Stereoselective Anti-Cancer Activities of Ginsenoside Rg3 on Triple Negative Breast Cancer Cell Models
Abstract
:1. Introduction
2. Results
2.1. Interaction of Rg3 with AQP1
2.1.1. Molecular Docking of Rg3
2.1.2. Stereoselectivity of Rg3 in Inhibiting AQP1 Water Channel
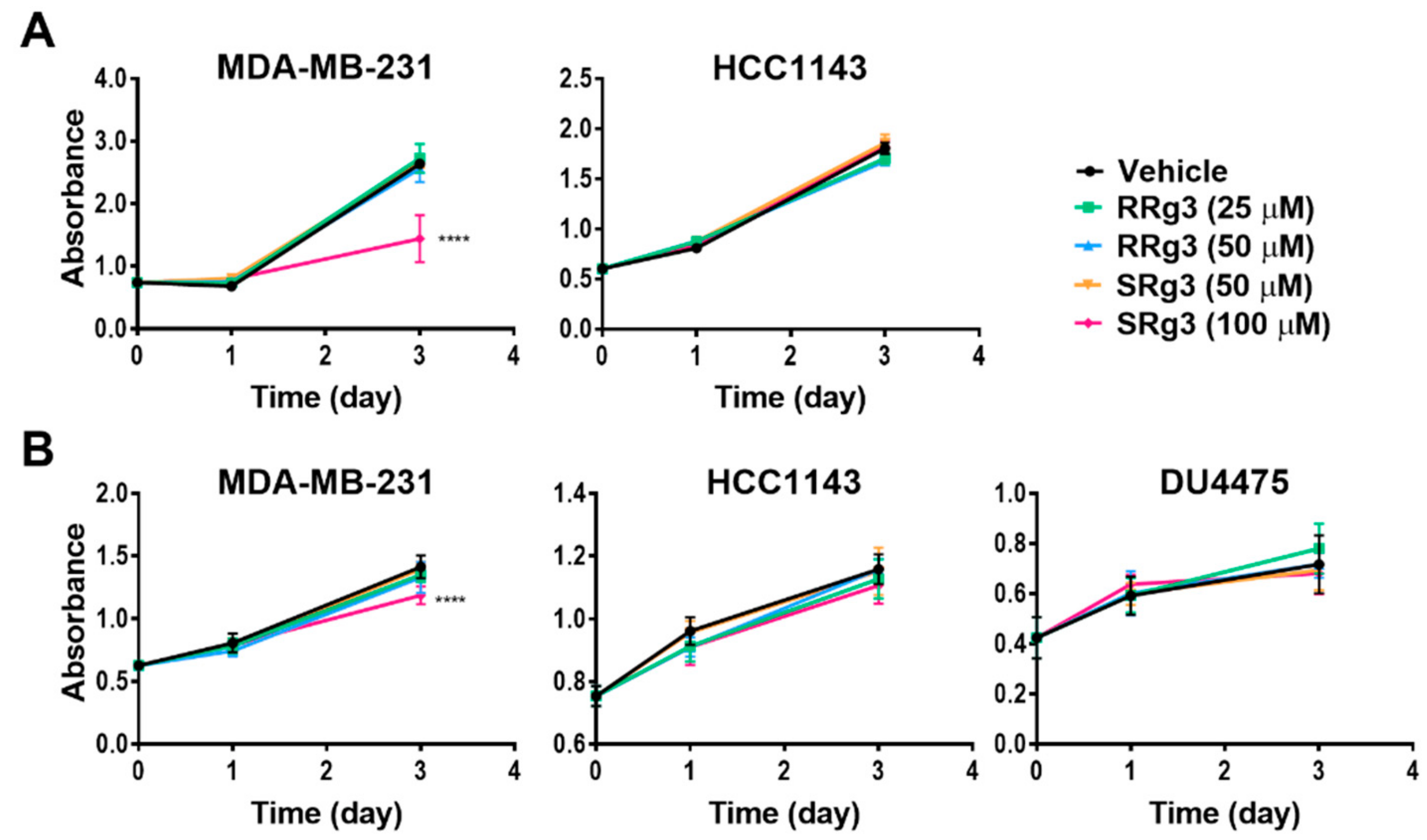
2.2. Rg3 Has Stereoselectivity and Cell Line-Specificity in Inhibition of Proliferation
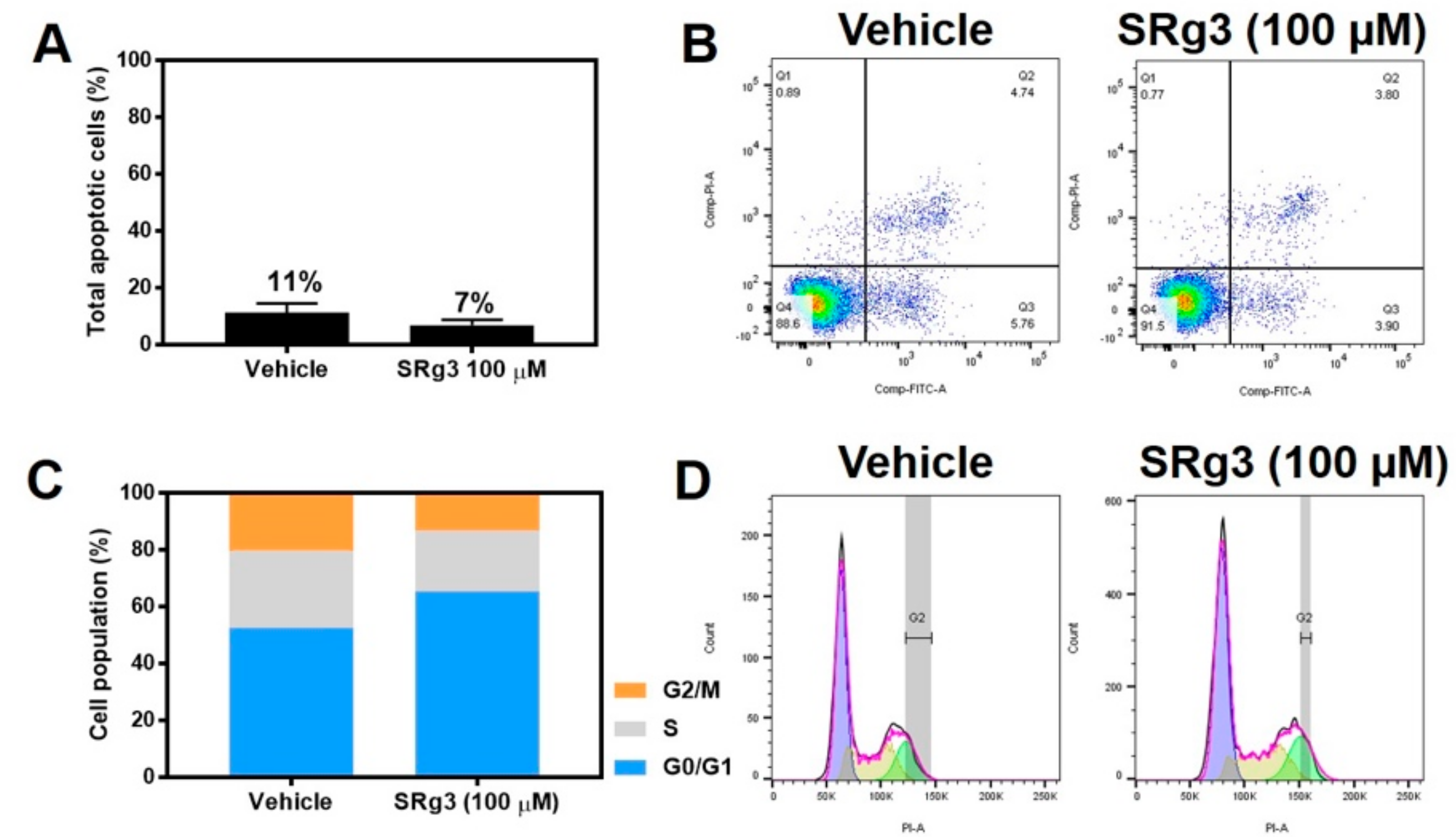
2.3. Cytostatic Effect of SRg3 Inhibits Cell Proliferation in MDA-MB-231 Cell Line without Inducing Apoptosis
2.4. Stereoselective Inhibition of Migration of MDA-MB-231 Cell Line
2.5. Stereoselective Inhibition of Invasion
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Molecular Docking of Rg3
4.3. Oocyte Expression System and Swelling Assay
4.4. Cell Culture
4.5. Quantitative PCR for Expression of AQP1
4.6. Proliferation Assay
4.7. Apoptosis Assay
4.8. Cell Cycle Analysis
4.9. Scratch Wound Closure Assay
4.10. Transwell Migration Assay
4.11. Spheroid Invasion Assay
4.12. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Yang, M.S.; Wu, M.Y. Chinese ginseng. In Nutraceuticals; Elsevier: Amsterdam, The Nederlands, 2016; pp. 693–705. [Google Scholar]
- Szczuka, D.; Nowak, A.; Zakłos-Szyda, M.; Kochan, E.; Szymańska, G.; Motyl, I.; Blasiak, J.J.N. American ginseng (panax quinquefolium l.) as a source of bioactive phytochemicals with pro-health properties. Nutrients 2019, 11, 1041. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Su, F.; Su, X.; Hu, T.; Hu, S. Stereospecific antioxidant effects of ginsenoside rg3 on oxidative stress induced by cyclophosphamide in mice. Fitoterapia 2012, 83, 636–642. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-Y.; Lim, K.-M.; Kim, S.-Y.; Bae, O.-N.; Noh, J.-Y.; Chung, S.-M.; Kim, K.; Shin, Y.-S.; Lee, M.-Y.; Chung, J.-H. Vascular smooth muscle dysfunction and remodeling induced by ginsenoside rg3, a bioactive component of ginseng. Toxicol. Sci. 2010, 117, 505–514. [Google Scholar] [CrossRef] [PubMed]
