Fighting Hypoxia to Improve PDT
Abstract
:1. Introduction
2. O2 vehicles
2.1. Hyperoxygenation/Hyperbaric Oxygenation (HBO)
2.2. Red Blood Cells (RBC) or Hemoglobin (Hb)
2.3. Perfluorocarbon
2.4. O2 Microbubbles
2.5. Other
3. Modification of Tumour Microenvironment (TME)
3.1. H2O2 Decomposition
3.1.1. MnO2
3.1.2. Catalase
3.1.3. Platinum (Pt) NPs
3.1.4. Others
3.2. Water Splitting
3.3. Destruction of Tumour Extracellular Matrix (ECM)
3.4. Decrease of Tumour O2 Consumption
3.5. Others
4. Combined Therapies
4.1. Chemo-PDT
4.1.1. Tirapazamine (TPZ)
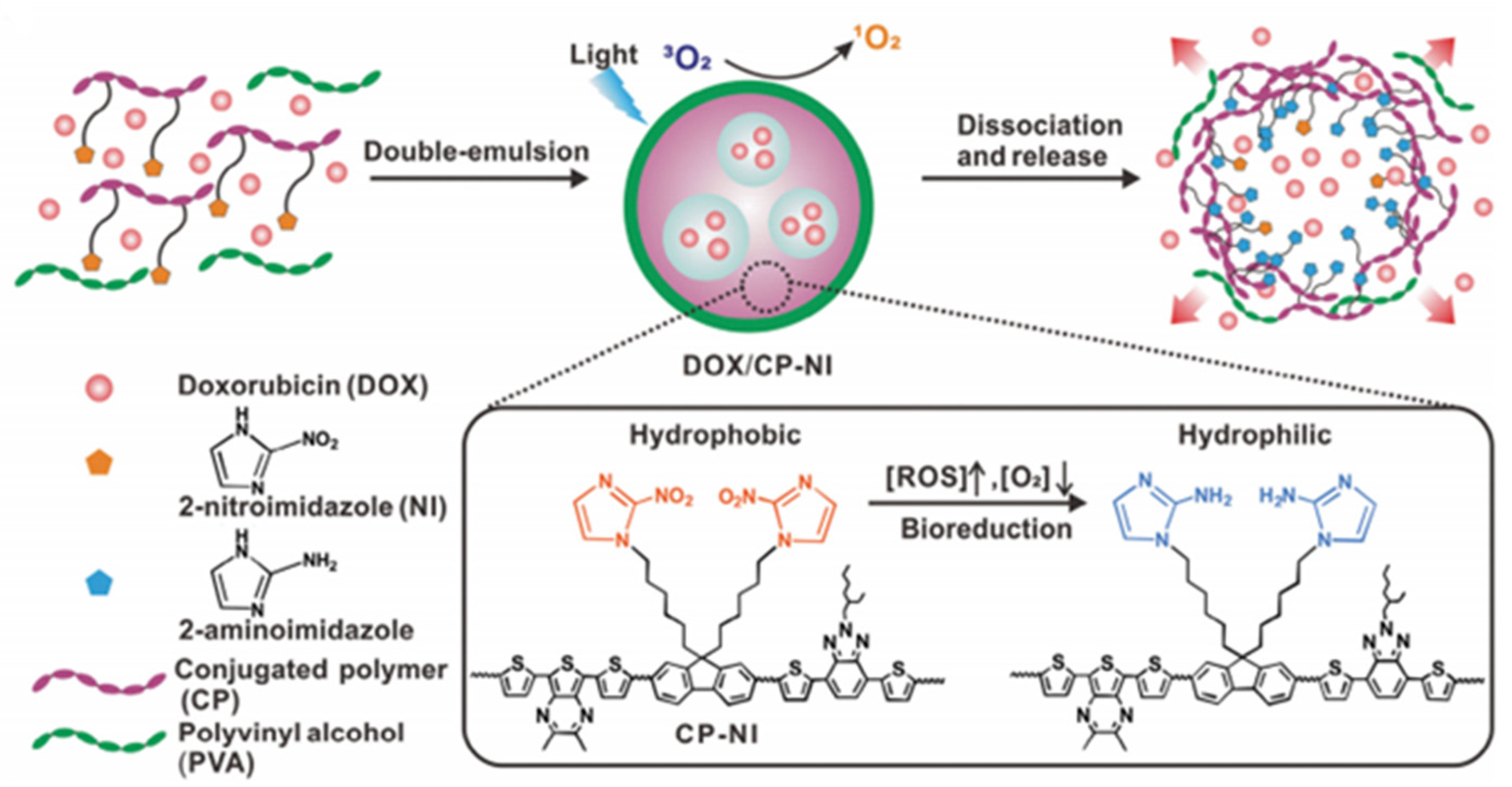
4.1.2. Doxorubicin (Dox)
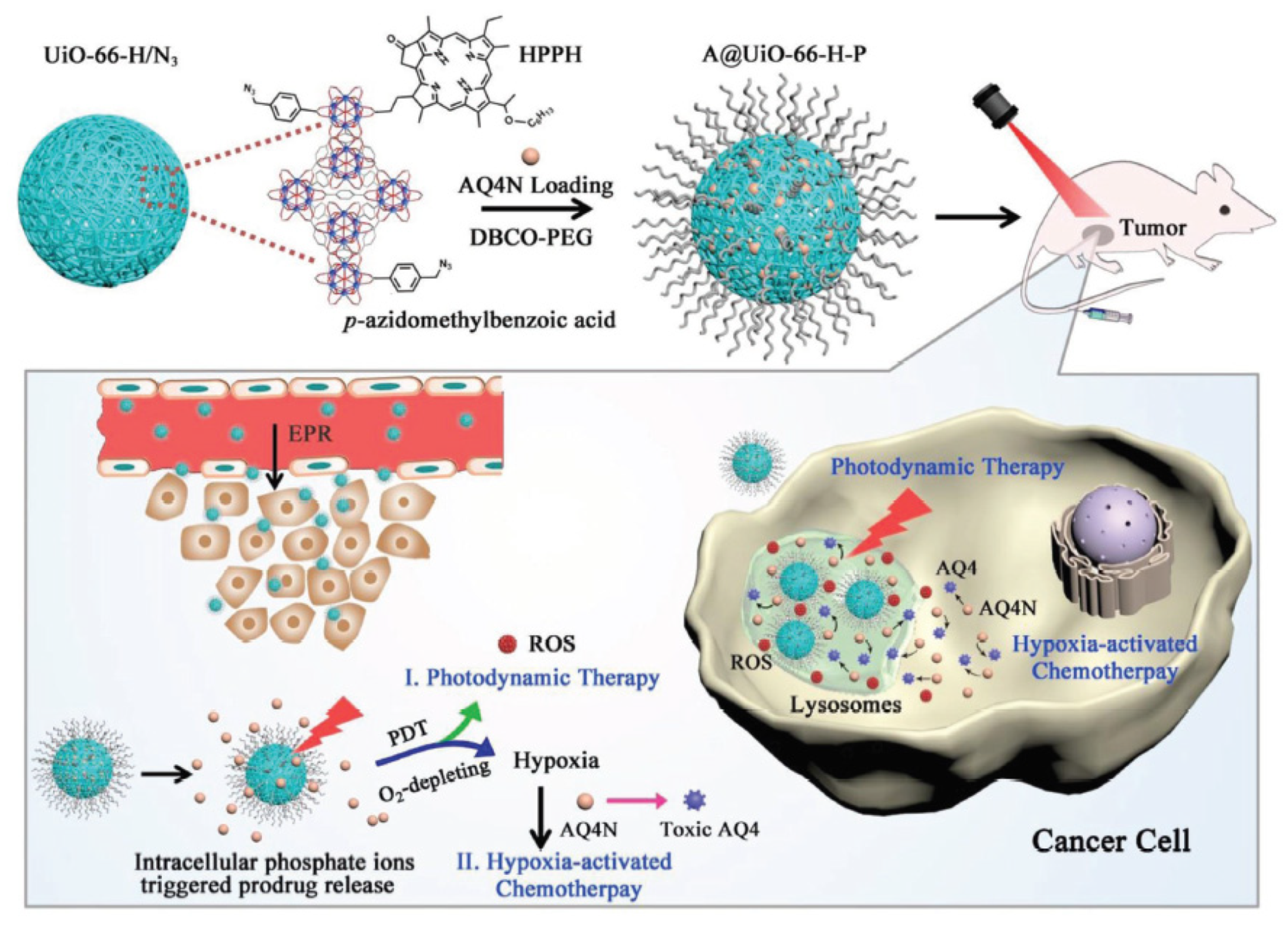
4.1.3. AQ4N
4.1.4. Platinium drugs
4.2. Antiangiogenic-PDT
4.2.1. HIF-1α inhibitors
4.2.2. VEGF Inhibitors
4.2.3. Others
4.3. PTT/PDT
4.4. Imuno-PDT
5. Hypoxia-Independent PDT
5.1. PDT Type I
5.2. O2 independent Cytotoxic Compounds
5.3. NO Donors
5.4. O2 Donor
5.5. Active Compounds in both Normoxic and Hypoxic Conditions
6. Hypoxia-Dependent PDT
6.1. Hypoxia-Reducible Compounds
6.1.1. Azobenzene (AZO)
6.1.2. Other
6.2. Environment-Accumulated Hypoxia Compounds
6.3. Other
7. Fractional PDT
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Semenza, G.L. Defining the role of hypoxia-inducible factor 1 in cancer biology and therapeutics. Oncogene 2009, 29, 625. [Google Scholar] [CrossRef] [PubMed]
- Hammond, E.M.; Asselin, M.C.; Forster, D.; O’Connor, J.P.B.; Senra, J.M.; Williams, K.J. The meaning, measurement and modification of hypoxia in the laboratory and the clinic. Clin. Oncol. 2014, 26, 277–288. [Google Scholar] [CrossRef] [PubMed]
- Dang, J.J.; He, H.; Chen, D.L.; Yin, L.C. Manipulating tumor hypoxia toward enhanced photodynamic therapy (PDT). Biomater. Sci. 2017, 5, 1500–1511. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Kwon, N.; Guo, T.; Liu, Z.; Yoon, J. Innovative strategies for hypoxic-tumor photodynamic therapy. Angew. Chem. Int. Ed. 2018, 57, 11522–11531. [Google Scholar] [CrossRef]
- Freitas, I.; Baronzio, G.F. Tumor hypoxia, reoxygenation and oxygenation strategies: Possible role in photodynamic therapy. J. Photochem. Photobiol. B Biol. 1991, 11, 3–30. [Google Scholar] [CrossRef]
- Frochot, C.; Mordon, S. Update of the situation of clinical photodynamic therapy in Europe in the 2003–2018 period. J. Porphyr. Phthalocyanines 2019, 23, 347–357. [Google Scholar] [CrossRef]
- Price, M.; Heilbrun, L.; Kessel, D. Effects of the oxygenation level on formation of different reactive oxygen species during photodynamic therapy. Photochem. Photobiol. 2013, 89, 683–686. [Google Scholar] [CrossRef]
- Freitas, I. Facing hypoxia: A must for photodynamic therapy. J. Photochem. Photobiol. B Biol. 1988, 2, 281–282. [Google Scholar] [CrossRef]
- Busch, T.M.; Hahn, S.M.; Wileyto, E.P.; Koch, C.J.; Fraker, D.L.; Zhang, P.; Putt, M.; Gleason, K.; Shin, D.B.; Emanuele, M.J.; et al. Hypoxia and photofrin uptake in the intraperitoneal carcinomatosis and sarcomatosis of photodynamic therapy patients. Clin. Cancer Res. 2004, 10, 4630–4638. [Google Scholar] [CrossRef]
- Busch, T.M.; Hahn, S.M.; Evans, S.M.; Koch, C.J. Depletion of tumor oxygenation during photodynamic therapy: Detection by the hypoxia marker EF3 2-(2-nitroimidazol-1 H -yl)-N-(3,3,3-trifluoropropyl) acetamide. Cancer Res. 2000, 60, 2636–2642. [Google Scholar]
- Hirsch, B.D.; Walz, N.C.; Meeker, B.E.; Arnfield, M.R.; Tulip, J.; McPhee, M.S.; Chapman, J.D. Photodynamic therapy-induced hypoxia in rat-tumors and normal-tissues. Photochem. Photobiol. 1987, 46, 847–852. [Google Scholar] [CrossRef] [PubMed]
- Busch, T.M. Hypoxia and perfusion labeling during photodynamic therapy. In Photodynamic Therapy: Methods and Protocols; Gomer, C.J., Ed.; Humana Press: Totowa, NJ, USA, 2010; Volume 635, pp. 107–120. [Google Scholar]
- Al-Waili, N.S.; Butler, G.J. Phototherapy and malignancy: Possible enhancement by iron administration and hyperbaric oxygen. Med. Hypotheses 2006, 67, 1148–1158. [Google Scholar] [CrossRef] [PubMed]
- Al-Waili, N.S.; Butler, G.J.; Beale, J.; Hamilton, R.W.; Lee, B.Y.; Lucas, P. Hyperbaric oxygen and malignancies: A potential role in radiotherapy, chemotherapy, tumor surgery and phototherapy. Med. Sci. Monit. 2005, 11, RA279–RA289. [Google Scholar] [PubMed]
- Li, Q.; Huang, C.; Liu, L.; Hu, R.; Qu, J. Enhancing Type I Photochemistry in Photodynamic Therapy Under Near Infrared Light by Using Antennae–Fullerene Complexes. Cytom. Part A 2018, 93, 997–1003. [Google Scholar] [CrossRef] [PubMed]
- Usacheva, M.; Swaminathan, S.K.; Kirtane, A.R.; Panyam, J. Enhanced photodynamic therapy and effective elimination of cancer stem cells using surfactant-polymer nanoparticles. Mol. Pharm. 2014, 11, 3186–3195. [Google Scholar] [CrossRef]
- Ariffin, A.B.; Forde, P.F.; Jahangeer, S.; Soden, D.M.; Hinchion, J. Releasing pressure in tumors: What do we know so far and where do we go from here? A review. Cancer Res. 2014, 74, 2655–2662. [Google Scholar] [CrossRef]
- Fuchs, J.; Thiele, J. The role of oxygen in cutaneous photodynamic therapy. Free Radic. Biol. Med. 1998, 24, 835–847. [Google Scholar] [CrossRef]
- Moen, I.; Stuhr, L.E. Hyperbaric oxygen therapy and cancer—A review. Target. Oncol. 2012, 7, 233–242. [Google Scholar] [CrossRef]
- Hjelde, A.; Gederaas, O.A.; Krokan, H.E.; Brubakk, A.O. Lack of effect of hyperoxia on photodynamic therapy and lipid peroxidation in three different cancer cell lines. Med. Sci. Monit. 2005, 11, Br351–Br356. [Google Scholar]
- Mei, L.H.; Yang, G.; Fang, F. Hyperbaric oxygen combined with 5-aminolevulinic acid photodynamic therapy inhibitedhuman squamous cell proliferation. Biol. Pharm. Bull. 2019, 42, 394–400. [Google Scholar] [CrossRef]
