Development of a Dew/Frost Point Temperature Sensor Based on Tunable Diode Laser Absorption Spectroscopy and Its Application in a Cryogenic Wind Tunnel
Abstract
:1. Introduction
2. Principles of optical spectroscopy method
3. Absorption line selection
4. Experimental setup and data retrieve method
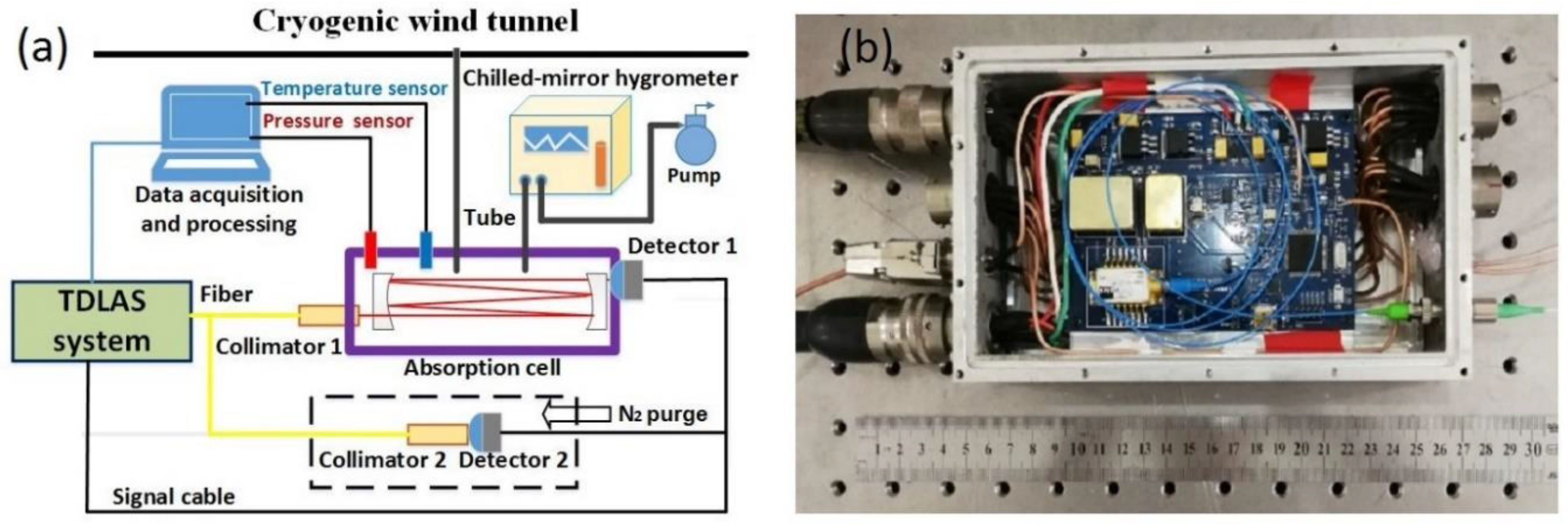
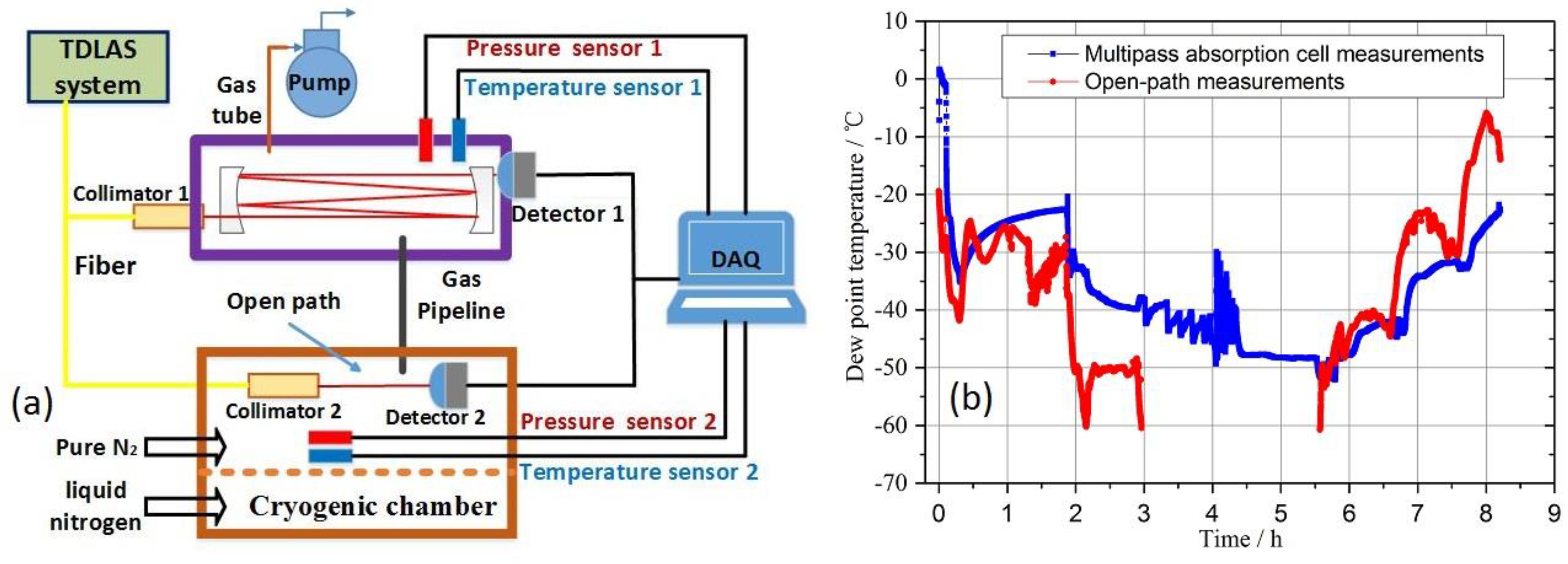
4.1. Experimental setup
4.2. The optical pathlength of multipass absorption cell
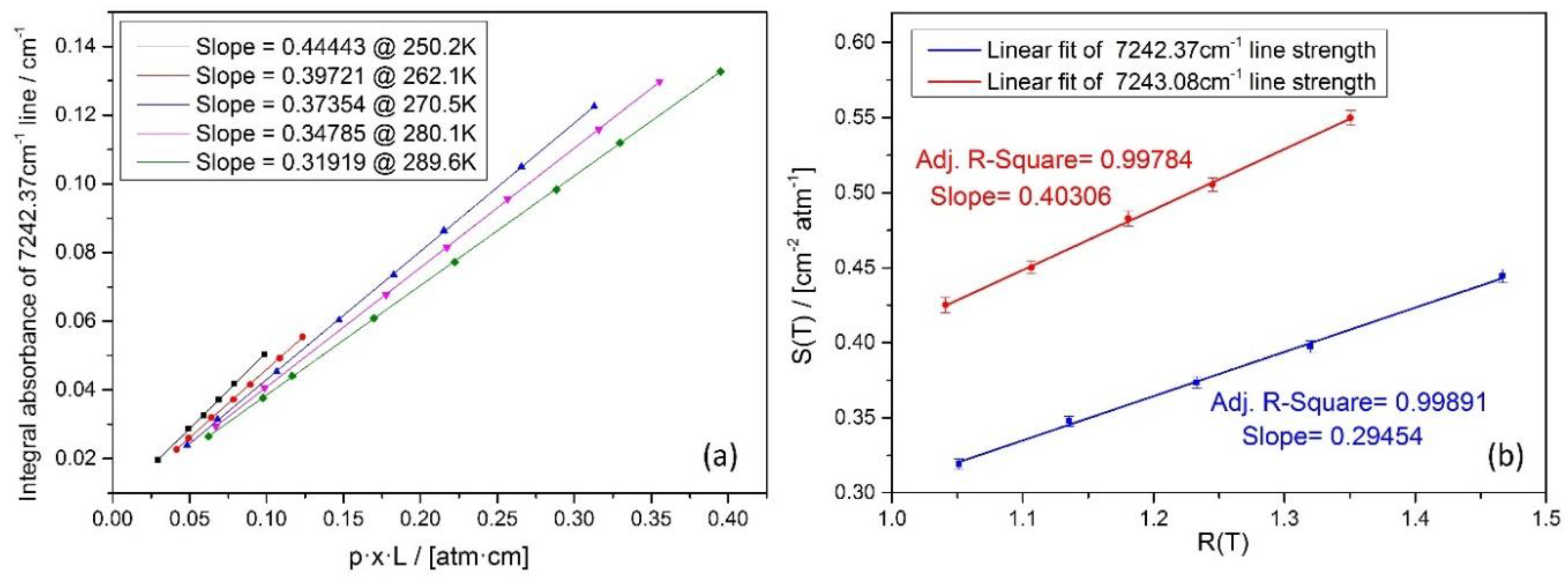
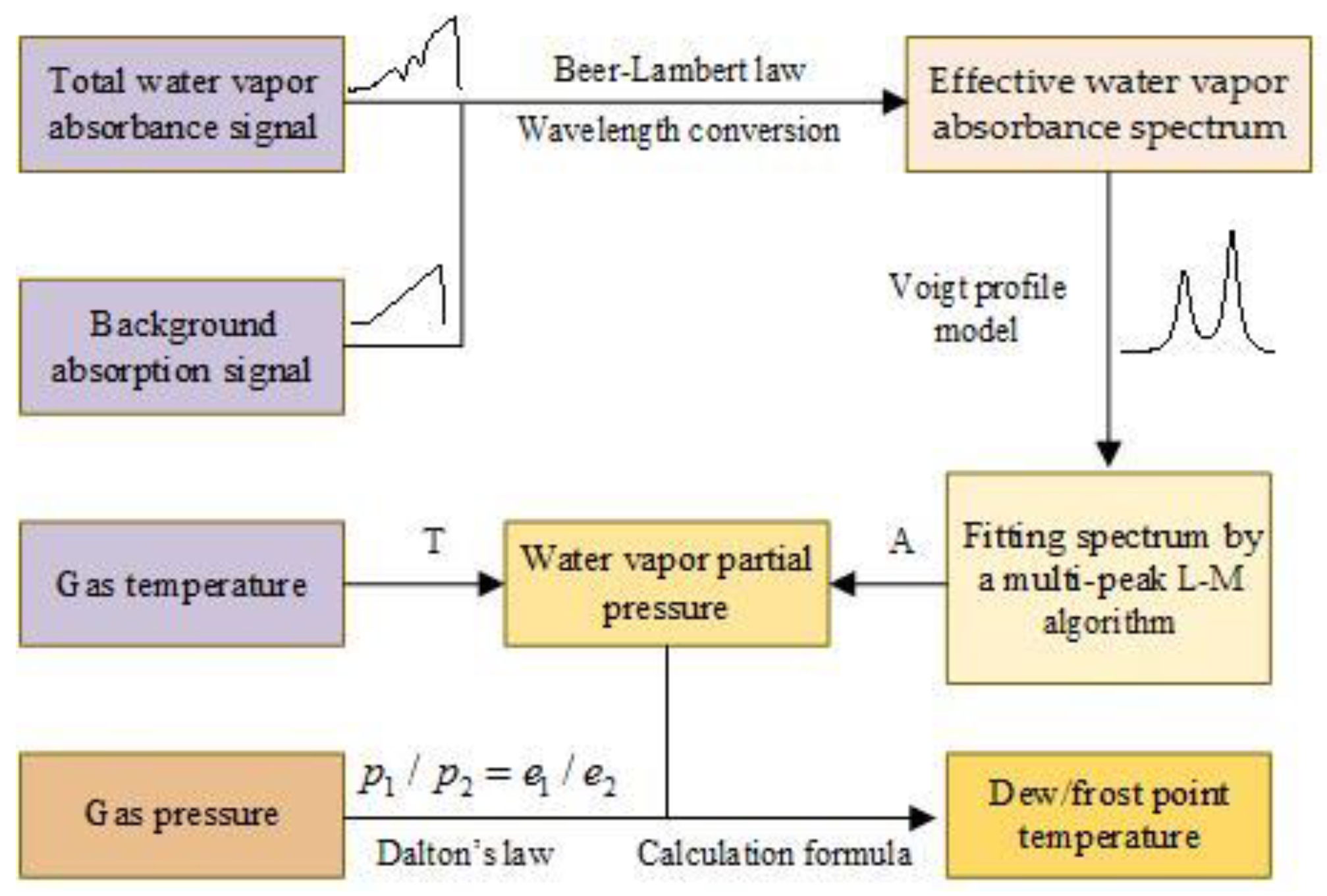
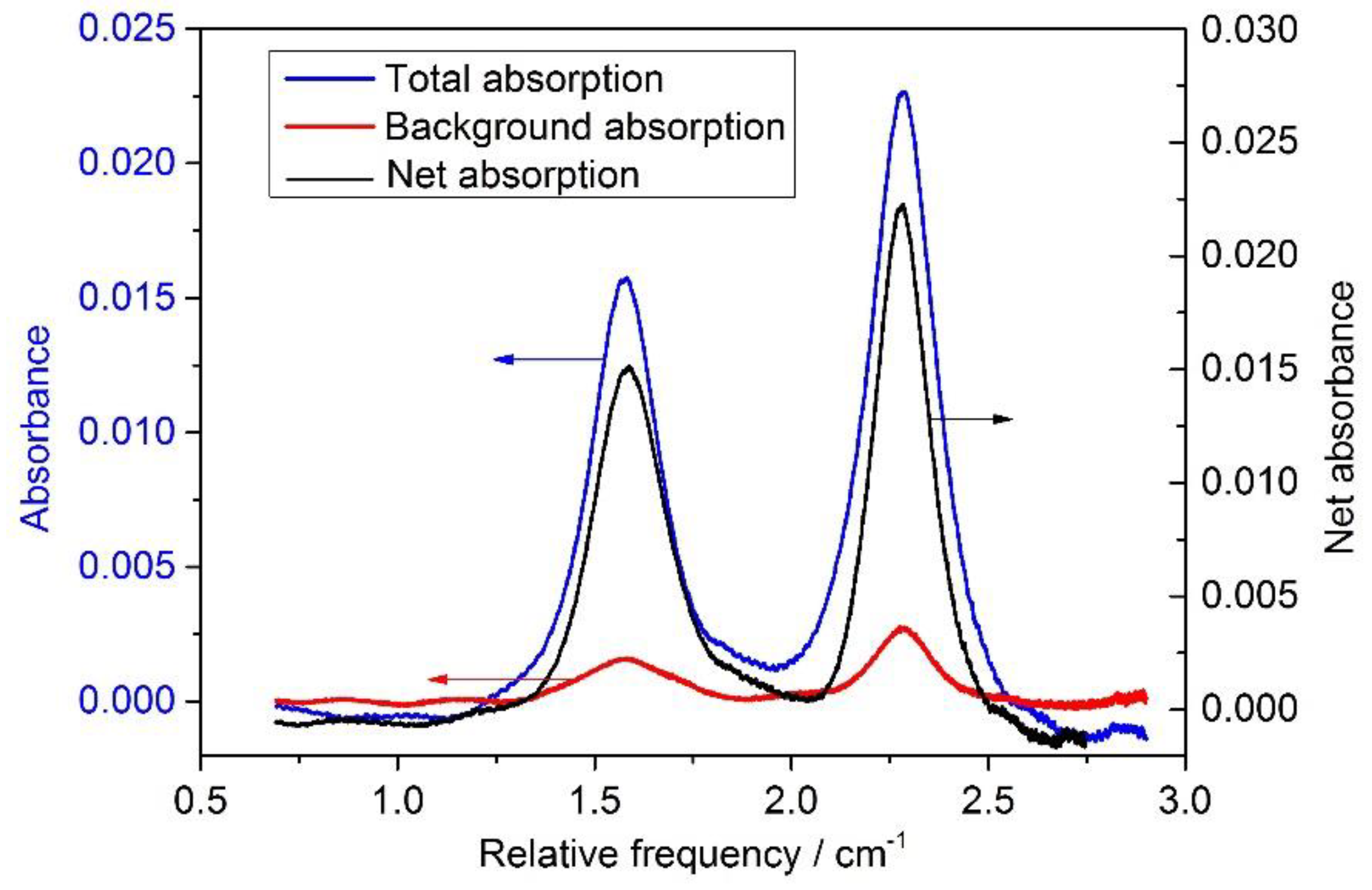
4.3. Data processing and evaluation
4.4. Uncertainty of the sensor
5. Results and discussion
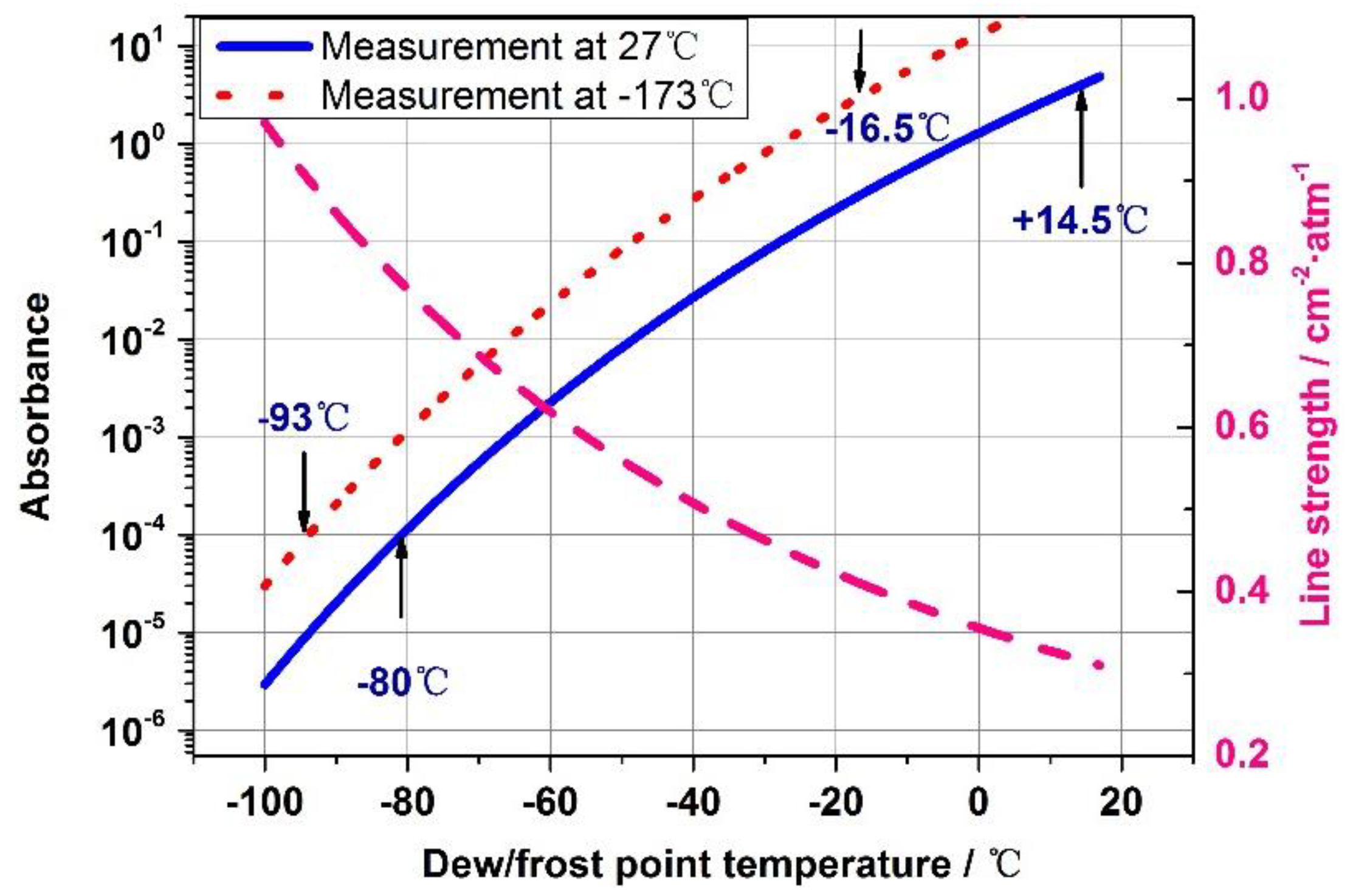
5.1. TDLAS dew/frost point temperature sensor detection limits analysis
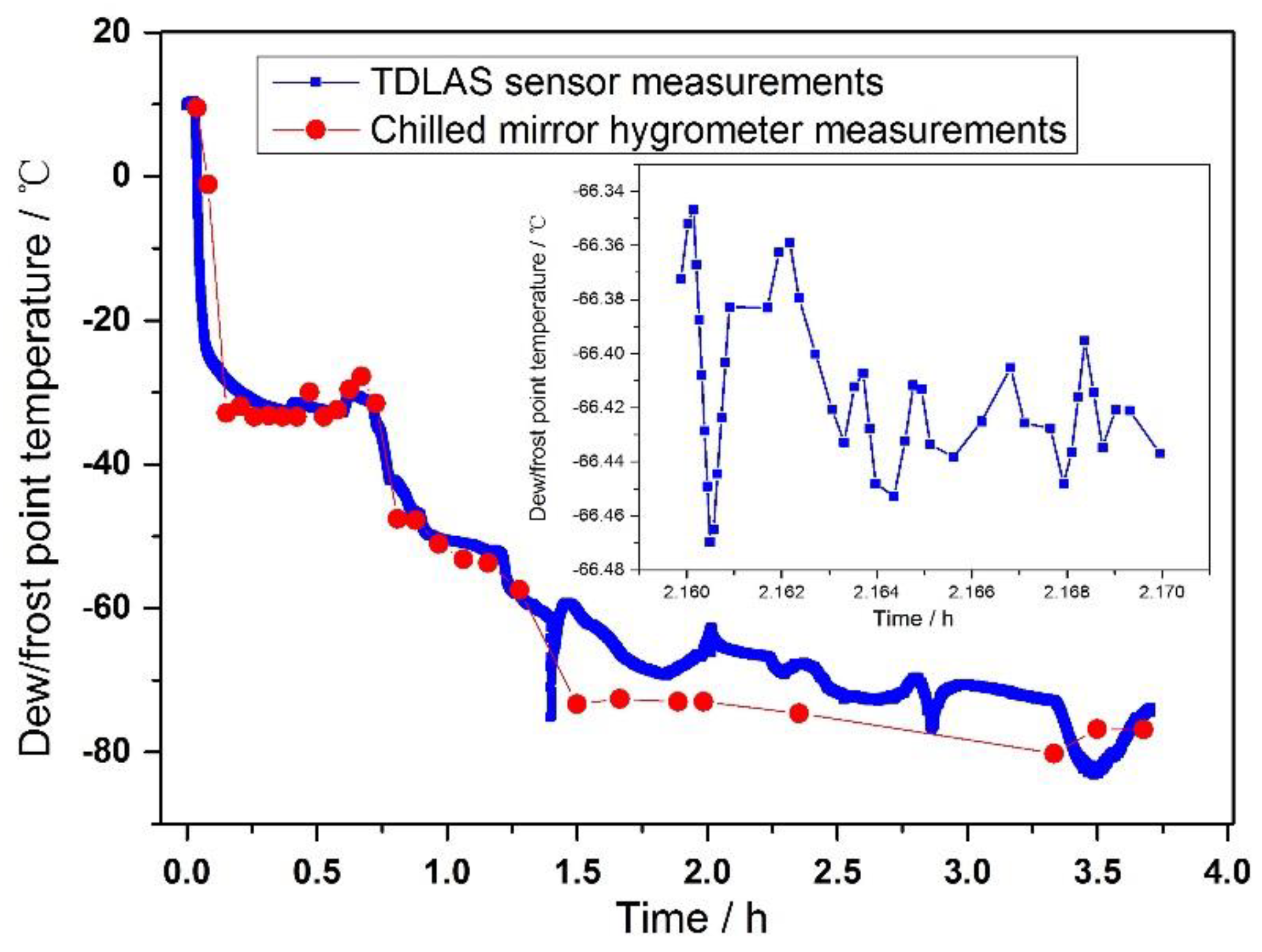
5.2. Measurement result
5.3. Deviation analysis
6. Conclusion and outlook
Author Contributions
Funding
Conflicts of Interest
References
