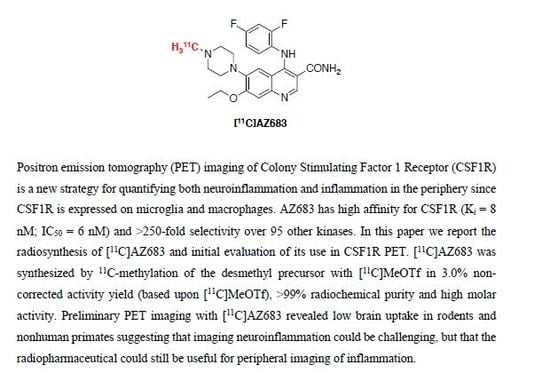
Synthesis and Initial In Vivo Evaluation of [11C]AZ683—A Novel PET Radiotracer for Colony Stimulating Factor 1 Receptor (CSF1R)
Abstract
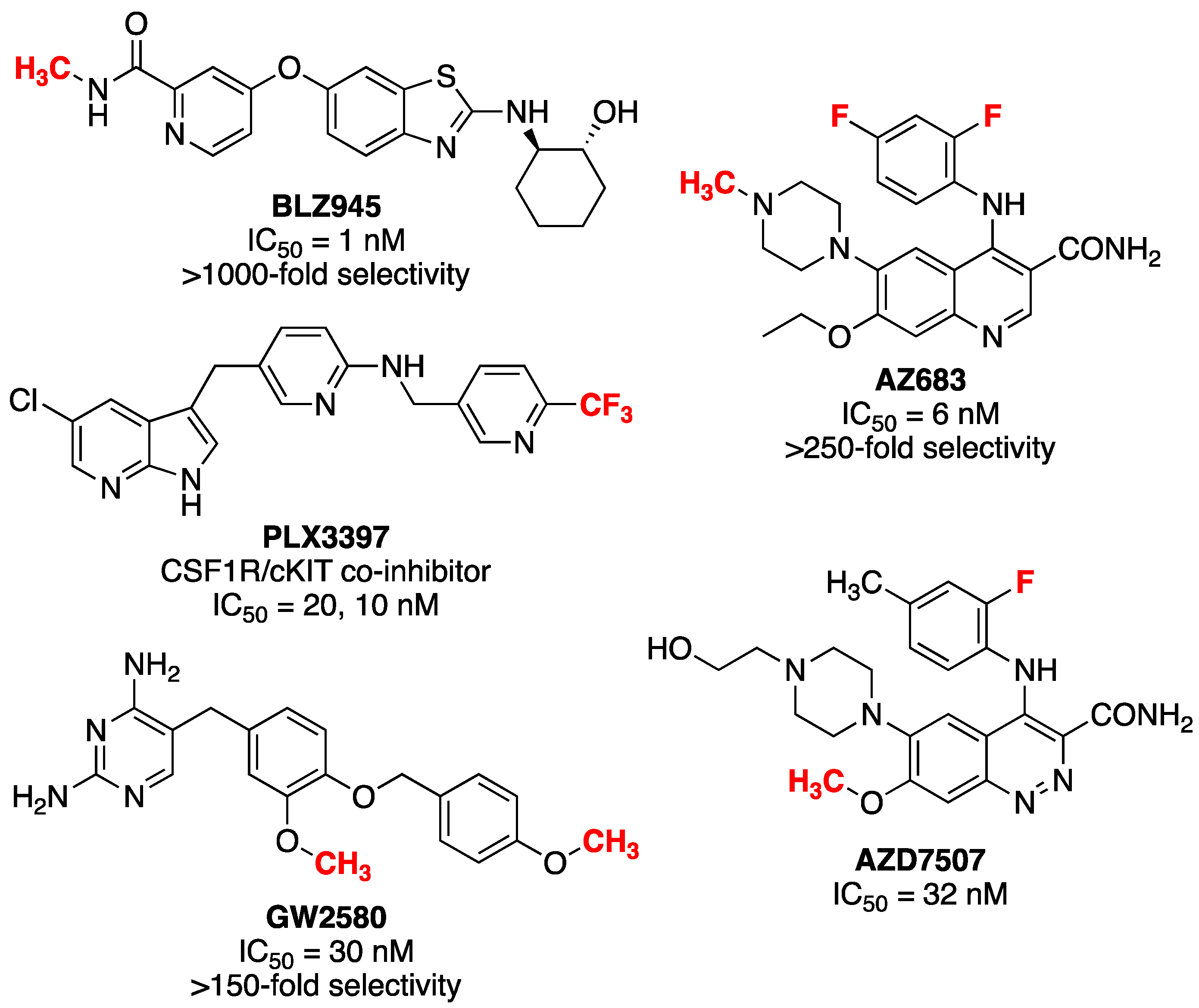
:1. Introduction
2. Results and Discussion
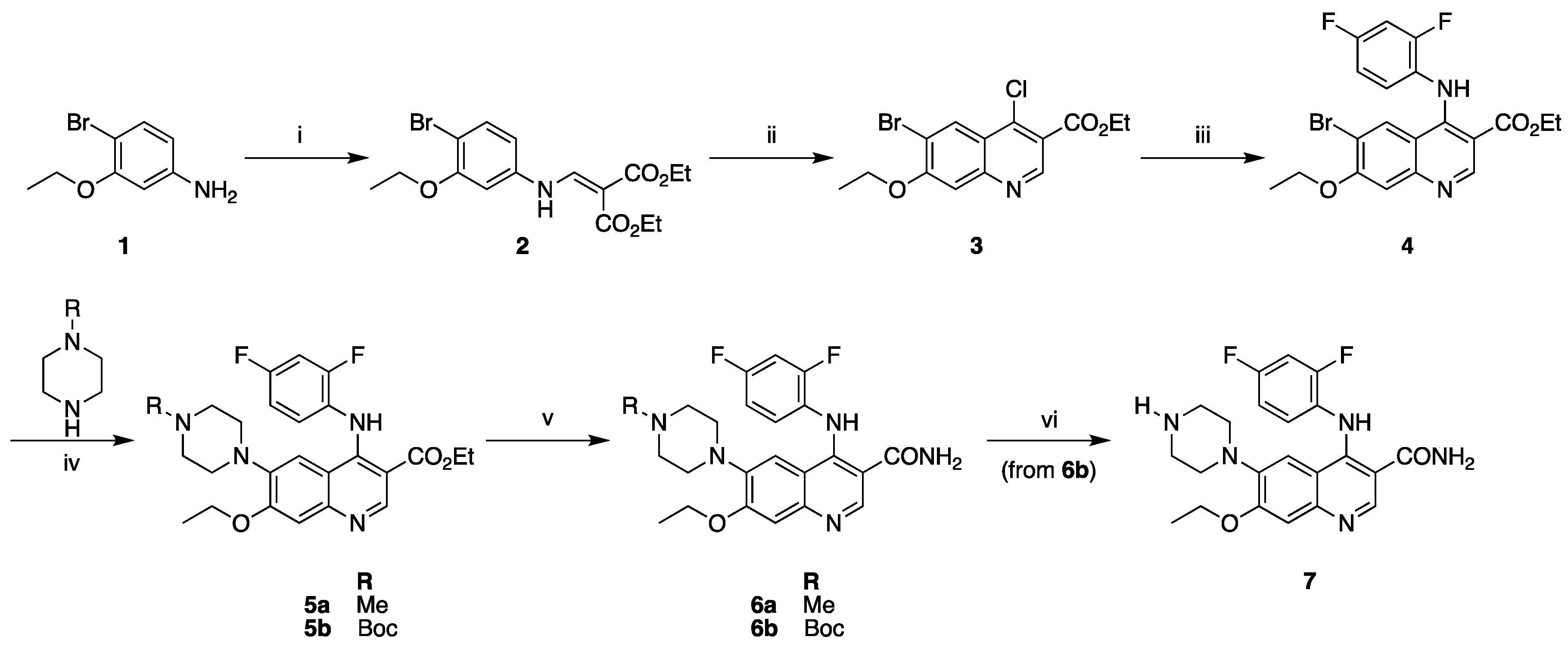
2.1. Synthesis of Reference Standard and Precursor
2.2. Radiosynthesis of [11C]AZ683
2.3. Preclinical PET Imaging
3. Materials and Methods
3.1. Synthesis
3.1.1. General Considerations
3.1.2. Compounds Synthesized
3.2. Radiochemistry
3.2.1. General Considerations
3.2.2. Radiosynthesis of [11C]AZ683
3.2.3. Quality Control Testing of [11C]AZ683
Visual inspection
Dose pH
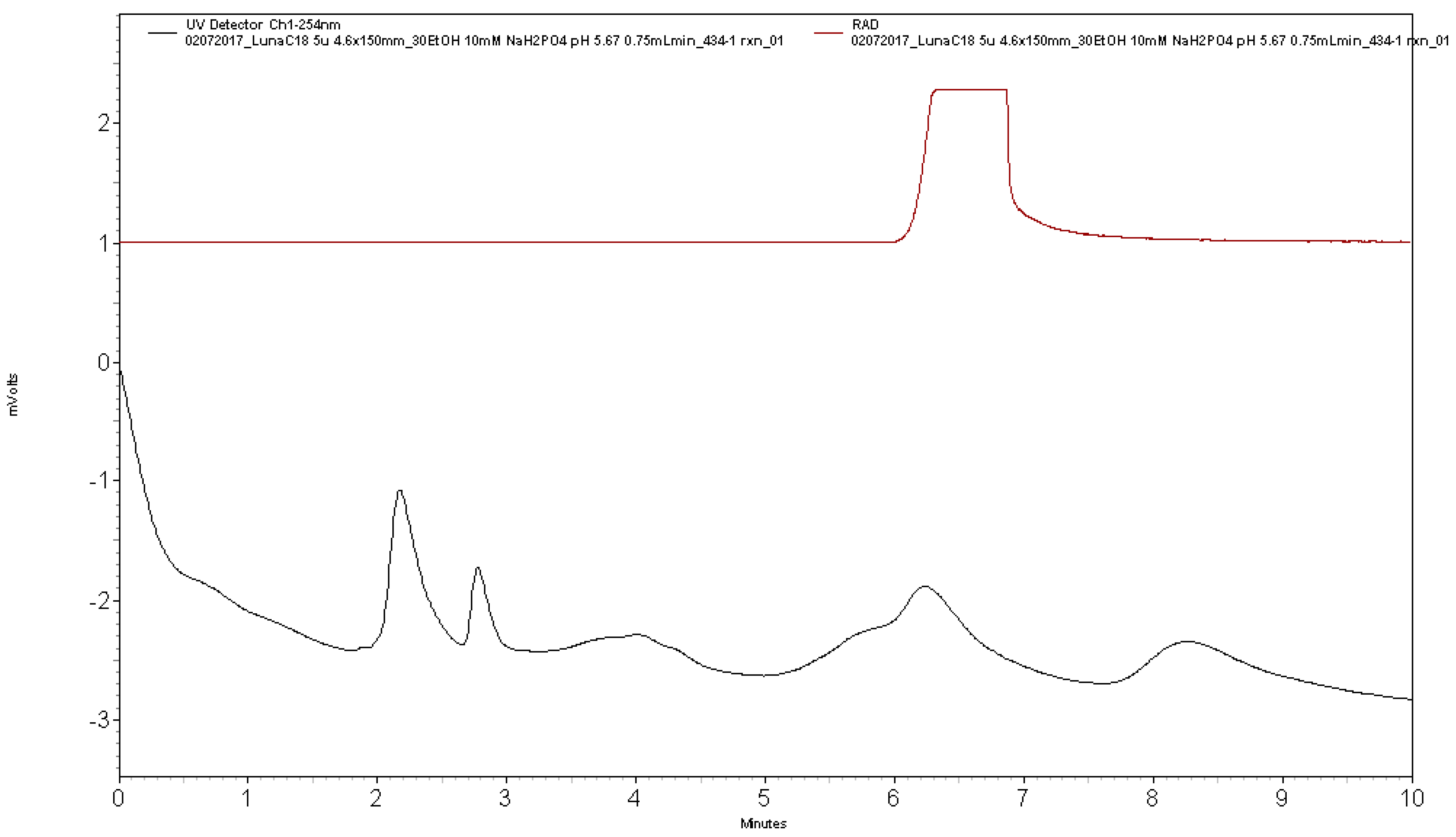
Analytical HPLC
3.3. Preclinical PET Imaging
3.3.1. General Considerations
3.3.2. Animal Husbandry and Housing
3.3.3. Rodent Imaging Protocol
3.3.4. Primate Imaging Protocol
3.3.5. PET Image Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Verstraete, K.; Savvides, S.N. Extracellular assembly and activation principles of oncogenic class III receptor tyrosine kinases. Nat. Rev. Cancer 2012, 12, 753–766. [Google Scholar] [CrossRef] [PubMed]
- Michell-Robinson Mackenzie, A.; Touil, H.; Healy Luke, M.; Durafourt Bryce, A.; Bar-Or, A.; Antel Jack, P.; Owen David, R.; Moore Craig, S. Roles of microglia in brain development, tissue maintenance and repair. Brain 2015, 138 (Pt 5), 1138–1159. [Google Scholar] [CrossRef] [Green Version]
- Nakamichi, Y.; Udagawa, N.; Takahashi, N. IL-34 and CSF-1: Similarities and differences. J. Bone Miner. Metab. 2013, 31, 486–495. [Google Scholar] [CrossRef] [PubMed]