- Chen, Q.; Beckers, J.; Hetzel, F.W. Modification of tumor response by manipulation of tumor oxygenation. In Optical Methods for Tumor Treatment and Detection: Mechanisms and Techniques in Photodynamic Therapy, Proceedings of SPIE—The International Society for Optical Engineering, San Jose, CA, USA, 23 January 1999; Dougherty, T.J., Katzir, A., Eds.; International Society for Optics and Photonics: San Diego, CA, USA, 1999; Volume 3592, pp. 60–64. [Google Scholar]
- Chen, Q.; Huang, Z.; Chen, H.; Shapiro, H.; Beckers, J.; Hetzel, F.W. Improvement of tumor response to photodynamic therapy by manipulation of tumor oxygenation in an In vivo model system. In Proceedings of the Photonics Asia, Shanghai, China, 12 september 2002; Volume 4916, pp. 43–52. [Google Scholar]
- Hetzel, F.W.; Shakil, A.; Beckers, J.; Chen, Q. Hyperoxygenation enhances photodynamic therapy tumor cure. In Proceedings of the International Symposium on Biomedical Optics (BiOS 2001), San Jose, CA, USA, 9 April 2001; Dougherty, T.J., Ed.; Volume 4248, pp. 56–59. [Google Scholar]
- Huang, Z.; Chen, Q.; Shakil, A.; Chen, H.; Beckers, J.; Shapiro, H.; Hetzel, F.W. Hyperoxygenation enhances the tumor cell killing of photofrin-mediated photodynamic therapy. Photochem. Photobiol. 2003, 78, 496–502. [Google Scholar] [CrossRef]
- Li, J.Q.; Huang, J.Z.; Ao, Y.X.; Li, S.Y.; Miao, Y.; Yu, Z.Z.; Zhu, L.T.; Lan, X.L.; Zhu, Y.H.; Zhang, Y.; et al. Synergizing upconversion nanophotosensitizers with hyperbaric oxygen to remodel the extracellular matrix for enhanced photodynamic cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 22985–22996. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Anegg, U.; Tomaselli, F.; Rehak, P.; Sankin, O.; Fell, B.; Renner, H.; Pinter, H.; Smolle-Juttner, F.M.; Friehs, G.B. Does hyperbaric oxygen enhance the effect of photodynamic therapy in patients with advanced esophageal carcinoma? A clinical pilot study. Endoscopy 2000, 32, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Maier, A.; Anegg, U.; Fell, B.; Rehak, P.; Ratzenhofer, B.; Tomaselli, F.; Sankin, O.; Pinter, H.; Smolle-Juttner, F.M.; Friehs, G.B. Hyperbaric oxygen and photodynamic therapy in the treatment of advanced carcinoma of the cardia and the esophagus. Lasers Surg. Med. 2000, 26, 308–315. [Google Scholar] [CrossRef]
- Maier, A.; Tomaselli, F.; Anegg, U.; Rehak, P.; Fell, B.; Luznik, S.; Pinter, H.; Smolle-Juttner, F.M. Combined photodynamic therapy and hyperbaric oxygenation in carcinoma of the esophagus and the esophago-gastric junction. Eur. J. Cardiothorac. Surg. 2000, 18, 649–654. [Google Scholar] [CrossRef]
- Maier, A.; Tomaselli, F.; Matzi, V.; Rehak, P.; Pinter, H.; Smolle-Juttner, F.M. Photosensitization with hematoporphyrin derivative compared to 5-aminolaevulinic acid for photodynamic therapy of esophageal carcinoma. Ann. Thorac. Surg. 2001, 72, 1136–1140. [Google Scholar] [CrossRef]
- Maier, A.; Tomaselli, F.; Matzi, V.; Rehak, P.; Pinter, H.; Smolle-Juttner, F.M. Does new photosensitizer improve photodynamic therapy in advanced esophageal carcinoma? Lasers Surg. Med. 2001, 29, 323–327. [Google Scholar] [CrossRef]
- Tomaselli, F.; Maier, A.; Pinter, H.; Stranzl, H.; Smolle-Juttner, F.M. Photodynamic therapy enhanced by hyperbaric oxygen in acute endoluminal palliation of malignant bronchial stenosis (clinical pilot study in 40 patients). Eur. J. Cardiothorac. Surg. 2001, 19, 549–554. [Google Scholar] [CrossRef]
- Tomaselli, F.; Maier, A.; Sankin, O.; Anegg, U.; Stranzl, U.; Pinter, H.; Kapp, K.; Smolle-Juttner, F.M. Acute effects of combined photodynamic therapy and hyperbaric oxygenation in lung cancer—A clinical pilot study. Lasers Surg. Med. 2001, 28, 399–403. [Google Scholar] [CrossRef]
- Schouwink, H.; Ruevekamp, M.; Oppelaar, H.; van Veen, R.; Baas, P.; Stewart, F.A. Photodynamic therapy for malignant mesothelioma: Preclinical studies for optimization of treatment protocols. Photochem. Photobiol. 2001, 73, 410–417. [Google Scholar] [CrossRef]
- Asimov, M.M. The biomedical effect of laser-induced photodissociation of oxyhemoglobin In vivo. Opt. Spectrosc. 2013, 115, 774–778. [Google Scholar] [CrossRef]
- Luo, Z.Y.; Zheng, M.B.; Zhao, P.F.; Chen, Z.; Siu, F.M.; Gong, P.; Gao, G.H.; Sheng, Z.H.; Zheng, C.F.; Ma, Y.F.; et al. Self-monitoring artificial red cells with sufficient oxygen supply for enhanced photodynamic therapy. Sci. Rep. 2016, 6, 23393. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.K.; Liu, L.L.; Liang, R.J.; Luo, Z.Y.; He, H.M.; Wu, Z.H.; Tian, H.; Zheng, M.B.; Ma, Y.F.; Cai, L.T. Bioinspired hybrid protein oxygen nanocarrier amplified photodynamic therapy for eliciting anti-tumor immunity and abscopal effect. ACS Nano 2018, 12, 8633–8645. [Google Scholar] [CrossRef]
- Tang, W.; Zhen, Z.P.; Wang, M.Z.; Wang, H.; Chuang, Y.J.; Zhang, W.Z.; Wang, G.D.; Todd, T.; Cowger, T.; Chen, H.M.; et al. Red blood cell-facilitated photodynamic therapy for cancer treatment. Adv. Funct. Mater. 2016, 26, 1757–1768. [Google Scholar] [CrossRef]
- Wang, P.Y.; Li, X.M.; Yao, C.; Wang, W.X.; Zhao, M.Y.; El -Toni, A.M.; Zhang, F. Orthogonal near-infrared upconversion co-regulated site-specific O2 delivery and photodynamic therapy for hypoxia tumor by using red blood cell microcarriers. Biomaterials 2017, 125, 90–100. [Google Scholar] [CrossRef] [PubMed]
- Cao, H.Q.; Wang, L.; Yang, Y.; Li, J.; Qi, Y.F.; Li, Y.; Li, Y.; Wang, H.; Li, J.B. An assembled nanocomplex for improving both therapeutic efficiency and treatment depth in photodynamic therapy. Angew. Chem. Int. Ed. 2018, 57, 7759–7763. [Google Scholar] [CrossRef] [PubMed]