- Liao, D.X.; Huang, Z.L.; Chen, Z.H.; Tang, G.S. Review on large-scale cryogenic wind tunnel and key technologies. J. Exper. Fluid Mech. 2014, 28, 1–6. [Google Scholar] [CrossRef]
- Kilgore, W.A.; Balakrishna, S. Effects of Water Vapor on Cryogenic Wind Tunnels. In Advances in Cryogenic Engineering; Kittel, P., Ed.; Springer: Boston, MA, USA, 1994; Volume 39, pp. 99–105. ISBN 1-4615–2522–6. [Google Scholar]
- Paryz, R.W. Recent Developments at the NASA Langley Research Center National Transonic Facility. In Proceedings of the 49th AIAA Aerospace Sciences Meeting Including the New Horizons Forum and Aerospace Exposition, Orlando, FL, USA, 4–7 January 2011; pp. 1–13. [Google Scholar] [CrossRef]
- Brandondooley, J.; O’Neal, D. The Transient Response of Capacitive Thin-Film Polymer Humidity Sensors. Hvac R Res. 2008, 14, 663–682. [Google Scholar] [CrossRef]
- Lee, C.Y.; Lee, G.B. Humidity Sensors: A Review. Sens. Lett. 2005, 3, 1–15. [Google Scholar] [CrossRef]
- Rittersma, Z.M. Recent achievements in miniaturised humidity sensors—a review of transduction techniques. Sens. Actuators A. 2002, 96, 196–210. [Google Scholar] [CrossRef]
