ammonia Sensor Integrated with a Readout Circuit Using the CMOS-MEMS Technique. Sensors 2009, 9, 869–880. [Google Scholar] [CrossRef] [PubMed]
- Stamenov, P.; Madathil, R.; Coey, J.M.D. Dynamic response of ammonia sensors constructed from polyaniline nanofibre films with varying morphology. Sens. Actuators B Chem. 2012, 161, 989–999. [Google Scholar] [CrossRef]
- Sutar, D.S.; Padma, N.; Aswal, D.K.; Deshpande, S.K.; Gupta, S.K.; Yakhmi, J.V. Preparation of nanofibrous polyaniline films and their application as ammonia gas sensor. Sens. Actuators B Chem. 2007, 128, 286–292. [Google Scholar] [CrossRef]
- Virji, S.; Huang, J.; Kaner, R.B.; Weiller, B.H. Polyaniline Nanofiber Gas Sensors: Examination of Response Mechanisms. Nano Lett. 2004, 4, 491–496. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G. Recent advances in polyaniline research: Polymerization mechanisms, structural aspects, properties and applications. Synth. Met. 2013, 177, 1–47. [Google Scholar] [CrossRef]
- Dhand, C.; Das, M.; Datta, M.; Malhotra, B.D. Recent advances in polyaniline based biosensors. Biosens. Bioelectron. 2011, 26, 2811–2821. [Google Scholar] [CrossRef] [PubMed]
- Crowley, K.; Morrin, A.; Hernandez, A.; O’Malley, E.; Whitten, P.G.; Wallace, G.G.; Smyth, M.R.; Killard, A.J. Fabrication of an ammonia gas sensor using inkjet-printed polyaniline nanoparticles. Talanta 2008, 77, 710–717. [Google Scholar] [CrossRef]
- Jin, Z.; Su, Y.; Duan, Y. Development of a polyaniline-based optical ammonia sensor. Sens. Actuators B Chem. 2001, 72, 75–79. [Google Scholar] [CrossRef]
- Bavane, R.G.; Shrisat, M.D.; Mahajan, A.M. Ammonia gas sensing characteristics of chemically synthesized polyaniline matrix. Sens. Transducers 2010, 113, 63–70. [Google Scholar]
- Abdulrazzaq, O.; Bourdo, S.E.; Saini, V.; Bairi, V.G.; Dervishi, E.; Viswanathan, T.; Nima, Z.A.; Biris, A.S. Optimization of the Protonation Level of Polyaniline-Based Hole-Transport Layers in Bulk-Heterojunction Organic Solar Cells. Energy Technol. 2013, 1, 463–470. [Google Scholar] [CrossRef]
- Chabukswar, V.V.; Pethkar, S.; Athawale, A.A. Acrylic acid doped polyaniline as an ammonia sensor. Sens. Actuators B Chem. 2001, 77, 657–663. [Google Scholar] [CrossRef]
- Pawar, S.G.; Patil, S.L.; Chougule, M.A.; Raut, B.T.; Godase, P.R.; Mulik, R.N.; Sen, S.; Patil, V.B. New Method for Fabrication of CSA Doped PANi-TiO2 thin-Film Ammonia Sensor. IEEE Sens. J. 2011, 11, 2980–2985. [Google Scholar] [CrossRef]
- Bairi, V.G.; Warford, B.A.; Bourdo, S.E.; Biris, A.S.; Viswanathan, T. Synthesis and characterization of tanninsulfonic acid doped polyaniline-metal oxide nanocomposites. J. Appl. Polym. Sci. 2012, 124, 3320–3328. [Google Scholar] [CrossRef]
- Taylor, K.K.; Cole, C.V.; Berry, B.C.; Tito, V. Use of tanninsulfonic acid in the synthesis of water-dispersible polyaniline. J. Appl. Polym. Sci. 2007, 103, 2113–2119. [Google Scholar] [CrossRef]
- Tito, V. Corrosion Prevention of Cold Rolled Steel Using Water Dispersible Lignosulfonic Acid Doped Polyaniline. Available online: https://www.google.com/patents/US7179404 (accessed on 15 October 2015).