- Guo, X.M.; Qua, J.X.; Zhu, C.Q.; Li, W.; Luo, L.H.; Yang, J.; Yin, X.Y.; Li, Q.P.; Du, Y.Z.; Chen, D.W.; et al. Synchronous delivery of oxygen and photosensitizer for alleviation of hypoxia tumor microenvironment and dramatically enhanced photodynamic therapy. Drug Deliv. 2018, 25, 585–599. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Li, T.W.; Ruan, Z.; Yan, L.F. Polypeptide-based artificial erythrocytes conjugated with near infrared photosensitizers for imaging-guided photodynamic therapy. J. Mater. Sci. 2018, 53, 9368–9381. [Google Scholar] [CrossRef]
- Xu, X.; Cui, Y.C.; Bu, H.X.; Chen, J.M.; Li, Y.; Tang, G.P.; Wang, L.Q. A photosensitizer loaded hemoglobin-polymer conjugate as a nanocarrier for enhanced photodynamic therapy. J. Mater. Chem. B 2018, 6, 1825–1833. [Google Scholar] [CrossRef]
- Liu, W.L.; Liu, T.; Zou, M.Z.; Yu, W.Y.; Li, C.X.; He, Z.Y.; Zhang, M.K.; Liu, M.D.; Li, Z.H.; Feng, J.; et al. Aggressive man-made red blood cells for hypoxia-resistant photodynamic therapy. Adv. Mater. 2018, 30, 1802006. [Google Scholar] [CrossRef]
- Gao, S.T.; Zheng, P.L.; Li, Z.H.; Feng, X.C.; Yan, W.X.; Chen, S.Z.; Guo, W.S.; Liu, D.D.; Yang, X.J.; Wang, S.X.; et al. Biomimetic O2-Evolving metal-organic framework nanoplatform for highly efficient photodynamic therapy against hypoxic tumor. Biomaterials 2018, 178, 83–94. [Google Scholar] [CrossRef] [PubMed]
- Rapoport, N.; Nam, K.H.; Gupta, R.; Gao, Z.; Mohan, P.; Payne, A.; Todd, N.; Liu, X.; Kim, T.; Shea, J.; et al. Ultrasound-mediated tumor imaging and nanotherapy using drug loaded, block copolymer stabilized perfluorocarbon nanoemulsions. J. Control. Release 2011, 153, 4–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Young, L.H.; Jaffe, C.C.; Revkin, J.H.; McNulty, P.H.; Cleman, M. Metabolic and functional effects of perfluorocarbon distal perfusion during coronary angioplasty. Am. J. Cardiol. 1990, 65, 986–990. [Google Scholar] [CrossRef]
- Cheng, Y.H.; Cheng, H.; Jiang, C.X.; Qiu, X.F.; Wang, K.K.; Huan, W.; Yuan, A.; Wu, J.H.; Hu, Y.Q. Perfluorocarbon nanoparticles enhance reactive oxygen levels and tumour growth inhibition in photodynamic therapy. Nat. Commun. 2015, 6, 8785. [Google Scholar] [CrossRef] [PubMed]
- Day, R.A.; Estabrook, D.A.; Logan, J.K.; Sletten, E.M. Fluorous photosensitizers enhance photodynamic therapy with perfluorocarbon nanoemulsions. Chem. Commun. 2017, 53, 13043–13046. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, C.H.; Dong, H.F.; Wu, N.Q.; Cao, Y.; Zhang, X.J. Plasmonic resonance energy transfer enhanced photodynamic therapy with Au@SiO2@Cu2O/perfluorohexane nanocomposites. ACS Appl. Mater. Interfaces 2018, 10, 6991–7002. [Google Scholar] [CrossRef] [PubMed]
- Que, Y.R.; Liu, Y.J.; Tan, W.; Feng, C.; Shi, P.; Li, Y.J.; Huang, X. Enhancing photodynamic therapy efficacy by using fluorinated nanoplatform. ACS Macro Lett. 2016, 5, 168–173. [Google Scholar] [CrossRef]
- Sheng, D.L.; Liu, T.Z.; Deng, L.M.; Zhang, L.; Li, X.L.; Xu, J.; Hao, L.; Li, P.; Ran, H.T.; Chen, H.R.; et al. Perfluorooctyl bromide & indocyanine green co-loaded nanoliposomes for enhanced multimodal imaging-guided phototherapy. Biomaterials 2018, 165, 1–13. [Google Scholar]
- Song, X.J.; Feng, L.Z.; Liang, C.; Yang, K.; Liu, Z. Ultrasound triggered tumor oxygenation with oxygen-shuttle nanoperfluorocarbon to overcome hypoxia-associated resistance in cancer therapies. Nano Lett. 2016, 16, 6145–6153. [Google Scholar] [CrossRef]
- Tang, W.; Yang, Z.; Wang, S.; Wang, Z.T.; Song, J.B.; Yu, G.C.; Fan, W.P.; Dai, Y.L.; Wang, J.J.; Shan, L.L.; et al. Organic semiconducting photoacoustic nanodroplets for laser-activatable ultrasound imaging and combinational cancer therapy. ACS Nano 2018, 12, 2610–2622. [Google Scholar] [CrossRef]
- Tang, X.L.; Cheng, Y.H.; Huang, S.T.; Zhi, F.; Yuan, A.H.; Hu, Y.Q.; Wu, J.H. Overcome the limitation of hypoxia against photodynamic therapy to treat cancer cells by using perfluorocarbon nanodroplet for photosensitizer delivery. Biochem. Biophys. Res. Commun. 2017, 487, 483–487. [Google Scholar] [CrossRef]
- Tao, D.L.; Feng, L.Z.; Chao, Y.; Liang, C.; Song, X.J.; Wang, H.R.; Yang, K.; Liu, Z. Covalent organic polymers based on fluorinated porphyrin as oxygen nanoshuttles for tumor hypoxia relief and enhanced photodynamic therapy. Adv. Funct. Mater. 2018, 28, 1804901. [Google Scholar] [CrossRef]
- Wang, J.P.; Liu, L.; You, Q.; Song, Y.L.; Sun, Q.; Wang, Y.D.; Cheng, Y.; Tan, F.P.; Li, N. All-in-one theranostic nanoplatform based on hollow mosx for photothermally-maneuvered oxygen self-enriched photodynamic therapy. Theranostics 2018, 8, 955–971. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Li, J.M.; Yu, H.; Deng, K.; Zhou, W.; Wang, C.X.; Zhang, Y.; Li, K.H.; Zhuo, R.X.; Huang, S.W. Fluorinated polymeric micelles to overcome hypoxia and enhance photodynamic cancer therapy. Biomater. Sci. 2018, 6, 3096–3107. [Google Scholar] [CrossRef] [PubMed]
- Yu, M.; Xu, X.L.; Cai, Y.J.; Zou, L.Y.; Shuai, X.T. Perfluorohexane-cored nanodroplets for stimulations-responsive ultrasonography and O2-potentiated photodynamic therapy. Biomaterials 2018, 175, 61–71. [Google Scholar] [CrossRef]
- Yuan, P.; Ruan, Z.; Jiang, W.; Liu, L.; Dou, J.X.; Li, T.W.; Yan, L.F. Oxygen self-sufficient fluorinated polypeptide nanoparticles for NIR imaging-guided enhanced photodynamic therapy. J. Mater. Chem. B 2018, 6, 2323–2331. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, D.; Yang, K.; Sheng, D.L.; Tan, B.; Wang, Z.G.; Ran, H.T.; Yi, H.J.; Zhong, Y.X.; Lin, H.; et al. Mitochondria-targeted artificial “nano-RBCs” for amplified synergistic cancer phototherapy by a single NIR irradiation. Adv. Sci. 2018, 5, 1800049. [Google Scholar] [CrossRef]
- Zhao, C.Y.; Tong, Y.J.; Li, X.L.; Shao, L.H.; Chen, L.; Lu, J.Q.; Deng, X.W.; Wang, X.; Wu, Y. Photosensitive nanoparticles combining vascular-independent intratumor distribution and on-demand oxygen-depot delivery for enhanced cancer photodynamic therapy. Small 2018, 14, 1703045. [Google Scholar] [CrossRef]
