Portable Sensors Add Reliable Kinematic Measures to the Assessment of Upper Extremity Function
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Measurements
2.3. Study Design and Test Protocols
2.4. Data Processing
2.5. Statistics
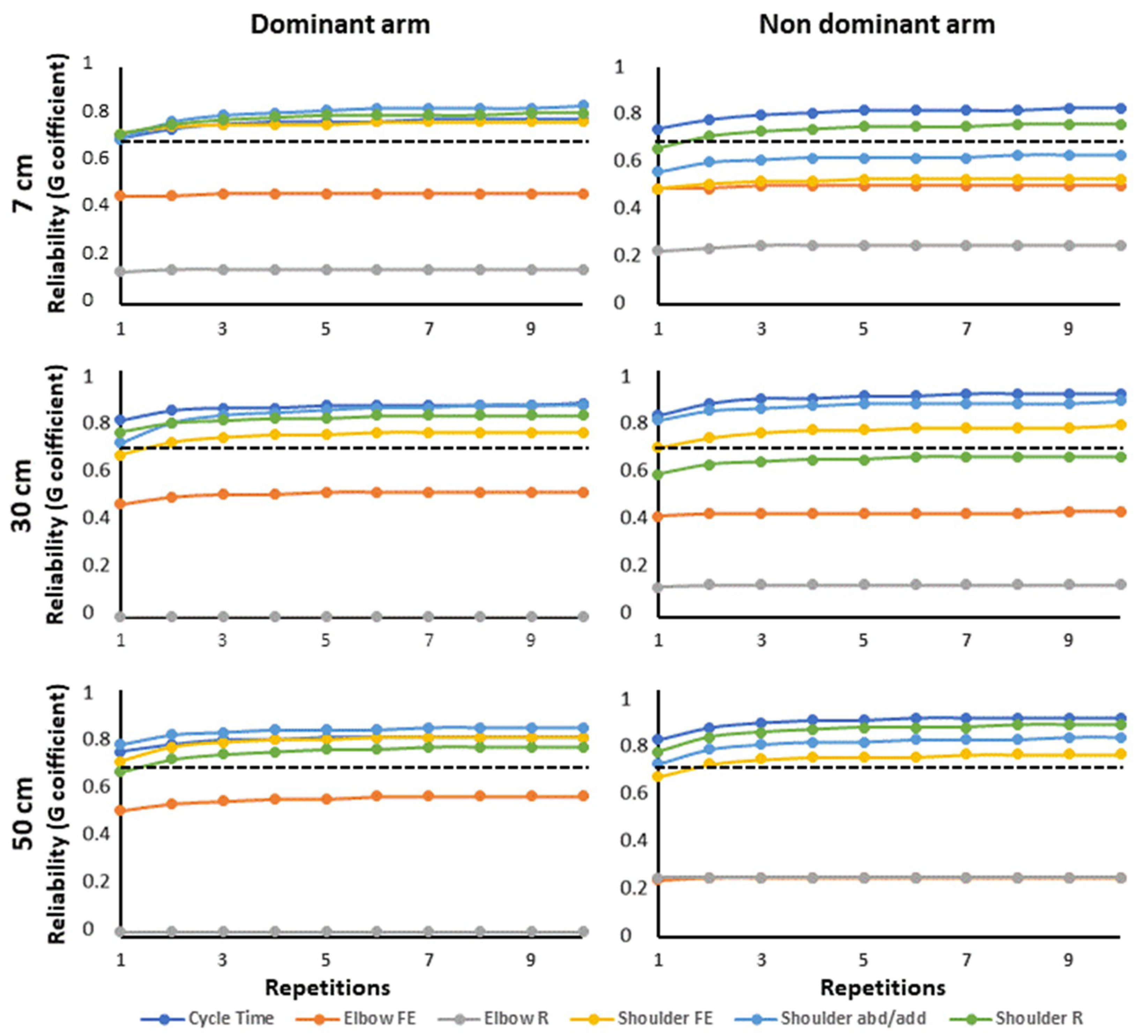
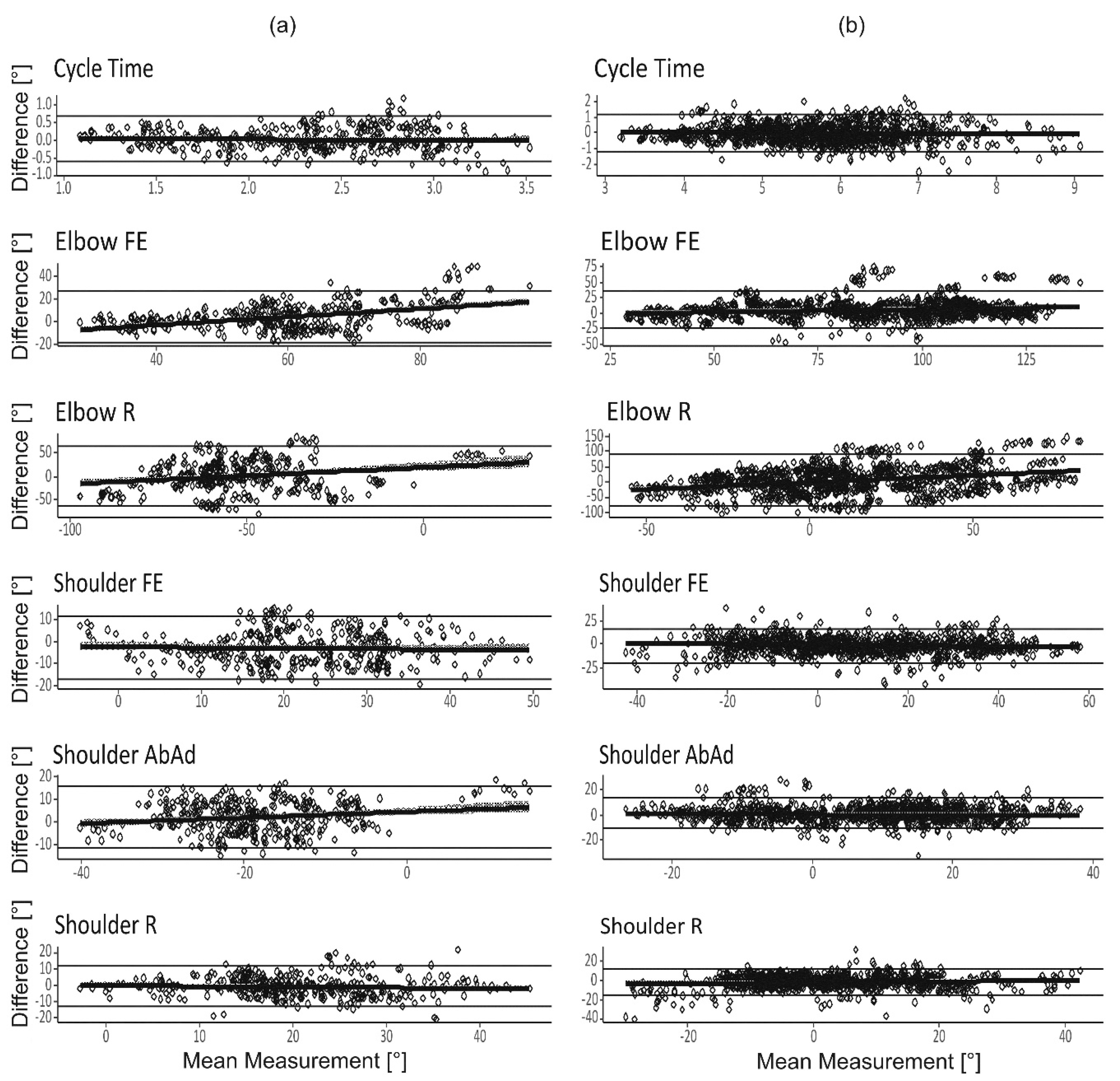
3. Results
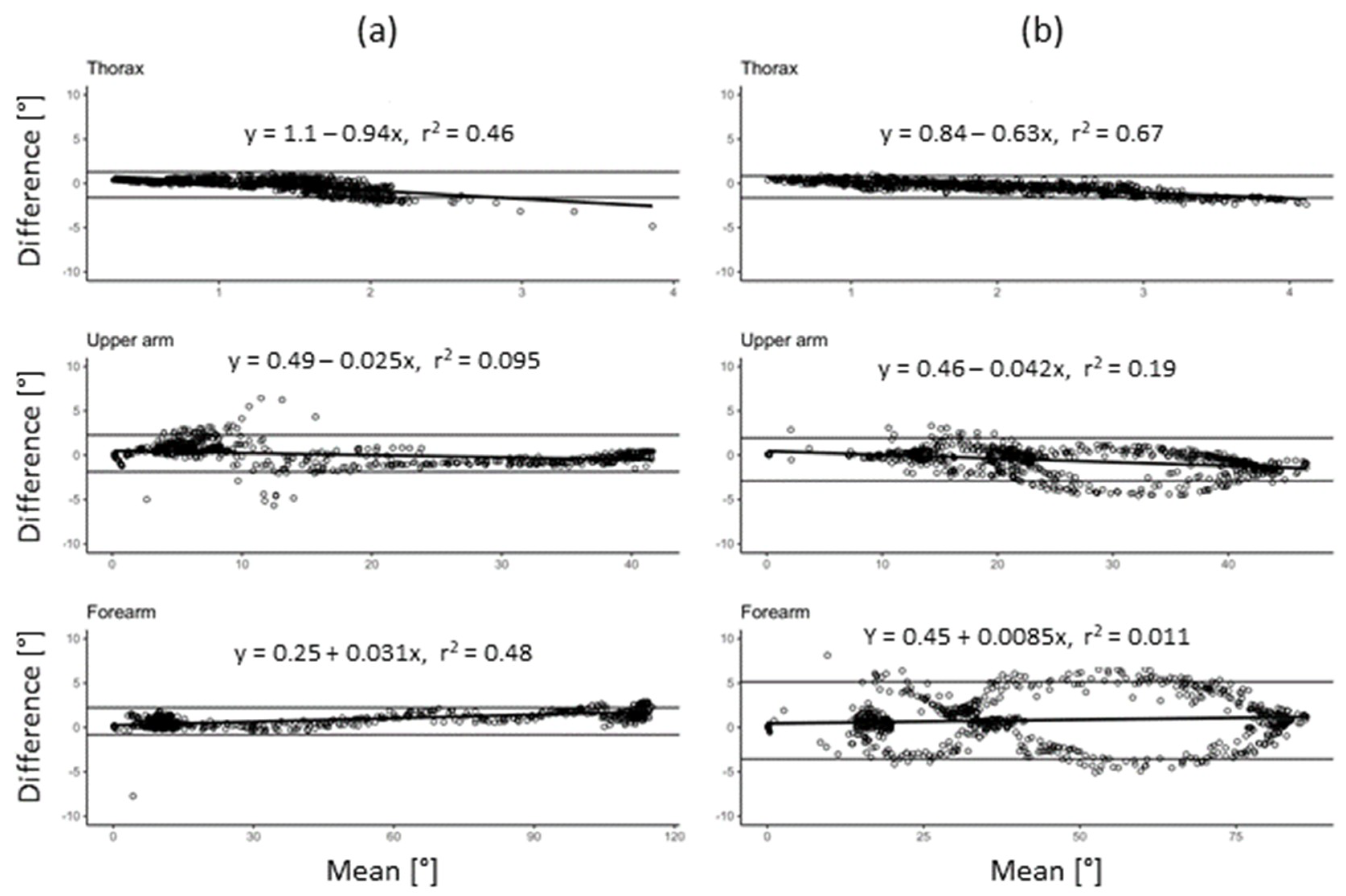
3.1. System Validity
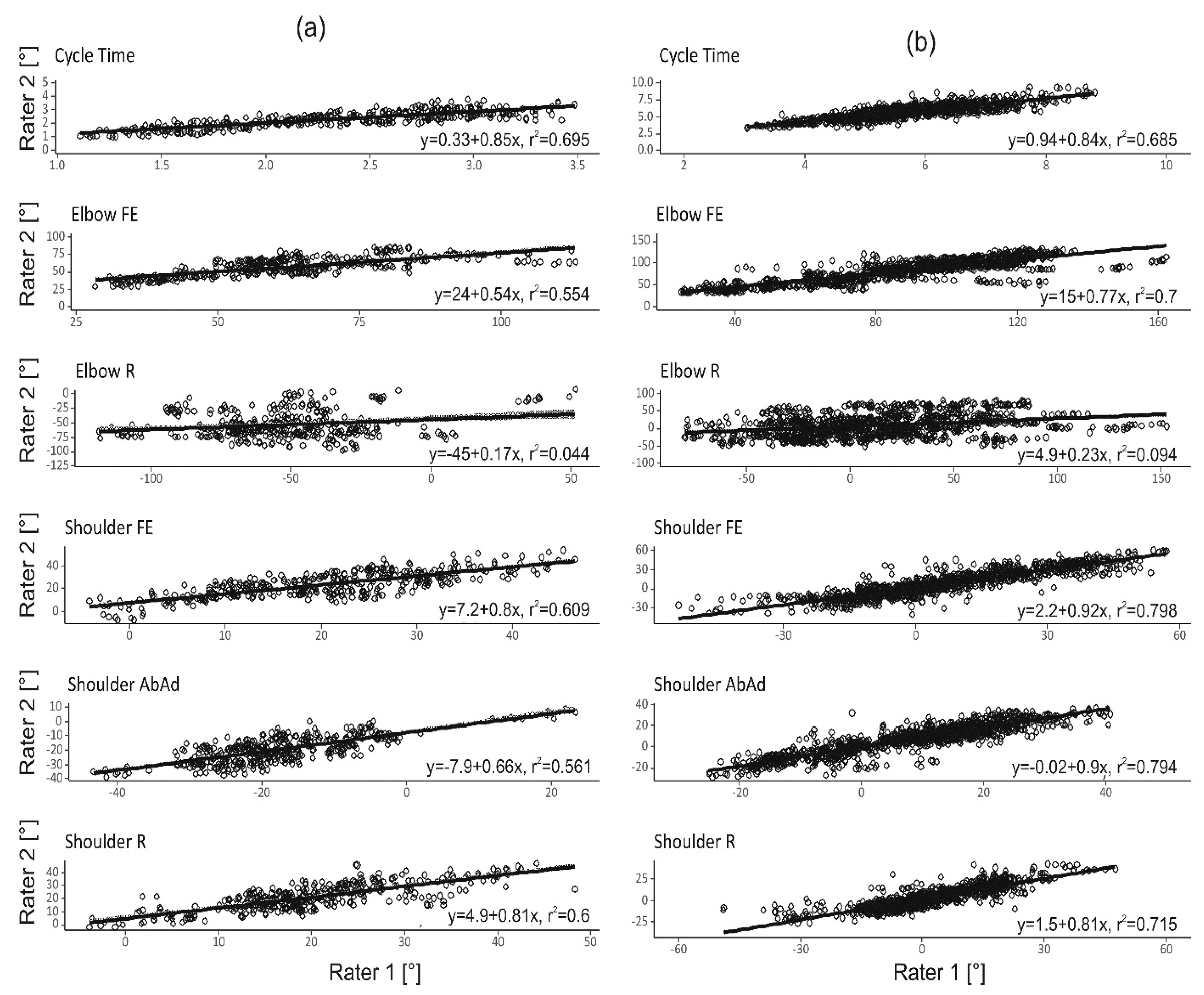
3.2. Inter-Rater and Within-Subject Reliability
4. Discussion
4.1. Within-Subject Reliability
4.2. Rater Dependency
4.3. Proposed Protocol Design for Increased Reliability
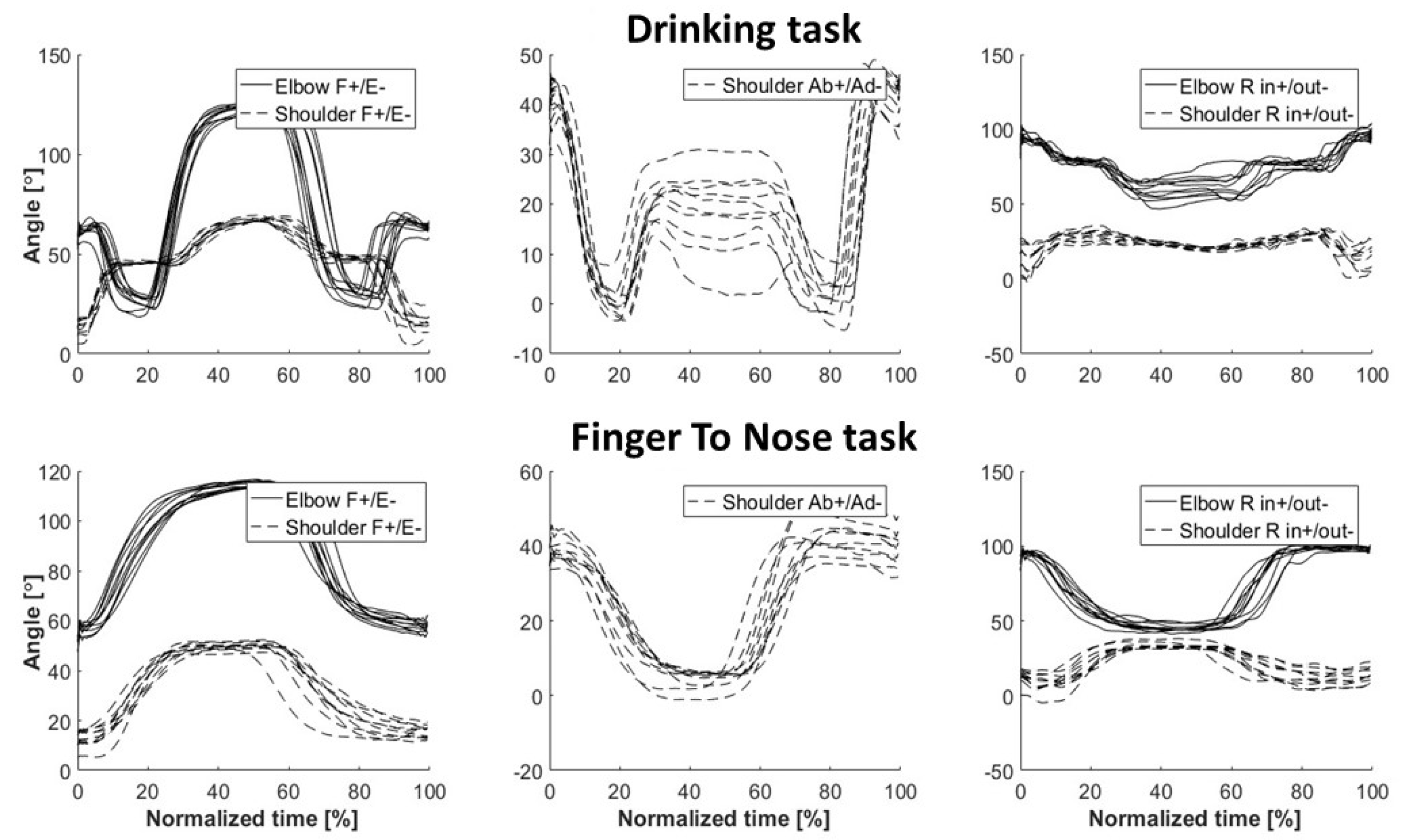
4.4. Kinematic Assessment of the Finger-To-Nose Task
4.5. Kinematic Assessment of the Drinking Task
4.6. Methodological Considerations and Strengths
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Side | Reliability | Cycle Time | Elbow FE | Elbow R | Shoulder FE | Shoulder AbAd | Shoulder R | |
|---|---|---|---|---|---|---|---|---|
| Dominant | Within-subject | 0.88 | 0.97 | 0.93 | 0.93 | 0.82 | 0.88 | |
| Inter-rater | 0.72 | 0.46 | 0.15 | 0.73 | 0.81 | 0.74 | ||
| G-coefficients | Overall | 0.88 | 0.64 | 0.26 | 0.87 | 0.90 | 0.89 | |
| Non-dominant | Within-subject | 0.88 | 0.97 | 0.93 | 0.90 | 0.88 | 0.86 | |
| Inter-rater | 0.75 | 0.49 | 0.25 | 0.50 | 0.59 | 0.67 | ||
| Overall | 0.90 | 0.67 | 0.40 | 0.69 | 0.77 | 0.86 | ||
| Dominant | Intercept | 5.11 *** | 56.22 *** | −12.61 * | 29.97 *** | −8.33 *** | 12.15 *** | |
| (0.18) | (4.80) | (4.96) | (2.72) | (1.98) | (2.45) | |||
| n = 399 | σ2rat | 0.00 | 20.72 | 0.00 | 3.32 | 0.00 | 2.33 | |
| σ2rep | 0.01 | 0.97 | 0.48 | 0.00 | 7.53 | 0.36 | ||
| σ2subj | 0.58 | 167.39 | 128.74 | 101.04 | 57.90 | 86.98 | ||
| Model and | σ2rep_rat | 0.00 | 0.00 | 0.00 | 0.41 | 0.00 | 0.00 | |
| variance | σ2subj_rat | 0.15 | 166.03 | 717.93 | 25.69 | 9.82 | 17.05 | |
| components | σ2subj_rep | 0.01 | 1.55 | 4.79 | 1.19 | 1.22 | 2.13 | |
| σ2residual | 0.08 | 10.10 | 60.34 | 8.84 | 5.92 | 12.41 | ||
| Non-dominant | Intercept | 5.11 *** | 60.41 *** | −13.04 ** | 29.66 *** | −4.29 * | 15.13 *** | |
| n = 377 | (0.17) | (4.53) | (4.40) | (2.53) | (2.17) | (1.99) | ||