- Sun, M.; Huang, C.; Wang, C.; Zheng, J.; Zhang, P.; Xu, Y.; Chen, H.; Shen, W. Ginsenoside rg3 improves cardiac mitochondrial population quality: Mimetic exercise training. Biochem. Biophys. Res. Commun. 2013, 441, 169–174. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-B.; Kwon, S.K.; Nagar, H.; Jung, S.-B.; Jeon, B.H.; Kim, C.S.; Oh, J.-H.; Song, H.-J.; Kim, C.-S. Rg3-enriched korean red ginseng improves vascular function in spontaneously hypertensive rats. J. Ginseng Res. 2014, 38, 244–250. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Fu, F.; Geng, M.; Jiang, Y.; Yang, J.; Jiang, W.; Wang, C.; Liu, K. Neuroprotective effect of 20 (s)-ginsenoside rg3 on cerebral ischemia in rats. Neurosci. Lett. 2005, 374, 92–97. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Hao, J.; Zhang, J.; Xia, W.; Dong, X.; Hu, X.; Kong, F.; Cui, X. Ginsenoside rg3 promotes beta-amyloid peptide degradation by enhancing gene expression of neprilysin. J. Pharm. Pharmacol. 2009, 61, 375–380. [Google Scholar] [CrossRef] [PubMed]
- He, B.; Chen, P.; Yang, J.; Yun, Y.; Zhang, X.; Yang, R.; Shen, Z. Neuroprotective effect of 20 (r)-ginsenoside rg3 against transient focal cerebral ischemia in rats. Neurosci. Lett. 2012, 526, 106–111. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Anderson, G.A.; Fernandez, T.G.; Dore, S. Efficacy and mechanism of panax ginseng in experimental stroke. Front. Neurosci. 2019, 13, 294. [Google Scholar] [CrossRef]
- Saba, E.; Kim, S.-H.; Kim, S.-D.; Park, S.-J.; Kwak, D.; Oh, J.-H.; Park, C.-K.; Rhee, M.H. Alleviation of diabetic complications by ginsenoside rg3-enriched red ginseng extract in western diet-fed ldl–/–mice. J. Ginseng Res. 2018, 42, 352–355. [Google Scholar] [CrossRef]
- Park, M.W.; Ha, J.; Chung, S.H. 20 (s)-ginsenoside rg3 enhances glucose-stimulated insulin secretion and activates ampk. Biol. Pharm. Bull. 2008, 31, 748–751. [Google Scholar] [CrossRef] [PubMed]
- Kang, K.S.; Yamabe, N.; Kim, H.Y.; Park, J.H.; Yokozawa, T. Therapeutic potential of 20 (s)-ginsenoside rg3 against streptozotocin-induced diabetic renal damage in rats. Eur. J. Pharmacol. 2008, 591, 266–272. [Google Scholar] [CrossRef] [PubMed]