- Hu, D.R.; Zhong, L.; Wang, M.Y.; Li, H.H.; Qu, Y.; Liu, Q.Y.; Han, R.X.; Yuan, L.P.; Shi, K.; Peng, J.R.; et al. Perfluorocarbon-loaded and redox-activatable photosensitizing agent with oxygen supply for enhancement of fluorescence/photoacoustic imaging guided tumor photodynamic therapy. Adv. Funct. Mater. 2019, 29, 1806199. [Google Scholar] [CrossRef]
- Hu, H.M.; Yan, X.F.; Wang, H.; Tanaka, J.; Wang, M.Z.; You, W.; Li, Z.B. Perfluorocarbon-based O2 nanocarrier for efficient photodynamic therapy. J. Mater. Chem. B 2019, 7, 1116–1123. [Google Scholar] [CrossRef]
- Ma, S.N.; Zhou, J.; Zhang, Y.X.; Yang, B.; He, Y.Y.; Tian, C.; Xu, X.H.; Gu, Z.W. An oxygen self-sufficient fluorinated nanoplatform for relieved tumor hypoxia and enhanced photodynamic therapy of cancers. ACS Appl. Mater. Interfaces 2019, 11, 7731–7742. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.S.; Hwang, J.; Lee, K.; Choi, Y.; Kim, K.; Koo, H.J.; Hong, J.W.; Choi, J. Oxygen-carrying micro/nanobubbles: Composition, synthesis techniques and potential prospects in photo-triggered theranostics. Molecules 2018, 23, 2210. [Google Scholar] [CrossRef] [PubMed]
- Huang, W.C.; Shen, M.Y.; Chen, H.H.; Lin, S.C.; Chiang, W.H.; Wu, P.H.; Chang, C.W.; Chiang, C.S.; Chiu, H.C. Monocytic delivery of therapeutic oxygen bubbles for dual-modality treatment of tumor hypoxia. J. Control. Release 2015, 220, 738–750. [Google Scholar] [CrossRef] [PubMed]
- Song, R.Y.; Hu, D.H.; Chung, H.Y.; Sheng, Z.H.; Yao, S.H. Lipid-polymer bilaminar oxygen nanobubbles for enhanced photodynamic therapy of cancer. ACS Appl. Mater. Interfaces 2018, 10, 36805–36813. [Google Scholar] [CrossRef] [PubMed]
- Niu, N.; Zhang, Z.; Gao, X.; Chen, Z.J.; Li, S.J.; Li, J. Photodynamic therapy in hypoxia: Near-infrared-sensitive, self-supported, oxygen generation nano-platform enabled by upconverting nanoparticles. Chem. Eng. J. 2018, 352, 818–827. [Google Scholar] [CrossRef]
- Castells, M.; Thibault, B.; Delord, J.P.; Couderc, B. Implication of tumor microenvironment in chemoresistance: Tumor-associated stromal cells protect tumor cells from cell death. Int. J. Mol. Sci. 2012, 13, 9545–9571. [Google Scholar] [CrossRef]
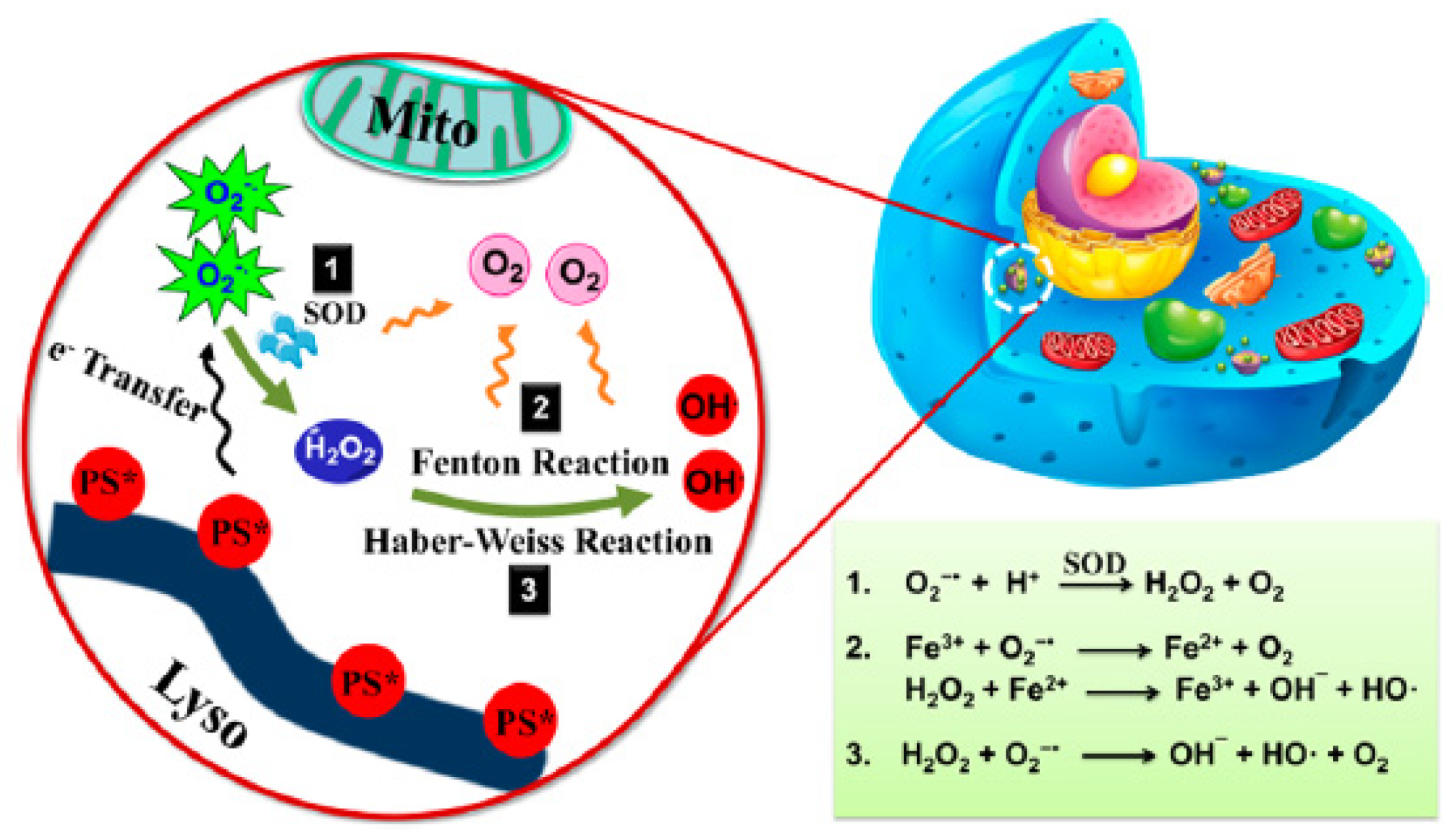
- Szatrowski, T.P.; Nathan, C.F. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991, 51, 794–798. [Google Scholar]
- Chen, Q.; Chen, J.; Liang, C.; Feng, L.; Dong, Z.; Song, X.; Song, G.; Liu, Z. Drug-induced co-assembly of albumin/catalase as smart nano-theranostics for deep intra-tumoral penetration, hypoxia relieve, and synergistic combination therapy. J. Control. Release 2017, 263, 79–89. [Google Scholar] [CrossRef]
- Liu, C.-P.; Wu, T.-H.; Liu, C.-Y.; Chen, K.-C.; Chen, Y.-X.; Chen, G.-S.; Lin, S.-Y. Self-Supplying O2 through the Catalase-Like Activity of Gold Nanoclusters for Photodynamic Therapy against Hypoxic Cancer Cells. Small 2017, 13, 1700278. [Google Scholar] [CrossRef]
- Policastro, L.; Molinari, B.; Larcher, F.; Blanco, P.; Podhajcer, O.L.; Costa, C.S.; Rojas, P.; Duran, H. Imbalance of antioxidant enzymes in tumor cells and inhibition of proliferation and malignant features by scavenging hydrogen peroxide. Mol. Carcinog. 2004, 39, 103–113. [Google Scholar] [CrossRef]
- Onumah, O.E.; Jules, G.E.; Zhao, Y.; Zhou, L.; Yang, H.; Guo, Z. Overexpression of catalase delays G0/G1- to S-phase transition during cell cycle progression in mouse aortic endothelial cells. Free Radic. Biol. Med. 2009, 46, 1658–1667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, X.L.; Shao, J.; Chen, Q.Y.; Li, C.H.; Kong, M.Y.; Fang, F.; Ji, L.; Boison, D.; Huang, T.; Gao, J.; et al. A Mn(II) complex of boradiazaindacene (BODIPY) loaded graphene oxide as both LED light and H2O2 enhanced anticancer agent. J. Inorg. Biochem. 2016, 159, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.W.; Dong, Z.L.; Fu, T.T.; Liu, J.J.; Chen, Q.; Li, Y.G.; Zhu, R.; Xu, L.G.; Liu, Z. Modulation of hypoxia in solid tumor microenvironment with MnO2 nanoparticles to enhance photodynamic therapy. Adv. Funct. Mater. 2016, 26, 5490–5498. [Google Scholar] [CrossRef]
- Chu, C.C.; Lin, H.R.; Liu, H.; Wang, X.Y.; Wang, J.Q.; Zhang, P.F.; Gao, H.Y.; Huang, C.; Zeng, Y.; Tan, Y.Z.; et al. Tumor microenvironment-triggered supramolecular system as an in situ nanotheranostic generator for cancer phototherapy. Adv. Mater. 2017, 29, 1605928. [Google Scholar] [CrossRef]
- Gao, S.; Wang, G.H.; Qin, Z.N.; Wang, X.Y.; Zhao, G.Q.; Ma, Q.J.; Zhu, L. Oxygen-generating hybrid nanoparticles to enhance fluorescent/photoacoustic/ultrasound imaging guided tumor photodynamic therapy. Biomaterials 2017, 112, 324–335. [Google Scholar] [CrossRef] [Green Version]
- Hao, Y.W.; Zhang, B.X.; Zheng, C.X.; Niu, M.Y.; Guo, H.C.; Zhang, H.L.; Chang, J.B.; Zhang, Z.Z.; Wang, L.; Zhang, Y. Multifunctional nanoplatform for enhanced photodynamic cancer therapy and magnetic resonance imaging. Colloids Surf. B. Biointerfaces 2017, 151, 384–393. [Google Scholar] [CrossRef]
- Kim, J.; Cho, H.R.; Jeon, H.; Kim, D.; Song, C.; Lee, N.; Choi, S.H.; Hyeon, T. Continuous O2-evolving MnFe2O4 nanoparticle-anchored mesoporous silica nanoparticles for efficient photodynamic therapy in hypoxic cancer. J. Am. Chem. Soc. 2017, 139, 10992–10995. [Google Scholar] [CrossRef]
- Liu, H.; Liu, Y.; Chu, C.C.; Tong, H.P.; Xue, W.; Wang, J.Q.; Liu, G.; Zhang, W.G. Intelligent albumin-stabilized manganese dioxide nanocomposites for tumor microenvironment responsive phototherapy. J. Biomed. Nanotechnol. 2017, 13, 1321–1332. [Google Scholar] [CrossRef]
- Ma, Z.F.; Jia, X.D.; Bai, J.; Ruan, Y.D.; Wang, C.; Li, J.M.; Zhang, M.C.; Jiang, X. MnO2 Gatekeeper: An intelligent and O2-evolving shell for preventing premature release of high cargo payload core, overcoming tumor hypoxia, and acidic H2O2-sensitive MRI. Adv. Funct. Mater. 2017, 27, 1604258. [Google Scholar] [CrossRef]