- El-Gamal, M.I.; Al-Ameen, S.K.; Al-Koumi, D.M.; Hamad, M.G.; Jalal, N.A.; Oh, C.-H. Recent advances of colony-stimulating factor-1 receptor (CSF-1R) kinase and its inhibitors. J. Med. Chem. 2018, 61, 5450–5466. [Google Scholar] [CrossRef] [PubMed]
- Burns, C.J.; Wilks, A.F. c-FMS inhibitors: A patent review. Expert Opin. Ther. Pat. 2011, 21, 147–165. [Google Scholar] [CrossRef] [PubMed]
- Spangenberg Elizabeth, E.; Lee Rafael, J.; Najafi Allison, R.; Rice Rachel, A.; Elmore Monica, R.P.; Blurton-Jones, M.; Green Kim, N.; West Brian, L. Eliminating microglia in Alzheimer’s mice prevents neuronal loss without modulating amyloid-β pathology. Brain 2016, 139 (Pt 4), 1265–1281. [Google Scholar] [CrossRef] [Green Version]
- Olmos-Alonso, A.; Schetters Sjoerd, T.T.; Sri, S.; Askew, K.; Mancuso, R.; Perry, V.H.; Gomez-Nicola, D.; Vargas-Caballero, M.; Holscher, C. Pharmacological targeting of CSF1R inhibits microglial proliferation and prevents the progression of Alzheimer’s-like pathology. Brain 2016, 139 (Pt 3), 891–907. [Google Scholar] [CrossRef] [Green Version]
- Asai, H.; Ikezu, S.; Tsunoda, S.; Medalla, M.; Luebke, J.; Haydar, T.; Wolozin, B.; Butovsky, O.; Kugler, S.; Ikezu, T. Depletion of microglia and inhibition of exosome synthesis halt tau propagation. Nat. Neurosci. 2015, 18, 1584–1593. [Google Scholar] [CrossRef] [Green Version]
- Pyonteck, S.M.; Akkari, L.; Schuhmacher, A.J.; Bowman, R.L.; Sevenich, L.; Quail, D.F.; Olson, O.C.; Quick, M.L.; Huse, J.T.; Teijeiro, V.; et al. CSF-1R inhibition alters macrophage polarization and blocks glioma progression. Nat. Med. 2013, 19, 1264–1272. [Google Scholar] [CrossRef] [Green Version]