| σ2rat | 0.00 | 17.59 | 0.00 | 2.75 | 1.59 | 0.00 | ||
| σ2rep | 0.01 | 0.31 | 1.51 | 0.00 | 0.51 | 0.00 | ||
| σ2subj | 0.51 | 154.37 | 146.22 | 67.17 | 56.97 | 64.87 | ||
| σ2rep_rat | 0.00 | 0.00 | 0.32 | 0.26 | 0.10 | 0.00 | ||
| σ2subj_rat | 0.10 | 134.95 | 433.00 | 54.84 | 30.80 | 19.13 | ||
| σ2subj_rep | 0.00 | 1.45 | 8.57 | 1.65 | 2.91 | 0.68 | ||
| σ2residual | 0.07 | 8.45 | 32.93 | 11.19 | 9.16 | 13.18 |
| Side | Reliability | Cycle Time | Elbow FE | Elbow R | Shoulder FE | Shoulder AbAd | Shoulder R | |
|---|---|---|---|---|---|---|---|---|
| Dominant | Within-subject | 0.91 | 0.87 | 0.98 | 0.86 | 0.91 | 0.86 | |
| Inter-rater | 0.78 | 0.51 | 0.02 | 0.72 | 0.79 | 0.70 | ||
| Overall | 0.90 | 0.73 | 0.04 | 0.90 | 0.92 | 0.87 | ||
| G-coefficients | ||||||||
| Non-dominant | Within-subject | 0.89 | 0.94 | 0.97 | 0.87 | 0.85 | 0.86 | |
| Inter-rater | 0.83 | 0.25 | 0.24 | 0.67 | 0.73 | 0.76 | ||
| Overall | 0.94 | 0.39 | 0.39 | 0.85 | 0.89 | 0.92 | ||
| Dominant | Intercept | 6.32 *** | 106.47 *** | 17.23 ** | −8.03 *** | 15.69 *** | −3.65 * | |
| n = 395 | (0.21) | (2.58) | (5.91) | (2.36) | (1.83) | (1.75) | ||
| σ2rat | 0.00 | 3.17 | 0.00 | 0.00 | 0.00 | 0.58 | ||
| σ2rep | 0.00 | 0.02 | 0.00 | 0.13 | 0.00 | 1.25 | ||
| σ2subj | 0.77 | 74.92 | 25.21 | 100.03 | 61.76 | 46.20 | ||
| σ2rep_rat | 0.00 | 0.00 | 1.48 | 0.00 | 0.00 | 0.00 | ||
| Model and | σ2subj_rat | 0.17 | 51.27 | 1342.60 | 20.93 | 10.28 | 12.11 | |
| variance | σ2subj_rep | 0.03 | 0.00 | 4.31 | 0.00 | 0.49 | 0.38 | |
| components | σ2residual | 0.06 | 18.56 | 24.63 | 18.96 | 6.63 | 8.23 | |
| Non-dominant | Intercept | 6.28 *** | 114.38 *** | 29.04 *** | −11.99 *** | 16.16 *** | −4.74 ** | |
| n = 377 | (0.19) | (3.86) | (6.40) | (2.56) | (1.77) | |||
| σ2rat | 0.00 | 20.71 | 0.00 | 1.20 | 0.00 | 0.08 | ||
| σ2rep | 0.00 | 0.23 | 3.11 | 0.00 | 0.00 | 0.30 | ||
| σ2subj | 0.62 | 37.73 | 302.47 | 97.08 | 53.01 | 48.27 | ||
| σ2rep_rat | 0.00 | 0.00 | 0.80 | 0.00 | 0.12 | 0.00 | ||
| σ2subj_rat | 0.07 | 94.76 | 935.13 | 30.81 | 11.60 | 6.90 | ||
| σ2subj_rep | 0.02 | 3.66 | 5.39 | 2.83 | 2.25 | 0.18 | ||
| σ2residual | 0.07 | 6.41 | 22.83 | 16.34 | 8.68 | 8.61 |
References