- Ai, X.Z.; Hu, M.; Wang, Z.M.; Lyu, L.N.; Zhang, W.M.; Li, J.; Yang, H.H.; Lin, J.; Xing, B.G. Enhanced cellular ablation by attenuating hypoxia status and reprogramming tumor-associated macrophages via NIR light-responsive upconversion nanocrystals. Bioconj. Chem. 2018, 29, 928–938. [Google Scholar] [CrossRef]
- Gu, T.X.; Cheng, L.; Gong, F.; Xu, J.; Li, X.; Han, G.R.; Liu, Z. Upconversion composite nanoparticles for tumor hypoxia modulation and enhanced near-infrared-triggered photodynamic therapy. ACS Appl. Mater. Interfaces 2018, 10, 15494–15503. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.Q.; Jia, Q.Y.; Zheng, X.L.; Wen, Y.M.; Liu, W.M.; Zhang, H.Y.; Ge, J.C.; Wang, P.F. PEGylated carbon dot/MnO2 nanohybrid: A new pH/H2O2-driven, turn-on cancer nanotheranostics. Sci. China Mater. 2018, 61, 1325–1338. [Google Scholar] [CrossRef]
- He, Z.M.; Xiao, Y.; Zhang, J.R.; Zhang, P.H.; Zhu, J.J. In situ formation of large pore silica-MnO2 nanocomposites with H+/H2O2 sensitivity for O2-elevated photodynamic therapy and potential MR imaging. Chem. Commun. 2018, 54, 2962–2965. [Google Scholar] [CrossRef] [PubMed]
- Kapri, S.; Bhattacharyya, S. Molybdenum sulfide-reduced graphene oxide p-n heterojunction nanosheets with anchored oxygen generating manganese dioxide nanoparticles for enhanced photodynamic therapy. Chem. Sci. 2018, 9, 8982–8989. [Google Scholar] [CrossRef] [PubMed]
- Liang, R.J.; Liu, L.L.; He, H.M.; Chen, Z.K.; Han, Z.Q.; Luo, Z.Y.; Wu, Z.H.; Zheng, M.B.; Ma, Y.F.; Cai, L.T. Oxygen-boosted immunogenic photodynamic therapy with gold nanocages@manganese dioxide to inhibit tumor growth and metastases. Biomaterials 2018, 177, 149–160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.H.; Wu, Y.Y.; Meng, X.Q.; Li, S.P.; Zhang, J.L.; Gong, P.; Zhang, P.F.; Jiang, T.; Deng, G.J.; Li, W.J.; et al. Dye-anchored MnO nanoparticles targeting tumor and inducing enhanced phototherapy effect via mitochondria-mediated pathway. Small 2018, 14, 1801008. [Google Scholar] [CrossRef]
- Lin, T.S.; Zhao, X.Z.; Zhao, S.; Yu, H.; Cao, W.M.; Chen, W.; Wei, H.; Guo, H.Q. O2-generating MnO2 nanoparticles for enhanced photodynamic therapy of bladder cancer by ameliorating hypoxia. Theranostics 2018, 8, 990–1004. [Google Scholar] [CrossRef]
- Wang, R.G.; Zhao, M.Y.; Deng, D.; Ye, X.; Zhang, F.; Chen, H.; Kong, J.L. An intelligent and biocompatible photosensitizer conjugated silicon quantum dots-MnO2 nanosystem for fluorescence imaging-guided efficient photodynamic therapy. J. Mater. Chem. B 2018, 6, 4592–4601. [Google Scholar] [CrossRef]
- Zhu, H.J.; Li, J.C.; Qi, X.Y.; Chen, P.; Pu, K.Y. Oxygenic hybrid semiconducting nanoparticles for enhanced photodynamic therapy. Nano Lett. 2018, 18, 586–594. [Google Scholar] [CrossRef]
- Zhu, W.; Zhang, L.; Yang, Z.; Liu, P.; Wang, J.; Cao, J.; Shen, A.; Xu, Z. An efficient tumor-inducible nanotheranostics for magnetic resonance imaging and enhanced photodynamic therapy. Chem. Eng. J. 2019, 358, 969–979. [Google Scholar] [CrossRef]
- Liu, J.; Du, P.; Liu, T.; Cordova Wong, B.J.; Wang, W.; Ju, H.; Lei, J. A black phosphorus/manganese dioxide nanoplatform: Oxygen self-supply monitoring, photodynamic therapy enhancement and feedback. Biomaterials 2019, 192, 179–188. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Meng, X.D.; Wang, D.D.; Zhang, K.; Dai, W.H.; Dong, H.F.; Zhang, X.J. Intelligent MnO2/Cu2-xS for multimode imaging diagnostic and advanced single-laser irradiated photothermal/photodynamic therapy. ACS Appl. Mater. Interfaces 2018, 10, 17732–17741. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.H.; Chen, D.P.; Zou, J.H.; Shao, J.J.; Tang, H.; Xu, H.; Si, W.L.; Dong, X.C. Tumor microenvironment responsive oxygen-self-generating nanoplatform for dual-imaging guided photodynamic and photothermal therapy. ChemistrySelect 2018, 3, 4366–4373. [Google Scholar] [CrossRef]
- Liu, X.; Tian, K.; Zhang, J.; Zhao, M.; Liu, S.; Zhao, Q.; Huang, W. Smart NIR-light-mediated nanotherapeutic agents for enhancing tumor accumulation and overcoming hypoxia in synergistic cancer therapy. ACS Appl. Bio Mater. 2019, 2, 1225–1232. [Google Scholar] [CrossRef]
- Chen, Q.; Feng, L.Z.; Liu, J.J.; Zhu, W.W.; Dong, Z.L.; Wu, Y.F.; Liu, Z. Intelligent albumin-MnO2 nanoparticles as pH-/H2O2-responsive dissociable nanocarriers to modulate tumor hypoxia for effective combination therapy. Adv. Mater. 2016, 28, 7129–7136. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.T.; Li, S.H.; Liu, X.N.; Yang, C.Y.; Hu, N.; Dou, L.N.; Zhao, B.X.; Zhang, Q.Y.; Suo, Y.R.; Wang, J.L. Oxygen-generating MnO2 nanodots-anchored versatile nanoplatform for combined chemo-photodynamic therapy in hypoxic cancer. Adv. Funct. Mater. 2018, 28, 1706375. [Google Scholar] [CrossRef]
- Fan, W.P.; Bu, W.B.; Shen, B.; He, Q.J.; Cui, Z.W.; Liu, Y.Y.; Zheng, X.P.; Zhao, K.L.; Shi, J.L. Intelligent MnO2 nanosheets anchored with upconversion nanoprobes for concurrent pH-/H2O2-responsive UCL imaging and oxygen-elevated synergetic therapy. Adv. Mater. 2015, 27, 4155–4161. [Google Scholar] [CrossRef]
- He, D.G.; Hai, L.; He, X.; Yang, X.; Li, H.W. Glutathione-activatable and O2/Mn2+-evolving nanocomposite for highly efficient and selective photodynamic and gene-silencing dual therapy. Adv. Funct. Mater. 2017, 27, 1704089. [Google Scholar] [CrossRef]
- Jia, Q.Y.; Ge, J.C.; Liu, W.M.; Zheng, X.L.; Chen, S.Q.; Wen, Y.M.; Zhang, H.Y.; Wang, P.F. A magnetofluorescent carbon dot assembly as an acidic H2O2-driven oxygenerator to regulate tumor hypoxia for simultaneous bimodal imaging and enhanced photodynamic therapy. Adv. Mater. 2018, 30, 1706090. [Google Scholar] [CrossRef]