- Kee, J.-Y.; Hong, S.-H. Ginsenoside rg3 suppresses mast cell–mediated allergic inflammation via mitogen-activated protein kinase signaling pathway. J. Ginseng Res. 2019, 43, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Chen, J.; Su, F.; Su, X.; Hu, T.; Hu, S. Stereospecificity of ginsenoside rg3 in promotion of the immune response to ovalbumin in mice. Int. Immunol. 2012, 24, 465–471. [Google Scholar] [CrossRef] [PubMed]
- Wu, R.; Ru, Q.; Chen, L.; Ma, B.; Li, C. Stereospecificity of ginsenoside rg3 in the promotion of cellular immunity in hepatoma h22-bearing mice. J. Food Sci. 2014, 79, H1430–H1435. [Google Scholar] [CrossRef] [PubMed]
- Yi, Y.-S. Roles of ginsenosides in inflammasome activation. J. Ginseng Res. 2019, 43, 172–178. [Google Scholar] [CrossRef] [PubMed]
- Nakhjavani, M.; Hardingham, J.E.; Palethorpe, H.M.; Tomita, Y.; Smith, E.; Price, T.J.; Townsend, A.R. Ginsenoside rg3: Potential molecular targets and therapeutic indication in metastatic breast cancer. Medicines 2019, 6, 17. [Google Scholar] [CrossRef]
- Park, E.-H.; Kim, Y.-J.; Yamabe, N.; Park, S.-H.; Kim, H.-K.; Jang, H.-J.; Kim, J.H.; Cheon, G.J.; Ham, J.; Kang, K.S. Stereospecific anticancer effects of ginsenoside rg3 epimers isolated from heat-processed american ginseng on human gastric cancer cell. J. Ginseng Res. 2014, 38, 22–27. [Google Scholar] [CrossRef]
- Jeong, S.M.; Lee, J.-H.; Kim, J.-H.; Lee, B.-H.; Yoon, I.-S.; Lee, J.-H.; Kim, D.-H.; Rhim, H.; Kim, Y.; Nah, S.-Y. Stereospecificity of ginsenoside rg 3 action on ion channels. Mol. Cells 2004, 18, 383–389. [Google Scholar]
- Ismail-Khan, R.; Bui, M.M. A review of triple-negative breast cancer. Cancer Control 2010, 17, 173–176. [Google Scholar] [CrossRef]
- Kim, B.-M.; Kim, D.-H.; Park, J.-H.; Na, H.-K.; Surh, Y.-J. Ginsenoside rg3 induces apoptosis of human breast cancer (mda-mb-231) cells. J. Cancer Prev. 2013, 18, 177–185. [Google Scholar] [CrossRef] [PubMed]
- Kim, B.-M.; Kim, D.-H.; Park, J.-H.; Surh, Y.-J.; Na, H.-K. Ginsenoside rg3 inhibits constitutive activation of nf-κb signaling in human breast cancer (mda-mb-231) cells: Erk and akt as potential upstream targets. J. Cancer Prev. 2014, 19, 23. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.-Z.; Aung, H.H.; Zhang, B.; Sun, S.; Li, X.-L.; He, H.; Xie, J.-T.; He, T.-C.; Du, W.; Yuan, C.-S. Chemopreventive effects of heat-processed panax quinquefolius root on human breast cancer cells. Anticancer Res. 2008, 28, 2545–2551. [Google Scholar] [PubMed]