- Andreas, L.; McCormick, D.R.; Pirece, J.R. Treatment and After-Treatment of Metal with Amine Oxide-Containing Polyphenol Compounds. Available online: https://www.google.com/patents/US4970264 (accessed on 15 October 2015).
- Taylor, K.K.; Cole, C.V.; Berry, B.C.; Viswanathan, T. Tanninsulfonic-acid doped polyaniline for protection of cold-rolled steel. Mater. Perform. 2007, 46, 38–42. [Google Scholar]
- Bourdo, S.E.; Saini, V.; Piron, J.; Al-Brahim, I.; Boyer, C.; Rioux, J.; Bairi, V.; Biris, A.S.; Viswanathan, T. Photovoltaic Device Performance of Single-Walled Carbon Nanotube and Polyaniline Films on n-Si: Device Structure Analysis. ACS Appl. Mater. Inter. 2011, 4, 363–368. [Google Scholar] [CrossRef] [PubMed]
- Dhawale, D.S.; Salunkhe, R.R.; Patil, U.M.; Gurav, K.V.; More, A.M.; Lokhande, C.D. Room temperature liquefied petroleum gas (LPG) sensor based on p-polyaniline/n-TiO2 heterojunction. Sens. Actuators B Chem. 2008, 134, 988–992. [Google Scholar] [CrossRef]
- Huyen, D.N.; Tung, N.T.; Thien, N.D.; Thanh, L.H. Effect of TiO2 on the Gas Sensing Features of TiO2/PANi Nanocomposites. Sensors 2011, 11, 1924–1931. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Gong, J.; He, G.; Deng, Y. Fabrication of polyaniline/titanium dioxide composite nanofibers for gas sensing application. Mater. Chem. Phys. 2011, 129, 477–482. [Google Scholar] [CrossRef]
- Pawar, S.G.; Chougule, M.A.; Patil, S.L.; Raut, B.T.; Godse, P.R.; Sen, S.; Patil, V.B. Room Temperature Ammonia Gas Sensor Based on Polyaniline-TiO2 Nanocomposite. IEEE Sens. J. 2011, 11, 3417–3423. [Google Scholar] [CrossRef]
- Srivastava, S.; Kumar, S.; Singh, V.N.; Singh, M.; Vijay, Y.K. Synthesis and characterization of TiO2 doped polyaniline composites for hydrogen gas sensing. Int. J. Hydrog. Energy 2011, 36, 6343–6355. [Google Scholar] [CrossRef]
- Tai, H.; Jiang, Y.; Xie, G.; Yu, J. Preparation, Characterization and Comparative NH3-sensing Characteristic Studies of PANI/inorganic Oxides Nanocomposite Thin Films. J. Mater. Sci. Technol. 2010, 26, 605–613. [Google Scholar] [CrossRef]
- Tai, H.; Jiang, Y.; Xie, G.; Yu, J.; Chen, X.; Ying, Z. Influence of polymerization temperature on NH3 response of PANI/TiO2 thin film gas sensor. Sens. Actuators B Chem. 2008, 129, 319–326. [Google Scholar] [CrossRef]
- Ćirić-Marjanović, G. Recent advances in polyaniline composites with metals, metalloids and nonmetals. Synth. Met. 2013, 170, 31–56. [Google Scholar] [CrossRef]
- Pawar, S.G.; Chougule, M.A.; Sen, S.; Patil, V.B. Development of nanostructured polyaniline-titanium dioxide gas sensors for ammonia recognition. J. Appl. Polym. Sci. 2012, 125, 1418–1424. [Google Scholar] [CrossRef]
- Lin, Q.; Li, Y.; Yang, M. Gas sensing properties of layer-by-layer self-assembled ultrathin film of polyaniline/titanium dioxide. Synth. Met. 2012, 162, 2242–2249. [Google Scholar] [CrossRef]
- Sun, X.; Qiao, L.; Wang, X. A Novel Immunosensor Based on Au Nanoparticles and Polyaniline/Multiwall Carbon Nanotubes/Chitosan Nanocomposite Film Functionalized Interface. Nano Micro Lett. 2013, 5, 191–201. [Google Scholar] [CrossRef]