- Xie, W.Y.; Liu, B.; Xiao, S.H.; Li, H.; Wang, Y.R.; Cai, D.P.; Wang, D.D.; Wang, L.L.; Liu, Y.; Li, Q.H.; et al. High performance humidity sensors based on CeO2 nanoparticles. Sens. Actuators B. 2015, 215, 125–132. [Google Scholar] [CrossRef]
- Sakai, Y.; Sadaoka, Y.; Matsuguchi, M. Humidity sensors based on polymer thin films. Sens. Actuators B. 1996, 35, 85–90. [Google Scholar] [CrossRef]
- Nie, J.; Meng, X.; Li, N. Quartz crystal sensor using direct digital synthesis for dew point measurement. Measurement 2018, 117, 73–79. [Google Scholar] [CrossRef]
- Stehle, J.; Samarao, A.K.; Yama, G.; Krishnamoorthy, U.; Ambacher, O. Development of a Silicon-Only Capacitive Dew Point Sensor. IEEE Sens. J. 2017, 17, 7223–7230. [Google Scholar] [CrossRef]
- Kim, J.C.; Choi, B.I.; Woo, S.B.; Kim, Y.G.; Lee, S.W. The Effect of Temperature on Aluminum Oxide and Chilled Mirror Dew-point Hygrometers. J. Sens. Sci. Technol. 2017, 26, 50–55. [Google Scholar] [CrossRef] [Green Version]