- Coniglio, S.J.; Eugenin, E.; Dobrenis, K.; Stanley, E.R.; West, B.L.; Symons, M.H.; Segall, J.E. Microglial stimulation of glioblastoma invasion involves epidermal growth factor receptor (EGFR) and colony stimulating factor 1 receptor (CSF-1R) signaling. Mol. Med. 2012, 18, 519–527. [Google Scholar] [CrossRef]
- von Tresckow, B.; Morschhauser, F.; Ribrag, V.; Topp, M.S.; Chien, C.; Seetharam, S.; Aquino, R.; Kotoulek, S.; de Boer, C.J.; Engert, A. An open-label, multicenter, phase I/II study of JNJ-40346527, a CSF-1R inhibitor, in patients with relapsed or refractory hodgkin lymphoma. Clin. Cancer Res. 2015, 21, 1843–1850. [Google Scholar] [CrossRef]
- Elmore Monica, R.P.; Lee Rafael, J.; Green Kim, N.; West Brian, L. Characterizing newly repopulated microglia in the adult mouse: Impacts on animal behavior, cell morphology, and neuroinflammation. PLoS ONE 2015, 10, e0122912. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Player, M.R. Colony-stimulating factor-1 receptor inhibitors for the treatment of cancer and inflammatory disease. Curr. Top. Med. Chem. 2009, 9, 599–610. [Google Scholar] [CrossRef] [PubMed]
- Genovese Mark, C.; Hsia, E.; Belkowski Stanley, M.; Chien, C.; Masterson, T.; Thurmond Robin, L.; Manthey Carl, L.; Yan Xiaoyu, D.; Ge, T.; Franks, C.; et al. Results from a phase IIA parallel group study of JNJ-40346527, an oral CSF-1R inhibitor, in patients with active rheumatoid arthritis despite disease-modifying antirheumatic drug therapy. J. Rheumatol. 2015, 42, 1752–1760. [Google Scholar] [CrossRef] [PubMed]