- Wren, T.A.; Gorton, G.E., 3rd; Ounpuu, S.; Tucker, C.A. Efficacy of clinical gait analysis: A systematic review. Gait Posture 2011, 34, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Paulis, W.D.; Horemans, H.L.; Brouwer, B.S.; Stam, H.J. Excellent test-retest and inter-rater reliability for Tardieu Scale measurements with inertial sensors in elbow flexors of stroke patients. Gait Posture 2011, 33, 185–189. [Google Scholar] [CrossRef] [PubMed]
- van der Pas, S.C.; Verbunt, J.A.; Breukelaar, D.E.; van Woerden, R.; Seelen, H.A. Assessment of arm activity using triaxial accelerometry in patients with a stroke. Arch. Phys. Med. Rehabil. 2011, 92, 1437–1442. [Google Scholar] [CrossRef] [PubMed]
- McIntyre, A.; Viana, R.; Janzen, S.; Mehta, S.; Pereira, S.; Teasell, R. Systematic review and meta-analysis of constraint-induced movement therapy in the hemiparetic upper extremity more than six months post stroke. Top. Stroke Rehabil. 2012, 19, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Felix, K.; Gain, K.; Paiva, E.; Whitney, K.; Jenkins, M.E.; Spaulding, S.J. Upper Extremity Motor Learning among Individuals with Parkinson’s Disease: A Meta-Analysis Evaluating Movement Time in Simple Tasks. Parkinson’s Dis. 2012, 2012, 589152. [Google Scholar] [CrossRef] [PubMed]
- El-Zayat, B.F.; Efe, T.; Heidrich, A.; Wolf, U.; Timmesfeld, N.; Heyse, T.J.; Lakemeier, S.; Fuchs-Winkelmann, S.; Schofer, M.D. Objective assessment of shoulder mobility with a new 3D gyroscope—A validation study. BMC Musculoskelet. Disord. 2011, 12, 168. [Google Scholar] [CrossRef] [PubMed]
- Bohannon, R.W.; Smith, M.B. Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys. Ther. 1987, 67, 206–207. [Google Scholar] [CrossRef] [PubMed]
- McCrea, P.H.; Eng, J.J.; Hodgson, A.J. Biomechanics of reaching: Clinical implications for individuals with acquired brain injury. Disabil. Rehabil. 2002, 24, 534–541. [Google Scholar] [CrossRef] [PubMed]
- Alt Murphy, M.; Murphy, S.; Persson, H.C.; Bergstrom, U.B.; Sunnerhagen, K.S. Kinematic Analysis Using 3D Motion Capture of Drinking Task in People With and Without Upper-extremity Impairments. J. Vis. Exp. 2018. [Google Scholar] [CrossRef] [PubMed]