- Buchholz, B.; Kühnreich, B.; Smit, H.G.J.; Ebert, V. Validation of an extractive, airborne, compact TDL spectrometer for atmospheric humidity sensing by blind intercomparison. Appl. Phys. B 2012, 110, 249–262. [Google Scholar] [CrossRef] [Green Version]
- Buchholz, B.; Afchine, A.; AKlein, A.; Schiller, C.; Krämer, M.; Ebert, V. HAI, A new airborne, absolute, twin dual-channel, multi-phase TDLAS-hygrometer: background, design, setup, and first flight data. Atmos. Meas. Tech. 2017, 10, 35–57. [Google Scholar] [CrossRef]
- Thornberry, T.D.; Rollins, A.W.; Gao, R.S.; Watts, S.J.; McLaughlin, R.J.; Fahey, D.W. A two-channel, tunable diode laser-based hygrometer for measurement of water vapor and cirrus cloud ice water content in the upper troposphere and lower stratosphere. Atmos. Meas. Tech. 2014, 7, 211–224. [Google Scholar] [CrossRef]
- Yao, L.; Liu, W.Q.; Kan, R.F.; Xu, Z.Y.; Ruan, J.; Wang, L.; Jiang, Q. Research and development of a compact TDLAS system to measure scramjet combusition temperature. J. Exper. Fluid Mech. 2015, 29, 71–76. [Google Scholar] [CrossRef]