- Ries, C.H.; Cannarile, M.A.; Hoves, S.; Benz, J.; Wartha, K.; Runza, V.; Rey-Giraud, F.; Pradel, L.P.; Feuerhake, F.; Klaman, I.; et al. Targeting tumor-associated macrophages with anti-CSF-1R antibody reveals a strategy for cancer therapy. Cancer Cell 2014, 25, 846–859. [Google Scholar] [CrossRef] [PubMed]
- Ota, T.; Urakawa, H.; Kozawa, E.; Ikuta, K.; Hamada, S.; Tsukushi, S.; Shimoyama, Y.; Ishiguro, N.; Nishida, Y. Expression of colony-stimulating factor 1 is associated with occurrence of osteochondral change in pigmented villonodular synovitis. Tumor Biol. 2015, 36, 5361–5367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmore, M.R.P.; Najafi, A.R.; Koike, M.A.; Dagher, N.N.; Spangenberg, E.E.; Rice, R.A.; Kitazawa, M.; Matusow, B.; Nguyen, H.; West, B.L.; et al. Colony-stimulating factor 1 receptor signaling is necessary for microglia viability, unmasking a microglia progenitor cell in the adult brain. Neuron 2014, 82, 380–397. [Google Scholar] [CrossRef] [PubMed]
- Dagher, N.N.; Najafi, A.R.; Kayala, K.M.N.; Elmore, M.R.P.; White, T.E.; Medeiros, R.; Green, K.N.; West, B.L. Colony-stimulating factor 1 receptor inhibition prevents microglial plaque association and improves cognition in 3xTg-AD mice. J. Neuroinflamm. 2015, 12, 139. [Google Scholar] [CrossRef]
- Turkheimer, F.; Rizzo, G.; Bloomfield, P.; Howes, O.; Zanotti-Fregonara, P.; Bertoldo, A.; Veronese, M. The methodology of TSPO imaging with positron emission tomography. Biochem. Soc. Trans. 2015, 43, 586–592. [Google Scholar] [CrossRef] [Green Version]