- Chen, H.C.; Tian, J.W.; He, W.J.; Guo, Z.J. H2O2-Activatable and O2-evolving nanoparticles for highly efficient and selective photodynamic therapy against hypoxic tumor cells. J. Am. Chem. Soc. 2015, 137, 1539–1547. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, T.T.; Kirillov, A.M.; Liu, W.S.; Tang, Y. NIR light/H2O2-triggered nanocomposites for a highly efficient and selective synergistic photodynamic and photothermal therapy against hypoxic tumor cells. Chem. Commun. 2016, 52, 7939–7942. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.H.; Chen, Z.W.; Sheng, Z.H.; Gao, D.Y.; Yan, F.; Ma, T.; Zheng, H.R.; Hong, M. A catalase-loaded hierarchical zeolite as an implantable nanocapsule for ultrasound-guided oxygen self-sufficient photodynamic therapy against pancreatic cancer. Nanoscale 2018, 10, 17283–17292. [Google Scholar] [CrossRef] [PubMed]
- Cheng, H.; Zhu, J.Y.; Li, S.Y.; Zeng, J.Y.; Lei, Q.; Chen, K.W.; Zhang, C.; Zhang, X.Z. An O2 self-sufficient biomimetic nanoplatform for highly specific and efficient photodynamic therapy. Adv. Funct. Mater. 2016, 26, 7847–7860. [Google Scholar] [CrossRef]
- Yang, G.B.; Xu, L.G.; Xu, J.; Zhang, R.; Song, G.S.; Chao, Y.; Feng, L.Z.; Han, F.X.; Dong, Z.L.; Li, B.; et al. Smart nanoreactors for pH-responsive tumor homing, mitochondria-targeting, and enhanced photodynamic-immunotherapy of cancer. Nano Lett. 2018, 18, 2475–2484. [Google Scholar] [CrossRef] [PubMed]
- Shen, L.Y.; Huang, Y.; Chen, D.; Qiu, F.; Ma, C.; Jin, X.; Zhu, X.Y.; Zhou, G.Y.; Zhang, Z.Y. pH-Responsive aerobic nanoparticles for effective photodynamic therapy. Theranostics 2017, 7, 4537–4550. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.J.; Li, L.; Zheng, C.X.; Hao, Y.W.; Niu, M.Y.; Hu, Y.J.; Chang, J.B.; Zhang, Z.Z.; Wang, L. An intelligent dual stimuli-responsive photosensitizer delivery system with O2-supplying for efficient photodynamic therapy. Colloids Surf. B. Biointerfaces 2018, 167, 299–309. [Google Scholar] [CrossRef]
- Wu, P.; Cai, Z.; Chen, J.; Zhang, H.; Cai, C. Electrochemical measurement of the flux of hydrogen peroxide releasing from RAW 264.7 macrophage cells based on enzyme-attapulgite clay nanohybrids. Biosens. Bioelectron. 2011, 26, 4012–4017. [Google Scholar] [CrossRef]
- Lu, K.Y.; Lin, P.Y.; Chuang, E.Y.; Shih, C.M.; Cheng, T.M.; Lin, T.Y.; Sung, H.W.; Mi, F.L. H2O2-Depleting and O2-generating selenium nanoparticles for fluorescence imaging and photodynamic treatment of proinflammatory-activated macrophages. ACS Appl. Mater. Interfaces 2017, 9, 5158–5172. [Google Scholar] [CrossRef]
- Ouyang, J.; Wang, L.Q.; Chen, W.S.; Zeng, K.; Han, Y.J.; Xu, Y.; Xu, Q.F.; Deng, L.; Liu, Y.N. Biomimetic nanothylakoids for efficient imaging-guided photodynamic therapy for cancer. Chem. Commun. 2018, 54, 3468–3471. [Google Scholar] [CrossRef]
- Sheptovitsky, Y.G.; Brudvig, G.W. Isolation and characterization of spinach photosystem II membrane-associated catalase and polyphenol oxidase. Biochemistry (Mosc) 1996, 35, 16255–16263. [Google Scholar] [CrossRef]
- Wang, H.R.; Chao, Y.; Liu, J.J.; Zhu, W.W.; Wang, G.L.; Xu, L.G.; Liu, Z.G. Photosensitizer-crosslinked in-situ polymerization on catalase for tumor hypoxia modulation & enhanced photodynamic therapy. Biomaterials 2018, 181, 310–317. [Google Scholar] [PubMed]
- Yang, Y.; Zhu, W.J.; Feng, L.Z.; Chao, Y.; Yi, X.; Dong, Z.L.; Yang, K.; Tan, W.H.; Liu, Z.; Chen, M.W. G-Quadruplex-based nanoscale coordination polymers to modulate tumor hypoxia and achieve nuclear-targeted drug delivery for enhanced photodynamic therapy. Nano Lett. 2018, 18, 6867–6875. [Google Scholar] [CrossRef]
- Ruan, Y.D.; Jia, X.D.; Wang, C.; Zhen, W.Y.; Jiang, X. Mn-Fe layered double hydroxide nanosheets: A new photothermal nanocarrier for O2-evolving phototherapy. Chem. Commun. 2018, 54, 11729–11732. [Google Scholar] [CrossRef] [PubMed]
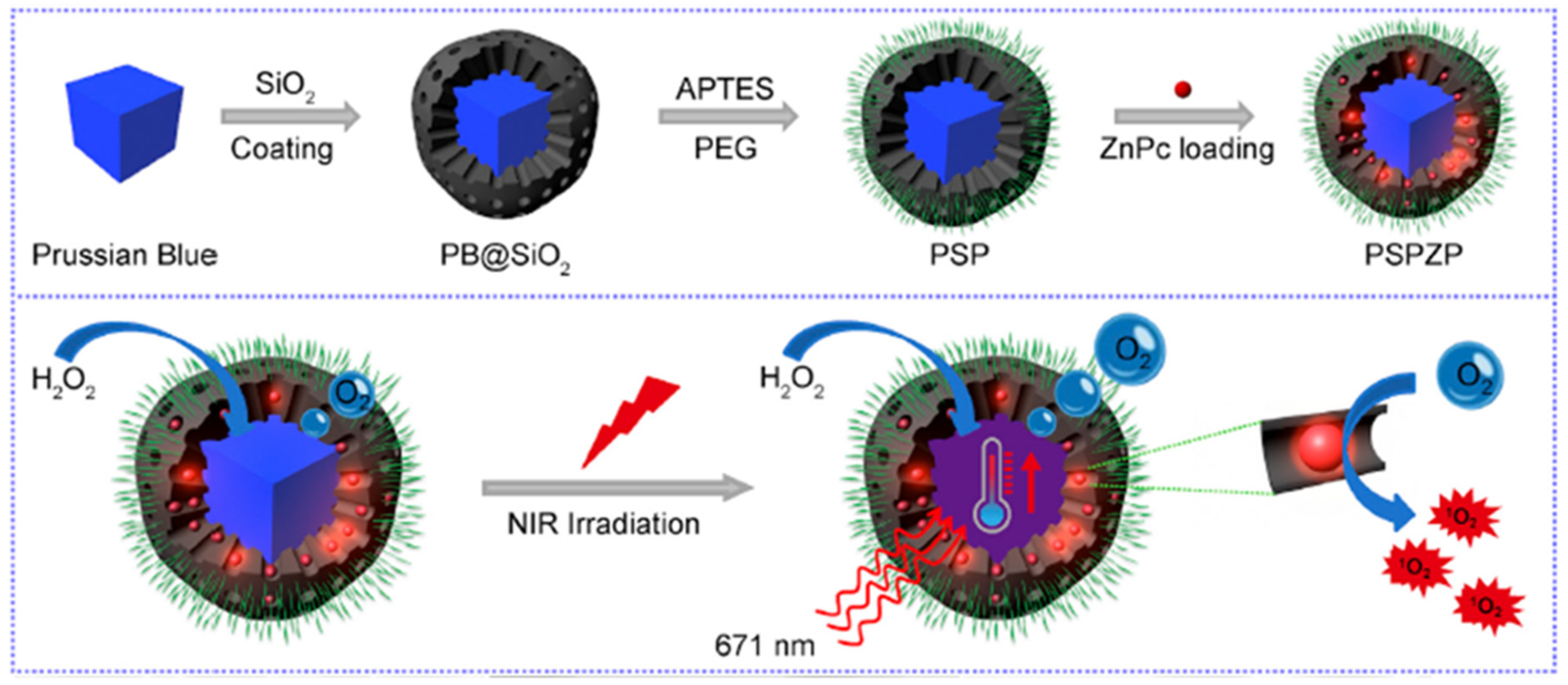
- Wang, D.D.; Shi, R.H.; Zhou, J.J.; Shi, S.X.; Wu, H.H.; Xu, P.P.; Wang, H.; Xia, G.L.; Barnhart, T.E.; Cai, W.B.; et al. Photo-enhanced singlet oxygen generation of prussian blue-based nanocatalyst for augmented photodynamic therapy. iScience 2018, 9, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.L.; Tian, W.; Wang, Q.; Zhao, Y.; Zhang, Y.L.; Tian, Y.; Tang, Y.X.; Wang, S.J.; Liu, Y.; Ni, Q.Q.; et al. Oxygen-evolving mesoporous organosilica coated prussian blue nanoplatform for highly efficient photodynamic therapy of tumors. Adv. Sci. 2018, 5, 1700847. [Google Scholar] [CrossRef]
- Ma, Z.F.; Zhang, M.C.; Jia, X.D.; Bai, J.; Ruan, Y.D.; Wang, C.; Sun, X.P.; Jiang, X. FeIII-doped two-dimensional C3N4 nanofusiform: A new O2-evolving and mitochondria-targeting photodynamic agent for mri and enhanced antitumor therapy. Small 2016, 12, 5477–5487. [Google Scholar] [CrossRef]
- Zhen, W.Y.; Bai, J.; Jia, X.D.; Wu, L.; Jiang, X.E. H2O2-Responsive MB-BSA-Fe(III) nanoparticles as oxygen generators for MRI-guided photodynamic therapy. J. Anal.Test. 2018, 2, 69–76. [Google Scholar] [CrossRef]
- Hu, F.; Mao, D.; Kenry; Wang, Y.X.; Wu, W.B.; Zhao, D.; Kong, D.L.; Liu, B. Metal-organic framework as a simple and general inert nanocarrier for photosensitizers to implement activatable photodynamic therapy. Adv. Funct. Mater. 2018, 28, 1707519. [Google Scholar] [CrossRef]
- Lan, G.X.; Ni, K.Y.; Xu, Z.W.; Veroneau, S.S.; Song, Y.; Lin, W.B. Nanoscale metal-organic framework overcomes hypoxia for photodynamic therapy primed cancer immunotherapy. J. Am. Chem. Soc. 2018, 140, 5670–5673. [Google Scholar] [CrossRef]