- Timothy, W., Jr. Large scale wind tunnel humidity sensor using laser diode absorption spectroscopy. Master’s Thesis, Vanderbilt University, Nashville, TN, USA, 2017. [Google Scholar]
- Wei, Y.B.; Chang, J.; Lian, J.; Wang, Q.; Wei, W. Study of a distributed feedback diode laser based hygrometer combined Herriot-gas cell and waterless optical components. Photonic Sens. 2016, 6, 214–220. [Google Scholar] [CrossRef] [Green Version]
- Rothman, L.S.; Gordon, I.E.; Babikov, Y.; Barbe, A.; Chris Benner, D.; Bernath, P.F.; Brik, M.; Bizzocchi, L.; Boudon, V.; Brown, L.R.; et al. The HITRAN2012 molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transfer. 2013, 130, 4–50. [Google Scholar] [CrossRef] [Green Version]
- Rothman, L.S.; Gordon, I.E.; Barber, R.J.; Dothe, H.; Gamache, R.R.; Goldman, A.; Perevalov, V.I.; Tashkun, S.A.; Tennyson, J. HITEMP, the high-temperature molecular spectroscopic database. J. Quant. Spectrosc. Radiat. Transfer. 2010, 111, 2139–2150. [Google Scholar] [CrossRef]