- Alt Murphy, M.; Willen, C.; Sunnerhagen, K.S. Kinematic variables quantifying upper-extremity performance after stroke during reaching and drinking from a glass. Neurorehabil. Neural Repair 2011, 25, 71–80. [Google Scholar] [CrossRef] [PubMed]
- de los Reyes-Guzman, A.; Gil-Agudo, A.; Penasco-Martin, B.; Solis-Mozos, M.; del Ama-Espinosa, A.; Perez-Rizo, E. Kinematic analysis of the daily activity of drinking from a glass in a population with cervical spinal cord injury. J. Neuroeng. Rehabil. 2010, 7, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ustinova, K.I.; Goussev, V.M.; Balasubramaniam, R.; Leven, M.F. Disruption of coordination between arm, trunk, and center of pressure displacement in patients with hemiparesis. Motor Control 2004, 8, 139–159. [Google Scholar] [CrossRef] [PubMed]
- Johansson, G.M.; Grip, H.; Levin, M.F.; Hager, C.K. The added value of kinematic evaluation of the timed finger-to-nose test in persons post-stroke. J. Neuroeng. Rehabil. 2017, 14, 11. [Google Scholar] [CrossRef]
- Gladstone, D.J.; Danells, C.J.; Black, S.E. The fugl-meyer assessment of motor recovery after stroke: A critical review of its measurement properties. Neurorehabil. Neural Repair 2002, 16, 232–240. [Google Scholar] [CrossRef] [PubMed]
- Fugl-Meyer, A.R.; Jaasko, L.; Leyman, I.; Olsson, S.; Steglind, S. The post-stroke hemiplegic patient. 1. a method for evaluation of physical performance. Scand. J. Rehabil. Med. 1975, 7, 13–31. [Google Scholar] [PubMed]
- Bergeron, D.; Vermette, A.; De La Sablonniere, J.; Cayer, A.M.; Laforce, R.; Bouchard, R.W. Finger-to-Nose Test Findings in Alzheimer’s Disease. J. Alzheimers Dis. 2017, 55, 1335–1337. [Google Scholar] [CrossRef]
- de Jong, L.D.; Nieuwboer, A.; Aufdemkampe, G. The hemiplegic arm: Interrater reliability and concurrent validity of passive range of motion measurements. Disabil. Rehabil. 2007, 29, 1442–1448. [Google Scholar] [CrossRef]
- Totty, M.S.; Wade, E. Muscle Activation and Inertial Motion Data for Noninvasive Classification of Activities of Daily Living. IEEE Trans. Bio-Med. Eng. 2018, 65, 1069–1076. [Google Scholar]