- Bernard-Gauthier, V.; Schirrmacher, R. 5-(4-((4-[18F]fluorobenzyl)oxy)-3-methoxybenzyl)pyrimidine-2,4-diamine: A selective dual inhibitor for potential PET imaging of Trk/CSF-1R. Bioorg. Med. Chem. Lett. 2014, 24, 4784–4790. [Google Scholar] [CrossRef]
- Naik, R.; Misheneva, V.; Minn, I.L.; Melnikova, T.; Mathews, W.; Dannals, R.; Pomper, M.; Savonenko, A.; Pletnikov, M.; Horti, A. PET tracer for imaging the macrophage colony stimulating factor receptor (CSF1R) in rodent brain. J. Nucl. Med. 2018, 59 (Suppl. 1), 547. [Google Scholar]
- Scott, D.A.; Balliet, C.L.; Cook, D.J.; Davies, A.M.; Gero, T.W.; Omer, C.A.; Poondru, S.; Theoclitou, M.-E.; Tyurin, B.; Zinda, M.J. Identification of 3-amido-4-anilinoquinolines as potent and selective inhibitors of CSF-1R kinase. Bioorg. Med. Chem. Lett. 2009, 19, 697–700. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Bell, K.J.; Campbell, C.T.; Cook, D.J.; Dakin, L.A.; Del Valle, D.J.; Drew, L.; Gero, T.W.; Hattersley, M.M.; Omer, C.A.; et al. 3-Amido-4-anilinoquinolines as CSF-1R kinase inhibitors 2: Optimization of the PK profile. Bioorg. Med. Chem. Lett. 2009, 19, 701–705. [Google Scholar] [CrossRef] [PubMed]
- Scott, D.A.; Dakin, L.A.; Del Valle, D.J.; Diebold, R.B.; Drew, L.; Gero, T.W.; Ogoe, C.A.; Omer, C.A.; Repik, G.; Thakur, K.; et al. 3-Amido-4-anilinocinnolines as a novel class of CSF-1R inhibitor. Bioorg. Med. Chem. Lett. 2011, 21, 1382–1384. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Hoareau, R.; Runkle, A.C.; Tluczek, L.J.M.; Hockley, B.G.; Henderson, B.D.; Scott, P.J.H. Highlighting the versatility of the Tracerlab synthesis modules. Part 2: Fully automated production of [11C]-labeled radiopharmaceuticals using a Tracerlab FXC-Pro. J. Label. Compd. Radiopharm. 2011, 54, 819–838. [Google Scholar] [CrossRef]