- Schreier, F. Computational aspects of speed-dependent Voigt profiles. J. Quant. Spectrosc. Radiat. Transfer. 2017, 187, 44–53. [Google Scholar] [CrossRef]
- Murphy, D.M.; Koop, T. Review of the vapour pressures of ice and supercooled water for atmospheric applications. Q. J. Roy. Meteor. Soc. 2010, 131, 1539–1565. [Google Scholar] [CrossRef]
- Greenspan, L. Functional equations for the enhancement factors for CO2-free moist air. J. Res. National Bur. Stand. A. Phys. Chem. 1976, 80, 41–44. [Google Scholar] [CrossRef]
- Xu, Z.Y.; Kan, R.F.; Ruan, J.; Yao, L.; Fan, X.L.; Liu, J.G. A Tunable Diode Laser Absorption Based Velocity Sensor for Local Field in Hypersonic Flows. Proceedings of Light, Energy and Environment, Leipzig, Germany, 14–17 November 2016. [Google Scholar] [CrossRef]
- Mchale, L.E.; Hecobian, A.; Yalin, A.P. Open-path cavity ring-down spectroscopy for trace gas measurements in ambient air. Opt. Express 2016, 24, 5523–5535. [Google Scholar] [CrossRef] [PubMed]


| Brand | Sensor type | Measurement range | Accuracy | Response time b | Operation environment |