- Chen, X.-P.; Qian, L.-L.; Jiang, H.; Chen, J.-H. Ginsenoside rg3 inhibits cxcr 4 expression and related migrations in a breast cancer cell line. Int. J. Clin. Oncol. 2011, 16, 519–523. [Google Scholar] [CrossRef] [PubMed]
- Yool, A.J.; Brown, E.A.; Flynn, G.A. Roles for novel pharmacological blockers of aquaporins in the treatment of brain oedema and cancer. Clin. Exp. Pharmacol. Physiol. 2010, 37, 403–409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yool, A.J. Functional domains of aquaporin-1: Keys to physiology, and targets for drug discovery. Curr. Pharm. Des. 2007, 13, 3212–3221. [Google Scholar] [CrossRef] [PubMed]
- Dorward, H.S.; Du, A.; Bruhn, M.A.; Wrin, J.; Pei, J.V.; Evdokiou, A.; Price, T.J.; Yool, A.J.; Hardingham, J.E. Pharmacological blockade of aquaporin-1 water channel by aqb013 restricts migration and invasiveness of colon cancer cells and prevents endothelial tube formation in vitro. J. Exp. Clin. Cancer Res. 2016, 35, 36–45. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, M.; Saadoun, S.; Verkman, A. Aquaporins and cell migration. Pflug. Arch.—Eur. J. Physiol. 2008, 456, 693–700. [Google Scholar] [CrossRef]
- Endo, M.; Jain, R.K.; Witwer, B.; Brown, D. Water channel (aquaporin 1) expression and distribution in mammary carcinomas and glioblastomas. Microvasc. Res. 1999, 58, 89–98. [Google Scholar] [CrossRef]
- Esteva-Font, C.; Jin, B.-J.; Verkman, A. Aquaporin-1 gene deletion reduces breast tumor growth and lung metastasis in tumor-producing mmtv-pyvt mice. FASEB J. 2014, 28, 1446–1453. [Google Scholar]
- Shi, Z.; Zhang, T.; Luo, L.; Zhao, H.; Cheng, J.; Xiang, J.; Zhao, C. Aquaporins in human breast cancer: Identification and involvement in carcinogenesis of breast cancer. J. Surg. Oncol. 2012, 106, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Otterbach, F.; Callies, R.; Adamzik, M.; Kimmig, R.; Siffert, W.; Schmid, K.W.; Bankfalvi, A. Aquaporin 1 (aqp1) expression is a novel characteristic feature of a particularly aggressive subgroup of basal-like breast carcinomas. Breast Cancer Res. Treat. 2010, 120, 67–76. [Google Scholar] [PubMed]