- CSF1R. Available online: https://www.proteinatlas.org/ENSG00000182578-CSF1R/tissue (accessed on 26 November 2018).
- Droin, N.; Solary, E. Editorial: CSF1R, CSF-1 and IL-34, a ménage à trois” conserved across vertebrates. J. Leukoc. Biol. 2010, 87, 745–747. [Google Scholar] [CrossRef] [PubMed]
- Cammermeyer, J. The life history of the microglial cell: A light microscopic study. In Neurosciences Research, 1st ed.; Ehrenpreis, S., Solnitzky, O.C., Eds.; Academic Press: New York, NY, USA; London, UK, 1970; Volume 3, pp. 43–129. [Google Scholar]
- Tiwari, A.K.; Ji, B.; Yui, J.; Fujinaga, M.; Yamasaki, T.; Xie, L.; Luo, R.; Shimoda, Y.; Kumata, K.; Zhang, Y.; et al. [18F]FEBMP: Positron emission tomography imaging of TSPO in a model of neuroinflammation in rats, and in vivo autoradiograms of the human brain. Theranostics 2015, 5, 961–969. [Google Scholar] [CrossRef] [PubMed]
- Sridharan, S.; Lepelletier, F.-X.; Trigg, W.; Banister, S.; Reekie, T.; Kassiou, M.; Gerhard, A.; Hinz, R.; Boutin, H. Comparative evaluation of three TSPO PET radiotracers in a LPS-induced model of mild neuroinflammation in rats. Mol. Imaging Biol. 2017, 19, 77–89. [Google Scholar] [CrossRef] [PubMed]
- Pajouhesh, H.; Lenz, G. Medicinal chemical properties of succesful central nervous system drugs. NeuroRX 2005, 2, 541–553. [Google Scholar] [CrossRef] [PubMed]
- cLogP and tSPA values were estimated using ChemDraw Professional 16.0 (PerkinElmer, Waltham, MA, USA) and pKa values were estimated using MarvinSketch (ChemAxon, Budapest, Hungary).