- Liu, J.T.; Du, P.; Mao, H.; Zhang, L.; Ju, H.X.; Lei, J.P. Dual-triggered oxygen self-supply black phosphorus nanosystem for enhanced photodynamic therapy. Biomaterials 2018, 172, 83–91. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, F.M.; Liu, C.Q.; Wang, Z.Z.; Kang, L.H.; Huang, Y.Y.; Dong, K.; Ren, J.S.; Qu, X.G. Nanozyme decorated metal-organic frameworks for enhanced photodynamic therapy. ACS Nano 2018, 12, 651–661. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.P.; Li, J.C.; Sun, D.; Li, Q.; Ma, J.Y.; Chen, X.L.; Zhu, X.; Zheng, N.F. A novel theranostic nanoplatform based on Pd@Pt-PEG-Ce6 for enhanced photodynamic therapy by modulating tumor hypoxia microenvironment. Adv. Funct. Mater. 2018, 28, 1706310. [Google Scholar] [CrossRef]
- Wang, X.S.; Zeng, J.Y.; Zhang, M.K.; Zeng, X.; Zhang, X.Z. A versatile Pt-based core-shell nanoplatform as a nanofactory for enhanced tumor therapy. Adv. Funct. Mater. 2018, 28, 1801783. [Google Scholar] [CrossRef]
- Cai, X.L.; Luo, Y.A.; Song, Y.; Liu, D.; Yan, H.Y.; Li, H.; Du, D.; Zhu, C.Z.; Lin, Y.H. Integrating in situ formation of nanozymes with three-dimensional dendritic mesoporous silica nanospheres for hypoxia-overcoming photodynamic therapy. Nanoscale 2018, 10, 22937–22945. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, J.; Deng, Y.Y.; Chen, W.S.; Xu, Q.F.; Wang, L.Q.; Liu, Z.J.; Tang, F.Y.; Deng, L.; Liu, Y.N. Marriage of artificial catalase and black phosphorus nanosheets for photodynamic antitumor therapy. J. Mater. Chem. B 2018, 6, 2057–2064. [Google Scholar] [CrossRef]
- Li, J.J.; Wei, K.; Zuo, S.; Xu, Y.X.; Zha, Z.S.; Ke, W.D.; Chen, H.B.; Ge, Z.S. Light-triggered clustered vesicles with self-supplied oxygen and tissue penetrability for photodynamic therapy against hypoxic tumor. Adv. Funct. Mater. 2017, 27, 1702108. [Google Scholar] [CrossRef]
- Wang, B.; Lin, W.M.; Mao, Z.W.; Gao, C.Y. Near-infrared light triggered photothermal therapy and enhanced photodynamic therapy with a tumor-targeting hydrogen peroxide shuttle. J. Mater. Chem. B 2018, 6, 3145–3155. [Google Scholar] [CrossRef]
- Manifold, R.N.; Anderson, C.D. Increased cutaneous oxygen availability by topical application of hydrogen peroxide cream enhances the photodynamic reaction to topical 5-aminolevulinic acid-methyl ester. Arch. Dermatol. Res. 2011, 303, 285–292. [Google Scholar] [CrossRef]
- Zhen, W.Y.; Liu, Y.; Jia, X.D.; Wu, L.; Wang, C.; Jiang, X. Reductive surfactant-assisted one-step fabrication of a BiOI/BiOIO3 heterojunction biophotocatalyst for enhanced photodynamic theranostics overcoming tumor hypoxia. Nanoscale Horiz. 2019, 4, 720–726. [Google Scholar] [CrossRef]
- Wu, X.; Yan, P.J.; Ren, Z.H.; Wang, Y.F.; Cai, X.J.; Li, X.; Deng, R.R.; Han, G.R. Ferric hydroxide-modified upconversion nanoparticles for 808 nm NIR-triggered synergetic tumor therapy with hypoxia modulation. ACS Appl. Mater. Interfaces 2019, 11, 385–393. [Google Scholar] [CrossRef]
- Jafari, T.; Moharreri, E.; Amin, A.S.; Miao, R.; Song, W.; Suib, S.L. Photocatalytic water splitting-The untamed dream: A review of recent advances. Molecules 2016, 21, 900. [Google Scholar] [CrossRef]
- Hu, L.D.; Wang, P.Y.; Zhao, M.Y.; Liu, L.; Zhou, L.; Li, B.H.; Albaqami, F.H.; El-Toni, A.M.; Li, X.M.; Xie, Y.; et al. Near-infrared rechargeable “optical battery” implant for irradiation-free photodynamic therapy. Biomaterials 2018, 163, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.H.; Zhang, Y.H.; Qiu, W.X.; Zhang, L.; Gao, F.; Li, B.; Xu, L.; Fan, J.X.; Li, Z.H.; Zhang, X.Z. Dual-stage light amplified photodynamic therapy against hypoxic tumor based on an O2 self-sufficient nanoplatform. Small 2017, 13, 1701621. [Google Scholar] [CrossRef] [PubMed]
- Sheng, Y.; Nesbitt, H.; Callan, B.; Taylor, M.A.; Love, M.; McHale, A.P.; Callan, J.F. Oxygen generating nanoparticles for improved photodynamic therapy of hypoxic tumours. J. Control. Release 2017, 264, 333–340. [Google Scholar] [CrossRef] [PubMed]
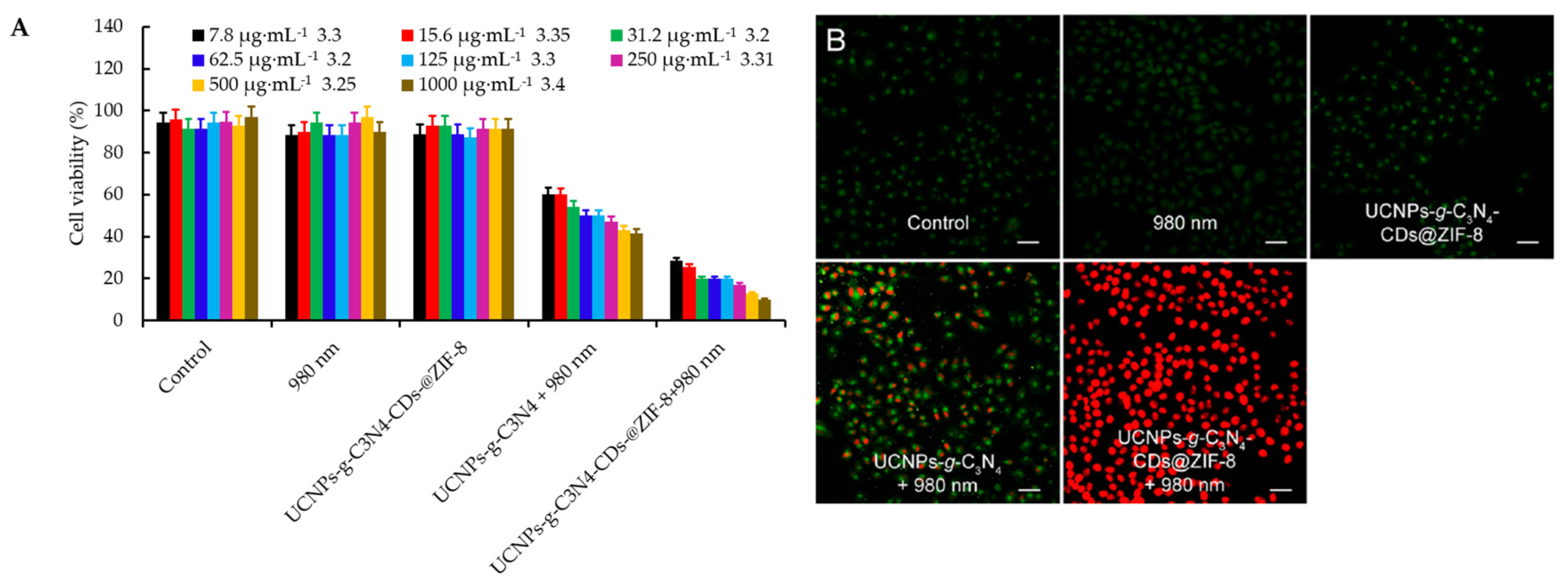
- Zheng, D.W.; Li, B.; Li, C.X.; Fan, J.X.; Lei, Q.; Li, C.; Xu, Z.S.; Zhang, X.Z. Carbon-dot-decorated carbon nitride nanoparticles for enhanced photodynamic therapy against hypoxic tumor via water splitting. ACS Nano 2016, 10, 8715–8722. [Google Scholar] [CrossRef]
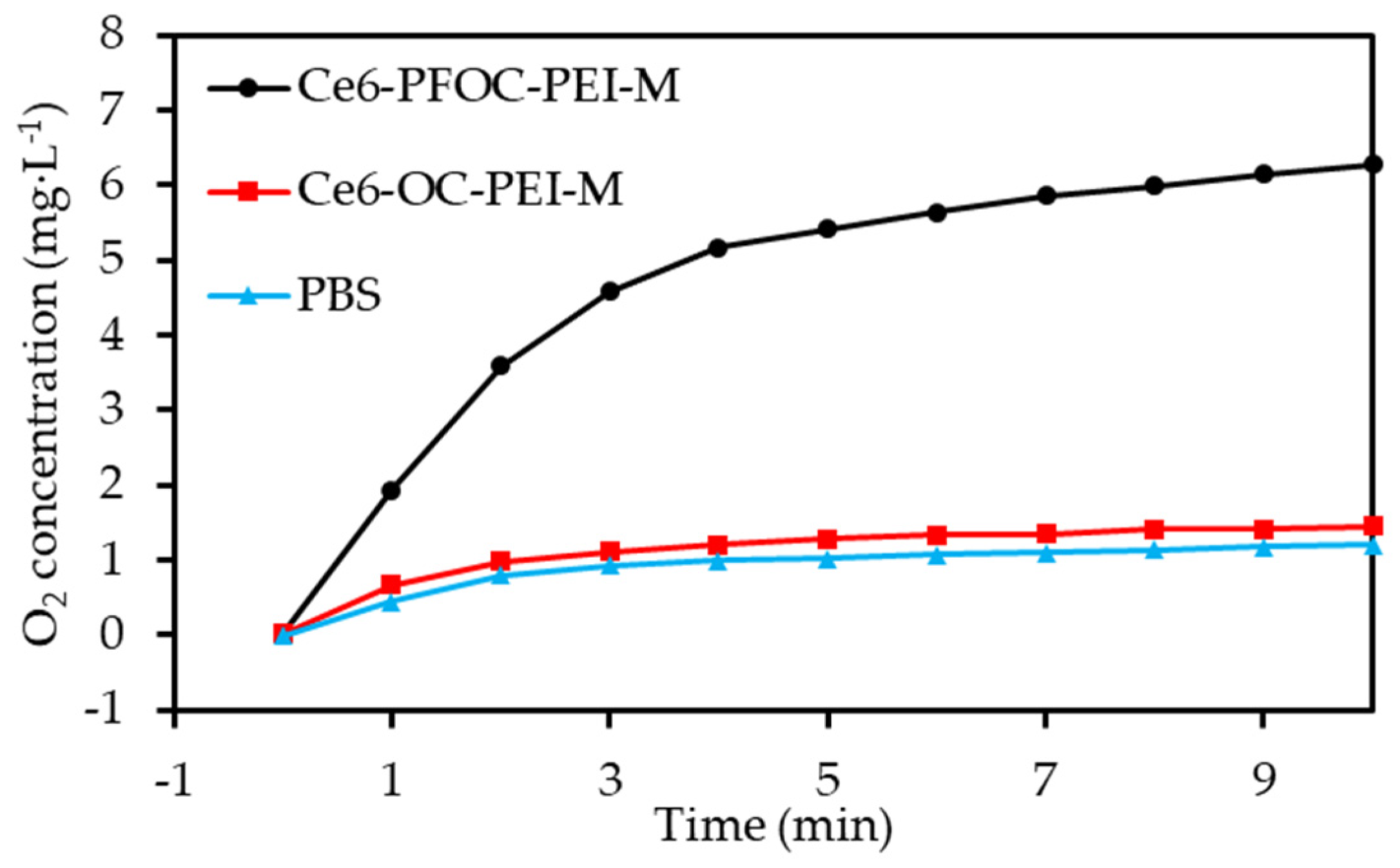
- Li, R.Q.; Zhang, C.; Xie, B.R.; Yu, W.Y.; Qiu, W.X.; Cheng, H.; Zhang, X.Z. A two-photon excited O2-evolving nanocomposite for efficient photodynamic therapy against hypoxic tumor. Biomaterials 2019, 194, 84–93. [Google Scholar] [CrossRef]
- Yang, D.; Yang, G.X.; Gai, S.L.; He, F.; Li, C.X.; Yang, P.P. Multifunctional theranostics for dual-modal photodynamic synergistic therapy via stepwise water splitting. ACS Appl. Mater. Interfaces 2017, 9, 6829–6838. [Google Scholar] [CrossRef]
- Zheng, D.W.; Li, B.; Xu, L.; Zhang, Q.L.; Fan, J.X.; Li, C.X.; Zhang, X.Z. Normalizing tumor microenvironment based on photosynthetic abiotic/biotic nanoparticles. ACS Nano 2018, 12, 6218–6227. [Google Scholar] [CrossRef]
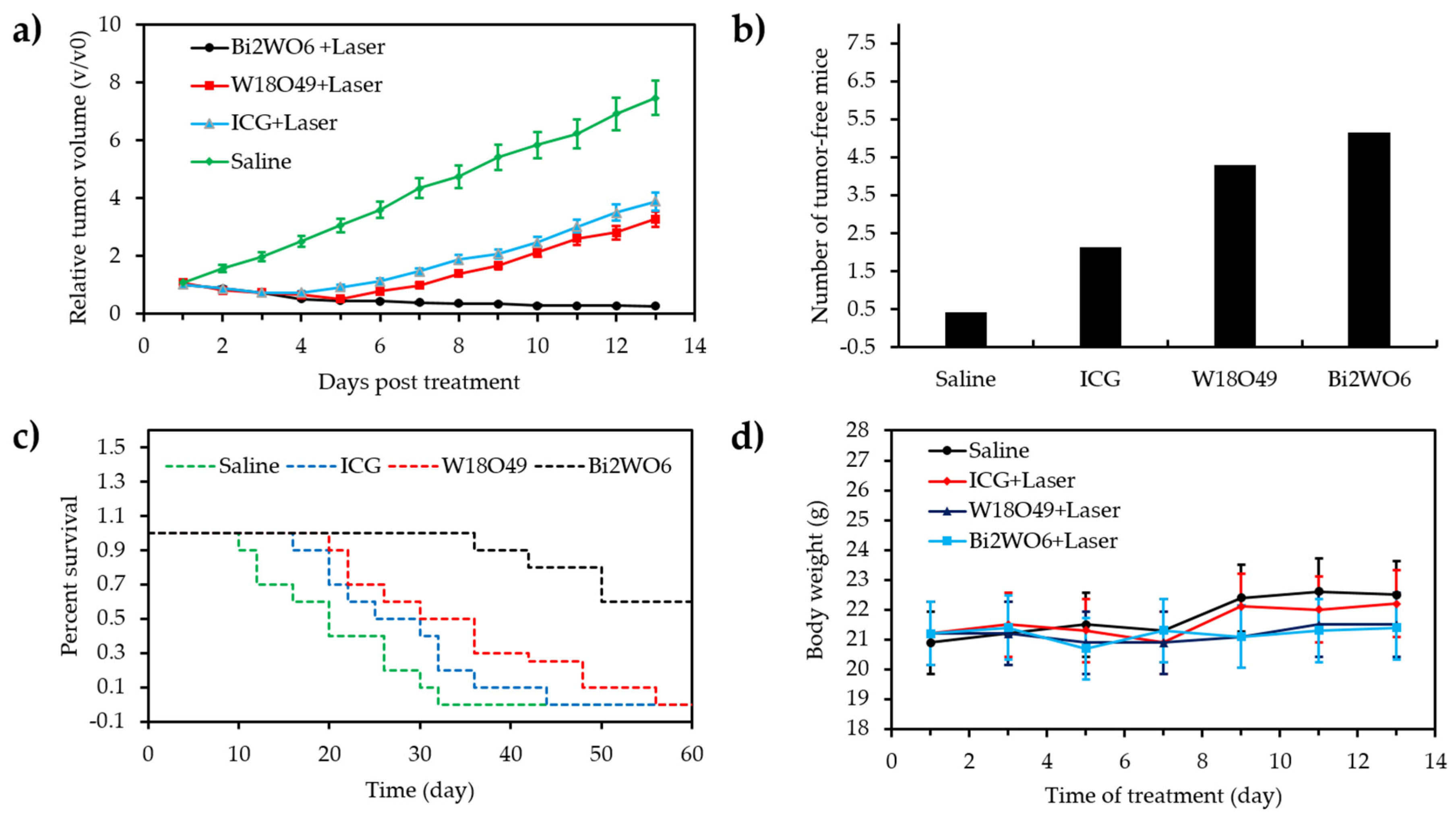
- Zhou, Y.; Zhang, Y.; Lin, M.; Long, J.; Zhang, Z.; Lin, H.; Wu, J.C.S.; Wang, X. Monolayered Bi2WO6 nanosheets mimicking heterojunction interface with open surfaces for photocatalysis. Nat. Commun. 2015, 6, 8340. [Google Scholar] [CrossRef]
- Zhang, C.; Ren, J.; Hua, J.S.; Xia, L.Y.; He, J.; Huo, D.; Hu, Y. Multifunctional Bi2WO6 nanoparticles for CT-guided photothermal and oxygen-free photodynamic therapy. ACS Appl. Mater. Interfaces 2018, 10, 1132–1146. [Google Scholar] [CrossRef]
- Fan, J.X.; Liu, M.D.; Li, C.X.; Hong, S.; Zheng, D.W.; Liu, X.H.; Chen, S.; Cheng, H.; Zhang, X.Z. A metal-semiconductor nanocomposite as an efficient oxygen-independent photosensitizer for photodynamic tumor therapy. Nanoscale Horiz. 2017, 2, 349–355. [Google Scholar] [CrossRef]