|---|---|---|---|---|---|
| GE (MMY30) | Aluminum oxide sensor | −90 ~ +10 °C | ± 2 °C (25 °C) | - | −40 ~ +50 °C |
| PhyMetrix (DewPatrol) | Nanopore sensor | −110 °C ~ +20 °C | ± 2 °C | 3 min | −20 ~ +60 °C Non-corrosive |
| Michell (Easidew) | Ceramic moisture sensor | −100 °C ~ +20 °C | ≤ ± 2 °C | 5 min (dry to wet) | −40 ~ +60 °C Non-corrosive |
| Vaisala (DMT340) | Capacitive thin-film polymer sensor | −70 °C ~ +80 °C | < ± 3 °C | 10 min (wet to dry) | −40 ~ +80 °C Non-corrosive |
| SIDPH (FM860) | Dual ceramic nano thin-film sensor | −110 °C ~ +20 °C | ± 2 °C | 1 min (dry to wet) | −40 ~ +65 °C Non-corrosive |
| MBW (373LX) | Chilled mirror hygrometry sensor | −95 °C ~ +20 °C | ± 0.1 °C | ~ 1 min | 15 ~ +35 °C Non-corrosive |
| Frequency (cm−1) | Lower state energy (cm−1) | Line intensity @296 K (cm−1/ (molecule·cm−2)) | Uncertainty of intensity | ||
|---|---|---|---|---|---|
| HITRAN 2012 | Our work | HITRAN 2012 | Our work | ||
| 7242.37075 | 42.3717 | 1.19 × 10−20 | 1.188 × 10−20 | ≥ 5% and ≤ 10% | 1.023% |