- Palethorpe, H.; Tomita, Y.; Smith, E.; Pei, J.; Townsend, A.; Price, T.; Young, J.; Yool, A.; Hardingham, J. The aquaporin 1 inhibitor bacopaside ii reduces endothelial cell migration and tubulogenesis and induces apoptosis. Int. J. Mol. Sci. 2018, 19, 653. [Google Scholar] [CrossRef] [PubMed]
- Smith, E.; Tomita, Y.; Palethorpe, H.M.; Howell, S.; Nakhjavani, M.; Townsend, A.R.; Price, T.J.; Young, J.P.; Hardingham, J.E. Reduced aquaporin-1 transcript expression in colorectal carcinoma is associated with promoter hypermethylation. Epigenetics 2019, 14, 158–170. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tomita, Y.; Palethorpe, H.M.; Smith, E.; Nakhjavani, M.; Townsend, A.R.; Price, T.J.; Yool, A.J.; Hardingham, J.E. Bumetanide-derived aquaporin 1 inhibitors, aqb013 and aqb050 inhibit tube formation of endothelial cells through induction of apoptosis and impaired migration in vitro. Int. J. Mol. Sci. 2019, 20, 1818. [Google Scholar] [CrossRef] [PubMed]
- Tomita, Y.; Dorward, H.; Yool, A.; Smith, E.; Townsend, A.; Price, T.; Hardingham, J. Role of aquaporin 1 signalling in cancer development and progression. Int. J. Mol. Sci. 2017, 18, 299. [Google Scholar] [CrossRef] [PubMed]
- Pei, J.V.; Kourghi, M.; De Ieso, M.L.; Campbell, E.M.; Dorward, H.S.; Hardingham, J.E.; Yool, A.J. Differential inhibition of water and ion channel activities of mammalian aquaporin-1 by two structurally related bacopaside compounds derived from the medicinal plant bacopa monnieri. Mol. Pharmacol. 2016, 90, 496–507. [Google Scholar] [CrossRef] [PubMed]
- De Ieso, M.L.; Yool, A.J. Mechanisms of aquaporin-facilitated cancer invasion and metastasis. Front. Chem. 2018, 6, 135. [Google Scholar]
- Dai, X.; Cheng, H.; Bai, Z.; Li, J. Breast cancer cell line classification and its relevance with breast tumor subtyping. J. Cancer 2017, 8, 3131. [Google Scholar] [CrossRef]
- Kao, J.; Salari, K.; Bocanegra, M.; Choi, Y.-L.; Girard, L.; Gandhi, J.; Kwei, K.A.; Hernandez-Boussard, T.; Wang, P.; Gazdar, A.F. Molecular profiling of breast cancer cell lines defines relevant tumor models and provides a resource for cancer gene discovery. PLoS ONE 2009, 4, e6146. [Google Scholar] [CrossRef]