- Chu, X.; Bleasby, K.; Evers, R. Species differences in drug transporters and implications for translating preclinical findings to humans. Expert Opin. Drug Metab. Toxicol. 2013, 9, 237–252. [Google Scholar] [CrossRef] [PubMed]
- Fawaz, M.V.; Brooks, A.F.; Rodnick, M.E.; Carpenter, G.M.; Shao, X.; Desmond, T.J.; Sherman, P.; Quesada, C.A.; Hockley, B.G.; Kilbourn, M.R.; et al. High affinity radiopharmaceuticals based upon lansoprazole for PET imaging of aggregated tau in Alzheimer’s disease and progressive supranuclear palsy: Synthesis, preclinical evaluation, and lead selection. ACS Chem. Neurosci. 2014, 5, 718–730. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Brooks, A.F.; Makaravage, K.J.; Zhang, H.; Sanford, M.S.; Scott, P.J.H.; Shao, X. Radiosynthesis of [11C]LY2795050 for preclinical and clinical PET imaging using Cu(II)-mediated cyanation. ACS Med. Chem. Lett. 2018, in press. [Google Scholar] [CrossRef]
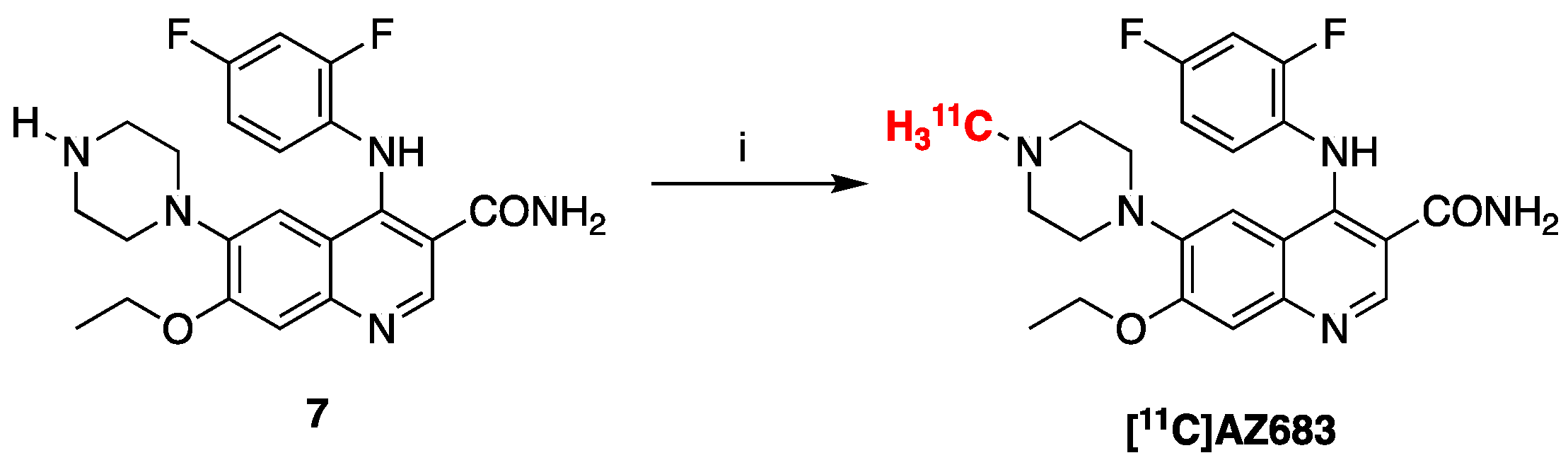

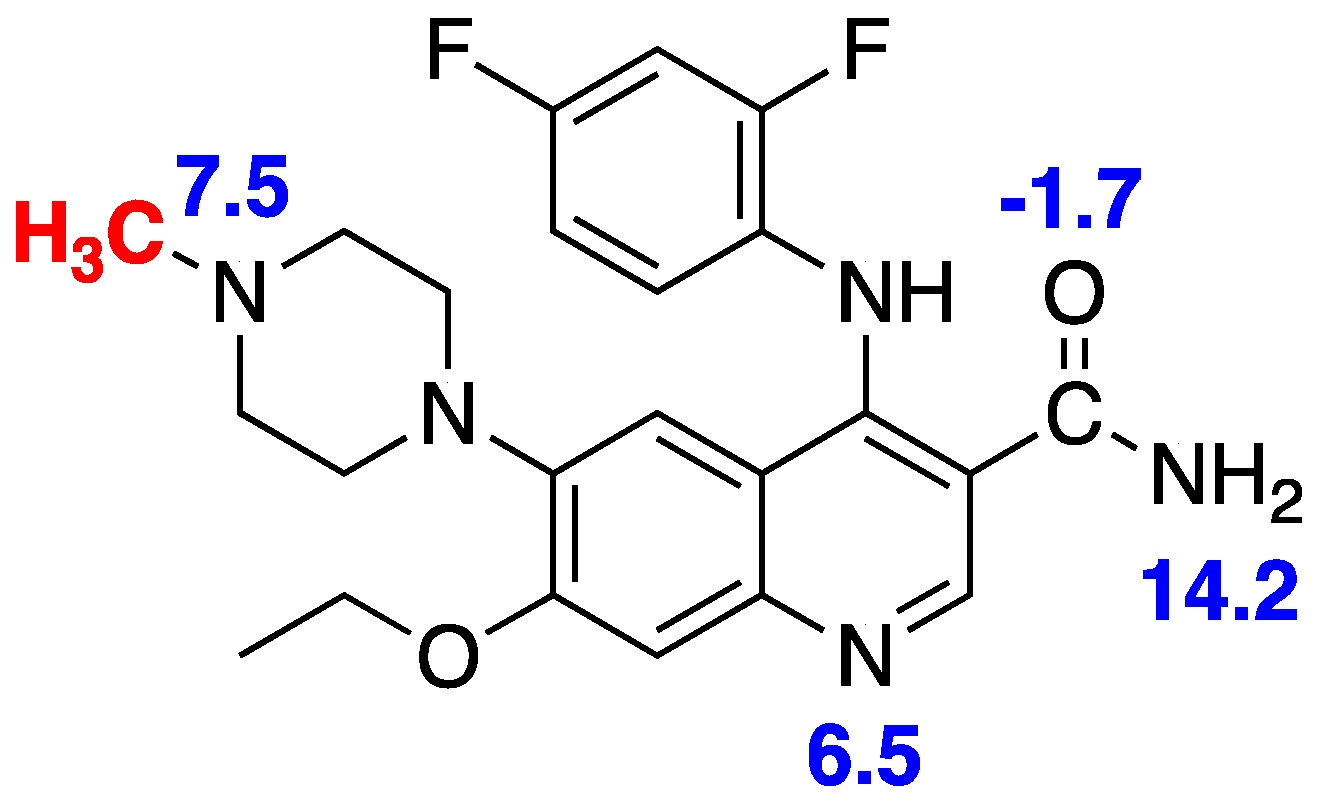


| Property | Preferred Value for Successful CNS Drugs [31] | [11C]AZ683 [23,32] |
|---|---|---|
| Activity | Low nM | Ki = 8 nM; IC50 = 6 nM |
| cLogP | <5 (Lipinski’s Ro5 [29]) <2.7 (optimized [29]) | 3.1 |
| tPSA | 60–70 Å2 | 83 Å2 |