- Perez, R.; Costa, U.; Torrent, M.; Solana, J.; Opisso, E.; Caceres, C.; Tormos, J.M.; Medina, J.; Gomez, E.J. Upper limb portable motion analysis system based on inertial technology for neurorehabilitation purposes. Sensors 2010, 10, 10733–10751. [Google Scholar] [CrossRef]
- Shavelson, R.J.; Webb, N.M.; Rowley, G.L. Generalizability theory. Am. Psychol. 1989, 44, 922–932. [Google Scholar] [CrossRef]
- Öhberg, F.; Lundström, R.; Grip, H. Comparative analysis of different adaptive filters for tracking lower segments of a human body using inertial motion sensors. Meas. Sci. Technol. 2013, 24, 12. [Google Scholar] [CrossRef]
- Ertzgaard, P.; Ohberg, F.; Gerdle, B.; Grip, H. A new way of assessing arm function in activity using kinematic Exposure Variation Analysis and portable inertial sensors—A validity study. Man. Ther. 2016, 21, 241–249. [Google Scholar] [CrossRef] [PubMed]
- El-Gohary, M.; McNames, J. Human Joint Angle Estimation with Inertial Sensors and Validation with A Robot Arm. IEEE Trans. Bio-Med. Eng. 2015, 62, 1759–1767. [Google Scholar] [CrossRef] [PubMed]
- Wu, G.; van der Helm, F.C.; Veeger, H.E.; Makhsous, M.; Van Roy, P.; Anglin, C.; Nagels, J.; Karduna, A.R.; McQuade, K.; Wang, X.; et al. ISB recommendation on definitions of joint coordinate systems of various joints for the reporting of human joint motion—Part II: Shoulder, elbow, wrist and hand. J. Biomech. 2005, 38, 981–992. [Google Scholar] [CrossRef] [PubMed]
- Soderkvist, I.; Wedin, P.A. Determining the movements of the skeleton using well-configured markers. J. Biomech. 1993, 26, 1473–1477. [Google Scholar] [CrossRef]
- de Vet Henrica, C.W.; Terwee Caroline, B.; Mokkink Lidwine, B.; Knol, D.L. Measurement in Medicine: A Practical Guide; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2011; p. x. 338p. [Google Scholar]
- Salvia, J.; Ysseldyke, J.E.; Witmer, S. Assessment in Special and Inclusive Education, 13th ed.; Cengage Learning: Boston, MA, USA, 2017. [Google Scholar]
- Molina Rueda, F.; Rivas Montero, F.M.; Perez de Heredia Torres, M.; Alguacil Diego, I.M.; Molero Sanchez, A.; Miangolarra Page, J.C. Movement analysis of upper extremity hemiparesis in patients with cerebrovascular disease: A pilot study. Neurologia 2012, 27, 343–347. [Google Scholar] [CrossRef]