- Chang, Q.; Zhao, K.; Chen, X.; Li, M.; Liu, J. Preparation of gold/polyaniline/multiwall carbon nanotube nanocomposites and application in ammonia gas detection. J. Mater. Sci. 2008, 43, 5861–5866. [Google Scholar] [CrossRef]
- Huang, X.; Hu, N.; Gao, R.; Yu, Y.; Wang, Y.; Yang, Z.; Siu-Wai, K.E.; Wei, H.; Zhang, Y. Reduced graphene oxide-polyaniline hybrid: Preparation, characterization and its applications for ammonia gas sensing. J. Mater. Chem. 2012, 22, 22488–22495. [Google Scholar] [CrossRef]
- Tai, H.; Jiang, Y.; Xie, G.; Yu, J.; Chen, X. Fabrication and gas sensitivity of polyaniline-titanium dioxide nanocomposite thin film. Sens. Actuators B Chem. 2007, 125, 644–650. [Google Scholar] [CrossRef]
- Gong, J.; Li, Y.; Hu, Z.; Zhou, Z.; Deng, Y. Ultrasensitive NH3 Gas Sensor from Polyaniline Nanograin Enchased TiO2 Fibers. J. Phys. Chem. C 2010, 114, 9970–9974. [Google Scholar] [CrossRef]
- Ma, X.; Wang, M.; Li, G.; Chen, H.; Bai, R. Preparation of polyaniline–TiO2 composite film with in situ polymerization approach and its gas-sensitivity at room temperature. Mater. Chem. Phys. 2006, 98, 241–247. [Google Scholar]
- Mattoso, L.H.C.; Manohar, S.K.; Macdiarmid, A.G.; Epstein, A.J. Studies on the chemical syntheses and on the characteristics of polyaniline derivatives. J. Polym. Sci. Part A Polym. Chem. 1995, 33, 1227–1234. [Google Scholar] [CrossRef]
- Rahy, A.; Yang, D.J. Synthesis of highly conductive polyaniline nanofibers. Mater. Lett. 2008, 62, 4311–4314. [Google Scholar] [CrossRef]
- Dimitriev, O.P. Doping of Polyaniline by Transition-Metal Salts. Macromolecules 2004, 37, 3388–3395. [Google Scholar] [CrossRef]
- Bairi, V.; Bourdo, S.; Moore, J.; Schnackenberg, L.; Berry, B.; Biris, A.; Viswanathan, T. Separation and spectroscopic/molecular weight analysis of crude and purified polyaniline(s). J. Polym. Res. 2013, 20, 1–8. [Google Scholar] [CrossRef]
- Zheng, J.; Li, G.; Ma, X.; Wang, Y.; Wu, G.; Cheng, Y. Polyaniline-TiO2 nano-composite-based trimethylamine QCM sensor and its thermal behavior studies. Sens. Actuators B Chem. 2008, 133, 374–380. [Google Scholar] [CrossRef]
© 2015 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bairi, V.G.; Bourdo, S.E.; Sacre, N.; Nair, D.; Berry, B.C.; Biris, A.S.; Viswanathan, T. Ammonia Gas Sensing Behavior of Tanninsulfonic Acid Doped Polyaniline-TiO2 Composite. Sensors 2015, 15, 26415-26429. https://0-doi-org.brum.beds.ac.uk/10.3390/s151026415
Bairi VG, Bourdo SE, Sacre N, Nair D, Berry BC, Biris AS, Viswanathan T. Ammonia Gas Sensing Behavior of Tanninsulfonic Acid Doped Polyaniline-TiO2 Composite. Sensors. 2015; 15(10):26415-26429. https://0-doi-org.brum.beds.ac.uk/10.3390/s151026415
Chicago/Turabian StyleBairi, Venu Gopal, Shawn E. Bourdo, Nicolas Sacre, Dev Nair, Brian C. Berry, Alexandru S. Biris, and Tito Viswanathan. 2015. "Ammonia Gas Sensing Behavior of Tanninsulfonic Acid Doped Polyaniline-TiO2 Composite" Sensors 15, no. 10: 26415-26429. https://0-doi-org.brum.beds.ac.uk/10.3390/s151026415