| Molecular weight | ≤450 g/mol | 441 g/mol |
| H-bond donors | ≤3 | 2 |
| H-bond acceptors | ≤7 | 6 |
| Rotatable bonds | <8 | 8 |
| Metabolic stability | T1/2 > 3.1 h | 2.1 h |
| Solubility | >60 µg/mL | 128 µg/mL |
| pKa | 7.5–10.5 | 6.5–7.5 |
| cLogP—(N + O) | >0 | −4 |
© 2018 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tanzey, S.S.; Shao, X.; Stauff, J.; Arteaga, J.; Sherman, P.; Scott, P.J.H.; Mossine, A.V. Synthesis and Initial In Vivo Evaluation of [11C]AZ683—A Novel PET Radiotracer for Colony Stimulating Factor 1 Receptor (CSF1R). Pharmaceuticals 2018, 11, 136. https://0-doi-org.brum.beds.ac.uk/10.3390/ph11040136
Tanzey SS, Shao X, Stauff J, Arteaga J, Sherman P, Scott PJH, Mossine AV. Synthesis and Initial In Vivo Evaluation of [11C]AZ683—A Novel PET Radiotracer for Colony Stimulating Factor 1 Receptor (CSF1R). Pharmaceuticals. 2018; 11(4):136. https://0-doi-org.brum.beds.ac.uk/10.3390/ph11040136
Chicago/Turabian StyleTanzey, Sean S., Xia Shao, Jenelle Stauff, Janna Arteaga, Phillip Sherman, Peter J. H. Scott, and Andrew V. Mossine. 2018. "Synthesis and Initial In Vivo Evaluation of [11C]AZ683—A Novel PET Radiotracer for Colony Stimulating Factor 1 Receptor (CSF1R)" Pharmaceuticals 11, no. 4: 136. https://0-doi-org.brum.beds.ac.uk/10.3390/ph11040136