- Alt Murphy, M.; Willen, C.; Sunnerhagen, K.S. Responsiveness of upper extremity kinematic measures and clinical improvement during the first three months after stroke. Neurorehabil. Neural Repair 2013, 27, 844–853. [Google Scholar] [CrossRef]
- Santos, G.L.; Russo, T.L.; Nieuwenhuys, A.; Monari, D.; Desloovere, K. Kinematic Analysis of a Drinking Task in Chronic Hemiparetic Patients Using Features Analysis and Statistical Parametric Mapping. Arch. Phys. Med. Rehabil. 2018, 99, 501–511. [Google Scholar] [CrossRef]
- Lee, J.A.; Hwang, P.W.; Kim, E.J. Upper extremity muscle activation during drinking from a glass in subjects with chronic stroke. J. Phys. Ther. Sci. 2015, 27, 701–703. [Google Scholar] [CrossRef] [Green Version]
- Bernstein, N.A. The Co-Ordination and Regulation of Movements; Pergamon Press: London, UK, 1967. [Google Scholar]
- Rodrigues, M.R.; Slimovitch, M.; Chilingaryan, G.; Levin, M.F. Does the Finger-to-Nose Test measure upper limb coordination in chronic stroke? J. Neuroeng. Rehabil. 2017, 14, 6. [Google Scholar] [CrossRef]
- Maurel, N.; Diop, A.; Gouelle, A.; Alberti, C.; Husson, I. Assessment of upper limb function in young Friedreich ataxia patients compared to control subjects using a new three-dimensional kinematic protocol. Clin. Biomech. 2013, 28, 386–394. [Google Scholar] [CrossRef]
- Jeon, H.J.; An, S.; Yoo, J.; Park, N.H.; Lee, K.H. The effect of Monkey Chair and Band exercise system on shoulder range of motion and pain in post-stroke patients with hemiplegia. J. Phys. Ther. Sci. 2016, 28, 2232–2237. [Google Scholar] [CrossRef] [Green Version]
- Eriks-Hoogland, I.E.; de Groot, S.; Post, M.W.; van der Woude, L.H. Passive shoulder range of motion impairment in spinal cord injury during and one year after rehabilitation. J. Rehabil. Med. 2009, 41, 438–444. [Google Scholar] [CrossRef] [Green Version]
- Muller, R.; Buttner, P. A critical discussion of intraclass correlation coefficients. Stat. Med. 1994, 13, 2465–2476. [Google Scholar] [CrossRef]
- Thies, S.B.; Tresadern, P.A.; Kenney, L.P.; Smith, J.; Howard, D.; Goulermas, J.Y.; Smith, C.; Rigby, J. Movement variability in stroke patients and controls performing two upper limb functional tasks: A new assessment methodology. J. Neuroeng. Rehabil. 2009, 6, 2. [Google Scholar] [CrossRef]