- Riaz, M.; van Jaarsveld, M.T.; Hollestelle, A.; Prager-van der Smissen, W.J.; Heine, A.A.; Boersma, A.W.; Liu, J.; Helmijr, J.; Ozturk, B.; Smid, M. Mirna expression profiling of 51 human breast cancer cell lines reveals subtype and driver mutation-specific mirnas. Breast Cancer Res. 2013, 15, R33. [Google Scholar] [CrossRef] [PubMed]
- Mobasheri, A.; Shakibaei, M.; Marples, D. Immunohistochemical localization of aquaporin 10 in the apical membranes of the human ileum: A potential pathway for luminal water and small solute absorption. Histochem. Cell Biol. 2004, 121, 463–471. [Google Scholar] [CrossRef] [PubMed]
- Migliati, E.; Meurice, N.; DuBois, P.; Fang, J.S.; Somasekharan, S.; Beckett, E.; Flynn, G.; Yool, A.J. Inhibition of aquaporin-1 and aquaporin-4 water permeability by a derivative of the loop diuretic bumetanide acting at an internal pore-occluding binding site. Mol. Pharmacol. 2009, 76, 105–112. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.-Y.; Guo, H.; Han, J.; Hao, F.; An, Y.; Xu, Y.; Xiaokaiti, Y.; Pan, Y.; Li, X.-J. Ginsenoside rg3 attenuates cell migration via inhibition of aquaporin 1 expression in pc-3m prostate cancer cells. Eur. J. Pharmacol. 2012, 683, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Shan, X.; Fu, Y.-S.; Aziz, F.; Wang, X.-Q.; Yan, Q.; Liu, J.-W. Ginsenoside rg3 inhibits melanoma cell proliferation through down-regulation of histone deacetylase 3 (hdac3) and increase of p53 acetylation. PLoS ONE 2014, 9, e115401. [Google Scholar] [CrossRef] [PubMed]
- Galán-Cobo, A.; Ramírez-Lorca, R.; Toledo-Aral, J.J.; Echevarría, M. Aquaporin-1 plays important role in proliferation by affecting cell cycle progression. J. Cell. Physiol. 2016, 231, 243–256. [Google Scholar] [CrossRef] [PubMed]
- Justus, C.R.; Leffler, N.; Ruiz-Echevarria, M.; Yang, L.V. In vitro cell migration and invasion assays. J. Vis. Exp. 2013, 752, 10–24. [Google Scholar]
- Liu, T.; Zhao, L.; Zhang, Y.; Chen, W.; Liu, D.; Hou, H.; Ding, L.; Li, X. Ginsenoside 20 (s)-rg3 targets hif-1α to block hypoxia-induced epithelial-mesenchymal transition in ovarian cancer cells. PLoS ONE 2014, 9, e103887. [Google Scholar] [CrossRef]