| 7243.07526 | 134.9016 | 1.61 × 10−20 | 1.626 × 10−20 | ≥ 5% and ≤ 10% | 1.312% |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nie, W.; Xu, Z.; Kan, R.; Ruan, J.; Yao, L.; Wang, B.; He, Y. Development of a Dew/Frost Point Temperature Sensor Based on Tunable Diode Laser Absorption Spectroscopy and Its Application in a Cryogenic Wind Tunnel. Sensors 2018, 18, 2704. https://0-doi-org.brum.beds.ac.uk/10.3390/s18082704
Nie W, Xu Z, Kan R, Ruan J, Yao L, Wang B, He Y. Development of a Dew/Frost Point Temperature Sensor Based on Tunable Diode Laser Absorption Spectroscopy and Its Application in a Cryogenic Wind Tunnel. Sensors. 2018; 18(8):2704. https://0-doi-org.brum.beds.ac.uk/10.3390/s18082704
Chicago/Turabian StyleNie, Wei, Zhenyu Xu, Ruifeng Kan, Jun Ruan, Lu Yao, Bin Wang, and Yabai He. 2018. "Development of a Dew/Frost Point Temperature Sensor Based on Tunable Diode Laser Absorption Spectroscopy and Its Application in a Cryogenic Wind Tunnel" Sensors 18, no. 8: 2704. https://0-doi-org.brum.beds.ac.uk/10.3390/s18082704