| Side | Reliability | Cycle Time | Elbow FE | Elbow R | Shoulder FE | Shoulder AbAd | Shoulder R | |
|---|---|---|---|---|---|---|---|---|
| Dominant | Within-subject | 0.89 | 0.91 | 0.97 | 0.89 | 0.92 | 0.91 | |
| Inter-rater | 0.84 | 0.77 | 0.15 | 0.78 | 0.67 | 0.73 | ||
| G-coefficients | Overall | 0.94 | 0.88 | 0.26 | 0.91 | 0.83 | 0.87 | |
| Non-dominant | Within-subject | 0.91 | 0.94 | 0.96 | 0.90 | 0.92 | 0.94 | |
| Inter-rater | 0.83 | 0.62 | 0.30 | 0.74 | 0.76 | 0.82 | ||
| Overall | 0.94 | 0.77 | 0.47 | 0.88 | 0.87 | 0.92 | ||
| Dominant | Intercept | 2.35 s *** | 58.66° *** | −53.66° *** | 22.42° *** | −20.08° *** | 20.72° *** | |
| n = 397 | (0.12) | (4.14) | (4.70) | (2.81) | (1.72) | (1.89) | ||
| σ2rat | 0.00 | 13.71 | 0.00 | 3.99 | 0.00 | 0.29 | ||
| σ2rep | 0.00 | 0.00 | 5.90 | 0.76 | 0.62 | 0.14 | ||
| σ2subj | 0.28 | 186.75 | 113.81 | 107.42 | 48.16 | 60.17 | ||
| Model and | σ2rep_rat | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 | |
| variance | σ2subj_rat | 0.03 | 37.48 | 629.23 | 17.02 | 18.76 | 16.16 | |
| components | σ2subj_rep | 0.01 | 2.72 | 1.10 | 2.20 | 0.57 | 0.92 | |
| σ2residual | 0.02 | 6.32 | 14.05 | 8.43 | 4.55 | 6.49 | ||
| Non-dominant | Intercept | 2.31 s *** | 61.35° *** | −54.47° *** | 21.87° *** | −16.26° *** | 19.94° *** | |
| n = 379 | (0.13) | (4.04) | (4.14) | (2.64) | (2.69) | (2.13) | ||
| σ2rat | 0.00 | 9.61 | 0.00 | 3.57 | 4.35 | 0.00 | ||
| σ2rep | 0.00 | 0.30 | 3.94 | 0.02 | 0.00 | 0.06 | ||
| σ2subj | 0.29 | 173.26 | 148.50 | 87.90 | 85.66 | 79.07 | ||
| σ2rep_rat | 0.00 | 0.00 | 0.00 | 0.14 | 0.09 | 0.00 | ||
| σ2subj_rat | 0.03 | 90.53 | 338.92 | 20.29 | 19.94 | 12.80 | ||
| σ2subj_rep | 0.00 | 1.89 | 2.41 | 1.24 | 1.60 | 1.42 | ||
| σ2residual | 0.03 | 4.74 | 14.53 | 6.52 | 3.47 | 4.69 |
| Side | Reliability | Cycle Time | Elbow FE | Elbow R | Shoulder FE | Shoulder AbAd | Shoulder R | |
|---|---|---|---|---|---|---|---|---|
| Dominant | Within-subject | 0.92 | 0.88 | 0.97 | 0.85 | 0.80 | 0.90 | |
| Inter-rater | 0.81 | 0.47 | 0.002 | 0.67 | 0.72 | 0.78 | ||
| G-coefficients | Overall | 0.93 | 0.68 | 0 | 0.86 | 0.93 | 0.90 | |