- Pelagalli, A.; Nardelli, A.; Fontanella, R.; Zannetti, A. Inhibition of aqp1 hampers osteosarcoma and hepatocellular carcinoma progression mediated by bone marrow-derived mesenchymal stem cells. Int. J. Mol. Sci. 2016, 17, 1102. [Google Scholar] [CrossRef]
- Qin, F.; Zhang, H.; Shao, Y.; Liu, X.; Yang, L.; Huang, Y.; Fu, L.; Gu, F.; Ma, Y. Expression of aquaporin1, a water channel protein, in cytoplasm is negatively correlated with prognosis of breast cancer patients. Oncotarget 2016, 7, 8143. [Google Scholar] [CrossRef]
- Kim, Y.-J.; Choi, W.-I.; Jeon, B.-N.; Choi, K.-C.; Kim, K.; Kim, T.-J.; Ham, J.; Jang, H.J.; Kang, K.S.; Ko, H. Stereospecific effects of ginsenoside 20-rg3 inhibits tgf-β1-induced epithelial–mesenchymal transition and suppresses lung cancer migration, invasion and anoikis resistance. Toxicology 2014, 322, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Shen, D.; Li, X.; Shan, X.; Wang, X.; Yan, Q.; Liu, J. Ginsenoside rg3 inhibits epithelial-mesenchymal transition (emt) and invasion of lung cancer by down-regulating fut4. Oncotarget 2016, 7, 1619. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Feng, X.; Song, X.; Zhou, H.; Zhao, Y.; Cheng, L.; Jia, L. Mir-493-5p attenuates the invasiveness and tumorigenicity in human breast cancer by targeting fut4. Oncol. Rep. 2016, 36, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Yan, X.; Lin, Y.; Liu, S.; Yan, Q. Fucosyltransferase iv (fut4) as an effective biomarker for the diagnosis of breast cancer. BioMed Pharmacother. 2015, 70, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Yool, A.J.; Morelle, J.; Cnops, Y.; Verbavatz, J.-M.; Campbell, E.M.; Beckett, E.A.; Booker, G.W.; Flynn, G.; Devuyst, O. Aqf026 is a pharmacologic agonist of the water channel aquaporin-1. J. Am. Soc. Nephrol. 2013, 24, 1045–1052. [Google Scholar] [CrossRef] [PubMed]
- Borrel, A. Development of Computational Methods to Predict Protein Pocket Druggability and profile Ligands Using Structural Data. Ph.D. Thesis, University of Helsinki, Helsinki, Finland, 2016. [Google Scholar]
- Smith, E.; Palethorpe, H.M.; Tomita, Y.; Pei, J.V.; Townsend, A.R.; Price, T.J.; Young, J.P.; Yool, A.J.; Hardingham, J.E. The purified extract from the medicinal plant bacopa monnieri, bacopaside ii, inhibits growth of colon cancer cells in vitro by inducing cell cycle arrest and apoptosis. Cells 2018, 7, 81. [Google Scholar] [CrossRef] [PubMed]
- Palethorpe, H.M.; Leach, D.A.; Need, E.F.; Drew, P.A.; Smith, E. Myofibroblast androgen receptor expression determines cell survival in co-cultures of myofibroblasts and prostate cancer cells in vitro. Oncotarget 2018, 9, 19100–19114. [Google Scholar] [CrossRef] [Green Version]
- Palethorpe, H.M.; Drew, P.A.; Smith, E. Androgen signaling in esophageal adenocarcinoma cell lines in vitro. Dig. Dis. Sci. 2017, 62, 3402–3414. [Google Scholar] [CrossRef]
- Kourghi, M.; Pei, J.V.; De Ieso, M.L.; Flynn, G.; Yool, A.J. Bumetanide derivatives aqb007 and aqb011 selectively block the aquaporin-1 ion channel conductance and slow cancer cell migration. Mol. Pharmacol. 2016, 89, 133–140. [Google Scholar] [CrossRef]
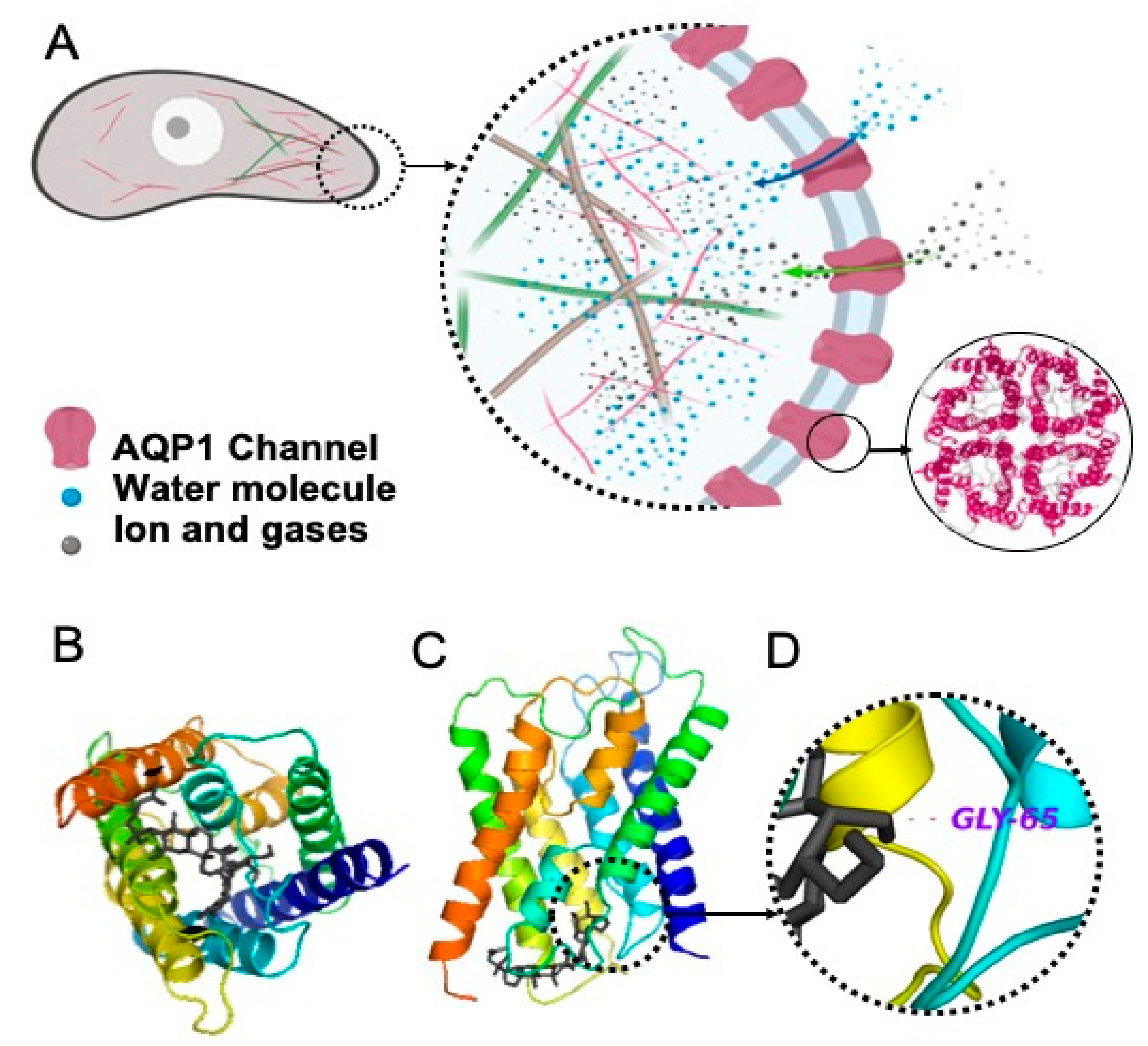



| Molecule | Binding Score (kJ mol−1) | |||
|---|---|---|---|---|
| AQP1 | AQP2 | AQP4 | AQP5 | |
| Ginsenoside Rg3 | −9.4 | −6.4 | −6.1 | −4 |
| Bacopaside I | −9.2 [38] | 7.4 | −5.2 | −6.9 |
| Bacopaside II | −9.3 [38] | 2.2 | −5.2 | −6.4 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nakhjavani, M.; Palethorpe, H.M.; Tomita, Y.; Smith, E.; Price, T.J.; Yool, A.J.; Pei, J.V.; Townsend, A.R.; Hardingham, J.E. Stereoselective Anti-Cancer Activities of Ginsenoside Rg3 on Triple Negative Breast Cancer Cell Models. Pharmaceuticals 2019, 12, 117. https://0-doi-org.brum.beds.ac.uk/10.3390/ph12030117
Nakhjavani M, Palethorpe HM, Tomita Y, Smith E, Price TJ, Yool AJ, Pei JV, Townsend AR, Hardingham JE. Stereoselective Anti-Cancer Activities of Ginsenoside Rg3 on Triple Negative Breast Cancer Cell Models. Pharmaceuticals. 2019; 12(3):117. https://0-doi-org.brum.beds.ac.uk/10.3390/ph12030117
Chicago/Turabian StyleNakhjavani, Maryam, Helen M. Palethorpe, Yoko Tomita, Eric Smith, Timothy J. Price, Andrea J. Yool, Jinxin V. Pei, Amanda R. Townsend, and Jennifer E. Hardingham. 2019. "Stereoselective Anti-Cancer Activities of Ginsenoside Rg3 on Triple Negative Breast Cancer Cell Models" Pharmaceuticals 12, no. 3: 117. https://0-doi-org.brum.beds.ac.uk/10.3390/ph12030117