| Non-Dominant | Within-subject | 0.90 | 0.96 | 0.96 | 0.87 | 0.90 | 0.87 | |
| Inter-rater | 0.84 | 0.42 | 0.14 | 0.72 | 0.81 | 0.60 | ||
| Overall | 0.95 | 0.60 | 0.23 | 0.87 | 0.93 | 0.79 | ||
| Dominant | Intercept | 5.65 s *** | 90.76° *** | 20.09° *** | 5.68° ** | 14.69° *** | −5.00° ** | |
| n = 397 | (0.20) | (2.77) | (5.62) | (1.76) | (1.64) | (1.68) | ||
| σ2rat | 0.00 | 0.00 | 0.00 | 0.00 | 0.91 | 0.00 | ||
| σ2rep | 0.00 | 2.38 | 0.00 | 0.16 | 0.00 | 1.04 | ||
| σ2subj | 0.71 | 100.31 | 0.00 | 53.09 | 41.88 | 48.74 | ||
| Model and | σ2rep_rat | 0.00 | 0.88 | 0.00 | 0.00 | 0.14 | 0.00 | |
| variance | σ2subj_rat | 0.10 | 92.30 | 1260.94 | 16.06 | 4.53 | 10.01 | |
| components | σ2subj_rep | 0.01 | 0.00 | 2.08 | 1.22 | 0.57 | 0.77 | |
| σ2residual | 0.06 | 23.05 | 36.25 | 10.46 | 11.02 | 4.44 | ||
| Non-dominant | Intercept | 5.68 s *** | 97.79° *** | 24.75° *** | 2.94° | 15.84° *** | −3.15° | |
| n = 376 | (0.19) | (2.96) | (5.38) | (2.37) | (1.74) | (1.62) | ||
| σ2rat | 0.00 | 0.00 | 0.00 | 0.22 | 0.00 | 0.00 | ||
| σ2rep | 0.00 | 0.03 | 3.67 | 0.00 | 0.00 | 0.00 | ||
| σ2subj | 0.62 | 99.10 | 124.19 | 90.38 | 53.51 | 39.08 | ||
| σ2rep_rat | 0.00 | 0.40 | 0.00 | 0.82 | 0.09 | 0.21 | ||
| σ2subj_rat | 0.05 | 133.03 | 832.17 | 24.28 | 6.91 | 19.92 | ||
| σ2subj_rep | 0.01 | 1.36 | 9.89 | 4.21 | 0.68 | 1.33 | ||
| σ2residual | 0.07 | 7.43 | 23.90 | 11.52 | 5.94 | 7.22 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Öhberg, F.; Bäcklund, T.; Sundström, N.; Grip, H. Portable Sensors Add Reliable Kinematic Measures to the Assessment of Upper Extremity Function. Sensors 2019, 19, 1241. https://0-doi-org.brum.beds.ac.uk/10.3390/s19051241
Öhberg F, Bäcklund T, Sundström N, Grip H. Portable Sensors Add Reliable Kinematic Measures to the Assessment of Upper Extremity Function. Sensors. 2019; 19(5):1241. https://0-doi-org.brum.beds.ac.uk/10.3390/s19051241
Chicago/Turabian StyleÖhberg, Fredrik, Tomas Bäcklund, Nina Sundström, and Helena Grip. 2019. "Portable Sensors Add Reliable Kinematic Measures to the Assessment of Upper Extremity Function" Sensors 19, no. 5: 1241. https://0-doi-org.brum.beds.ac.uk/10.3390/s19051241