- Gong, H.; Chao, Y.; Xiang, J.; Han, X.; Song, G.S.; Feng, L.Z.; Liu, J.J.; Yang, G.B.; Chen, Q.; Liu, Z. Hyaluronidase to enhance nanoparticle-based photodynamic tumor therapy. Nano Lett. 2016, 16, 2512–2521. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.J.; Tian, L.L.; Zhang, R.; Dong, Z.L.; Wang, H.R.; Liu, Z. Collagenase-encapsulated pH-responsive nanoscale coordination polymers for tumor microenvironment modulation and enhanced photodynamic nanomedicine. ACS Appl. Mater. Interfaces 2018, 10, 43493–43502. [Google Scholar] [CrossRef]
- Song, X.J.; Feng, L.Z.; Liang, C.; Gao, M.; Song, G.S.; Liu, Z. Liposomes co-loaded with metformin and chlorin e6 modulate tumor hypoxia during enhanced photodynamic therapy. Nano Res. 2017, 10, 1200–1212. [Google Scholar] [CrossRef]
- Zuo, H.Q.; Tao, J.X.; Shi, H.; He, J.; Zhou, Z.Y.; Zhang, C. Platelet-mimicking nanoparticles co-loaded with W18O49 and metformin alleviate tumor hypoxia for enhanced photodynamic therapy and photothermal therapy. Acta Biomater. 2018, 80, 296–307. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, Q.; Chen, J.; Dong, Z.; Zhang, R.; Liu, J.; Liu, Z. Tumor-pH-responsive dissociable albumin–tamoxifen nanocomplexes enabling efficient tumor penetration and hypoxia relief for enhanced cancer photodynamic therapy. Small 2018, 14, 1803262. [Google Scholar] [CrossRef]
- Xia, D.L.; Xu, P.P.; Luo, X.Y.; Zhu, J.F.; Gu, H.Y.; Huo, D.; Hu, Y. Overcoming hypoxia by multistage nanoparticle delivery system to inhibit mitochondrial respiration for photodynamic therapy. Adv. Funct. Mater. 2019, 29, 1807294. [Google Scholar] [CrossRef]
- Gui, L.; Zhou, J.H.; Zhou, L.; Wei, S.H. A smart copper-phthalocyanine framework nanoparticle for enhancing photodynamic therapy in hypoxic conditions by weakening cells through ATP depletion. J. Mater. Chem. B 2018, 6, 2078–2088. [Google Scholar] [CrossRef]
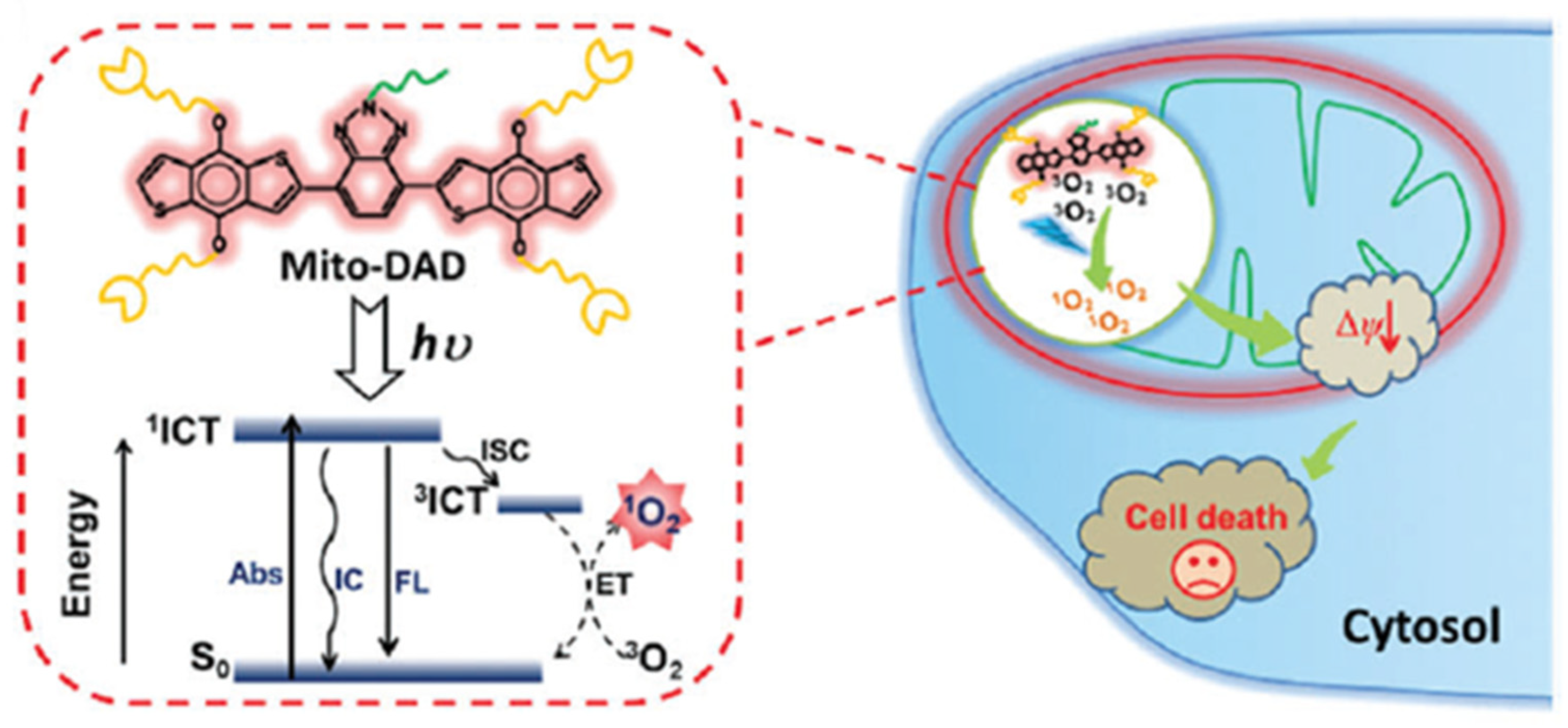
- Lv, W.; Zhang, Z.; Zhang, K.Y.; Yang, H.R.; Liu, S.J.; Xu, A.Q.; Guo, S.; Zhao, Q.; Huang, W. A mitochondria-targeted photosensitizer showing improved photodynamic therapy effects under hypoxia. Angew. Chem. Int. Ed. 2016, 55, 9947–9951. [Google Scholar] [CrossRef]
- Bayat Mokhtari, R.; Homayouni, T.S.; Baluch, N.; Morgatskaya, E.; Kumar, S.; Das, B.; Yeger, H. Combination therapy in combating cancer. Oncotarget 2017, 8, 38022–38043. [Google Scholar] [CrossRef]
- Brodin, N.P.; Guha, C.; Tome, W.A. Photodynamic therapy and its role in combined modality anticancer treatment. Technol. Cancer Res. Treat. 2015, 14, 355–368. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.Y.; Liu, Y.; Bu, W.B.; Cheng, C.; Zuo, C.J.; Xiao, Q.F.; Sun, Y.; Ni, D.L.; Zhang, C.; Liu, J.A.; et al. Hypoxia induced by upconversion-based photodynamic therapy: Towards highly effective synergistic bioreductive therapy in tumors. Angew. Chem. Int. Ed. 2015, 54, 8105–8109. [Google Scholar] [CrossRef] [PubMed]
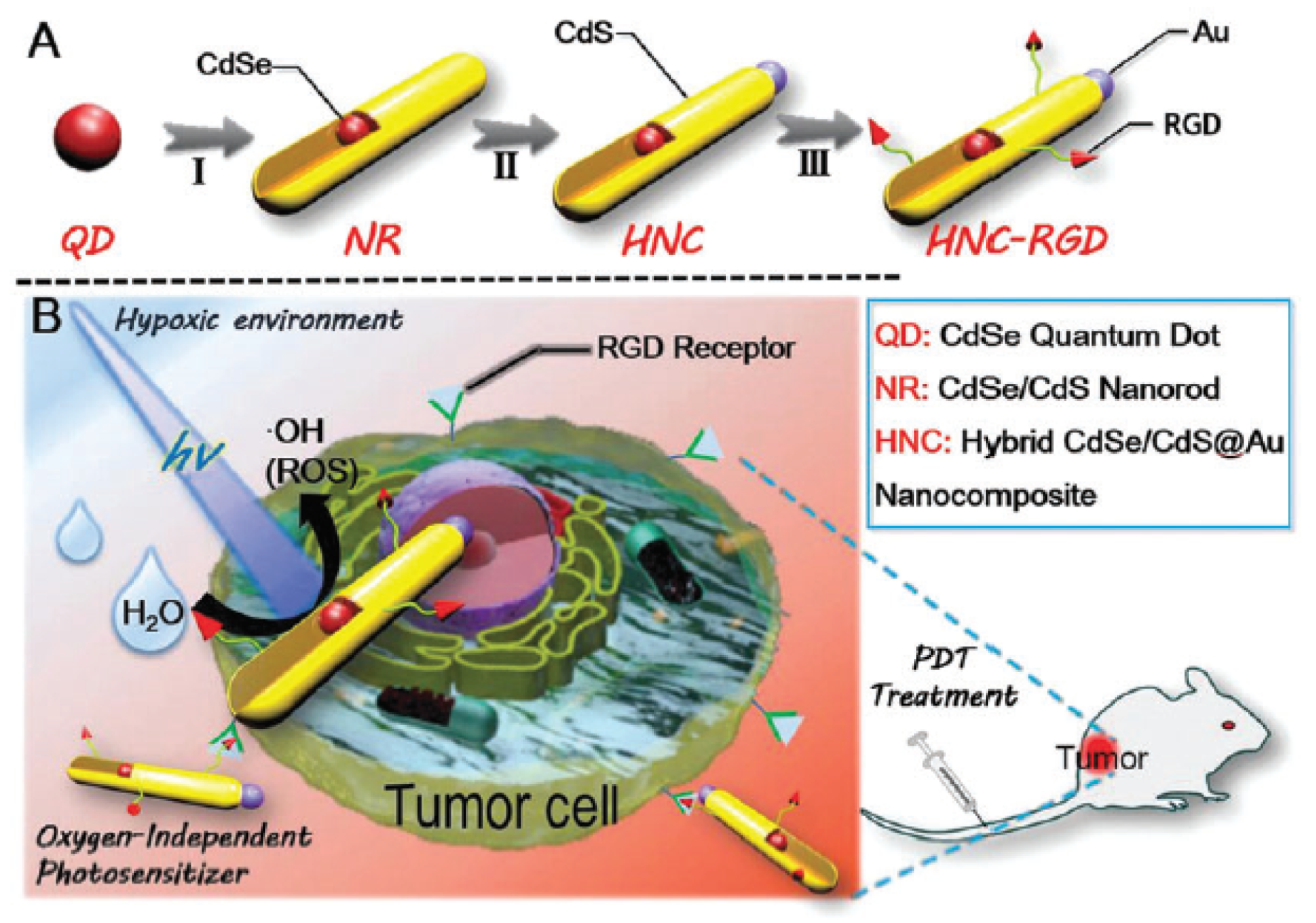
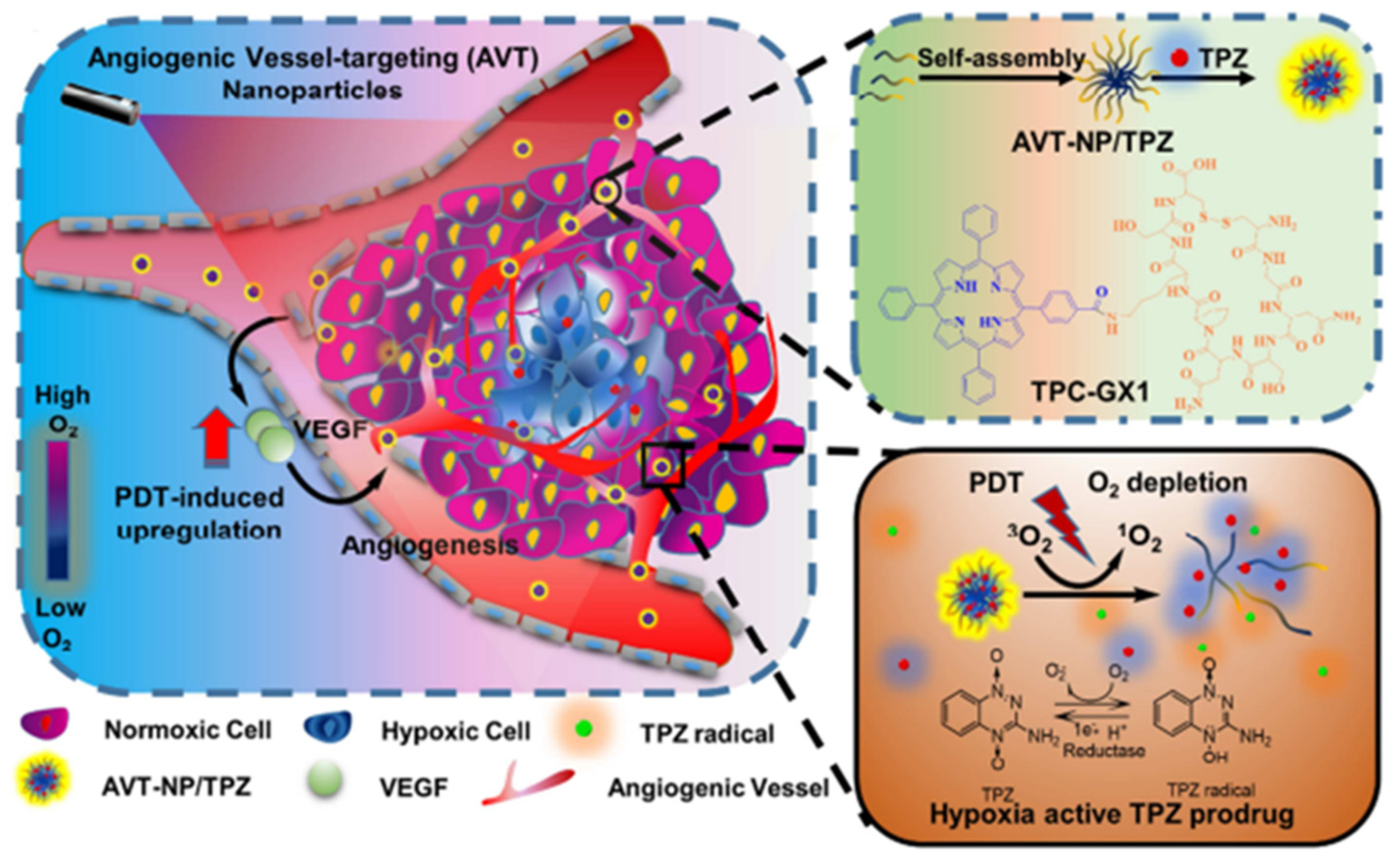
- Guo, D.B.; Xu, S.T.; Wang, N.; Jiang, H.Y.; Huang, Y.; Jin, X.; Xue, B.; Zhang, C.; Zhu, X.Y. Prodrug-embedded angiogenic vessel-targeting nanoparticle: A positive feedback amplifier in hypoxia-induced chemo-photo therapy. Biomaterials 2017, 144, 188–198. [Google Scholar] [CrossRef] [PubMed]
- Li, S.Y.; Cheng, H.; Qiu, W.X.; Zhang, L.; Wan, S.S.; Zeng, J.Y.; Zhang, X.Z. Cancer cell membrane-coated biomimetic platform for tumor targeted photodynamic therapy and hypoxia-amplified bioreductive therapy. Biomaterials 2017, 142, 149–161. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.Z.; Xie, Y.; Li, J.; Peng, Z.H.; Sheinin, Y.; Zhou, J.P.; Oupicky, D. Tumor-penetrating nanoparticles for enhanced anticancer activity of combined photodynamic and hypoxia-activated therapy. ACS Nano 2017, 11, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Liu, L.; Motevalli, S.M.; Wu, X.L.; Yang, X.H.; Li, X.L.; Han, L.; Magrini, A.; Guo, W.S.; Chang, J.; et al. Light-triggered retention and cascaded therapy of albumin-based theranostic nanomedicines to alleviate tumor adaptive treatment tolerance. Adv. Funct. Mater. 2018, 28, 1707291. [Google Scholar] [CrossRef]
- Liu, M.; Wang, L.; Zheng, X.H.; Liu, S.; Xie, Z.G. Hypoxia-triggered nanoscale metal-organic frameworks for enhanced anticancer activity. ACS Appl. Mater. Interfaces 2018, 10, 24638–24647. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, X.; Li, H.; Li, C.; Ding, H.; Zhang, M.; Guo, Y.; Sun, M. Near-infrared light triggered liposomes combining photodynamic and chemotherapy for synergistic breast tumor therapy. Colloids Surf. B. Biointerfaces 2019, 173, 564–570. [Google Scholar] [CrossRef]
- Zhang, K.; Zhang, Y.D.; Meng, X.D.; Lu, H.T.; Chang, H.; Dong, H.F.; Zhang, X.J. Light-triggered theranostic liposomes for tumor diagnosis and combined photodynamic and hypoxia-activated prodrug therapy. Biomaterials 2018, 185, 301–309. [Google Scholar] [CrossRef]
- Wang, Q.; Tian, L.; Xu, J.; Xia, B.; Li, J.; Lu, F.; Lu, X.; Wang, W.; Huang, W.; Fan, Q. Multifunctional supramolecular vesicles for combined photothermal/photodynamic/hypoxia-activated chemotherapy. Chem. Commun. 2018, 54, 10328–10331. [Google Scholar] [CrossRef]
- Qian, C.G.; Yu, J.C.; Chen, Y.L.; Hu, Q.Y.; Xiao, X.Z.; Sun, W.J.; Wang, C.; Feng, P.J.; Shen, Q.D.; Gu, Z. Light-activated hypoxia-responsive nanocarriers for enhanced anticancer therapy. Adv. Mater. 2016, 28, 3313–3320. [Google Scholar] [CrossRef] [PubMed]
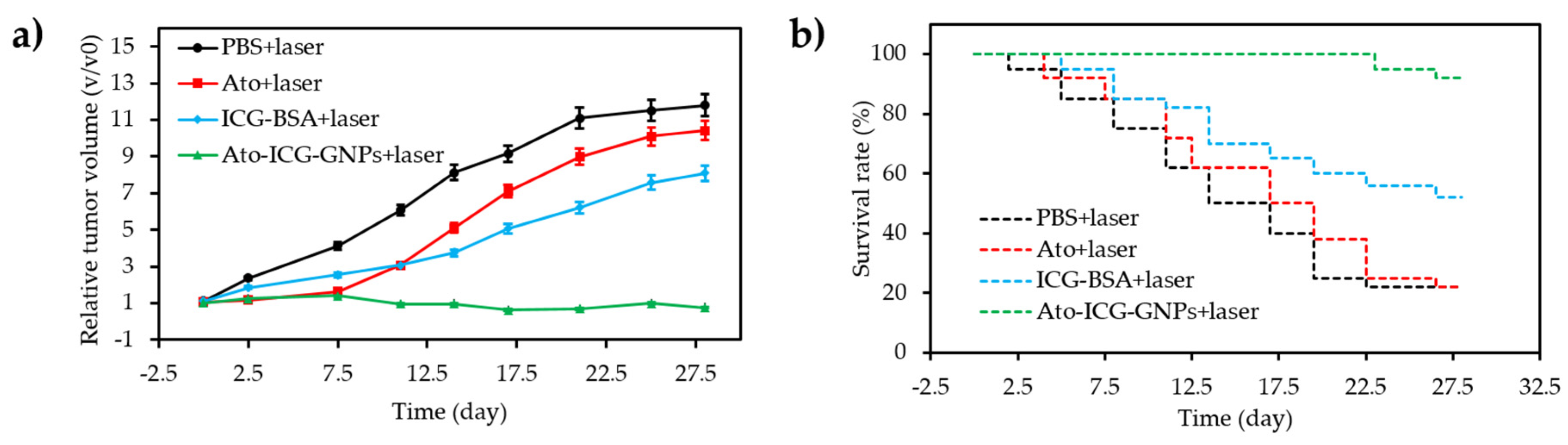
- Hu, D.R.; Chen, L.J.; Qu, Y.; Peng, J.R.; Chu, B.Y.; Shi, K.; Hao, Y.; Zhong, L.; Wang, M.Y.; Qian, Z.Y. Oxygen-generating hybrid polymeric nanoparticles with encapsulated doxorubicin and chlorin e6 for trimodal imaging-guided combined chemo-photodynamic therapy. Theranostics 2018, 8, 1558–1574. [Google Scholar] [CrossRef] [PubMed]
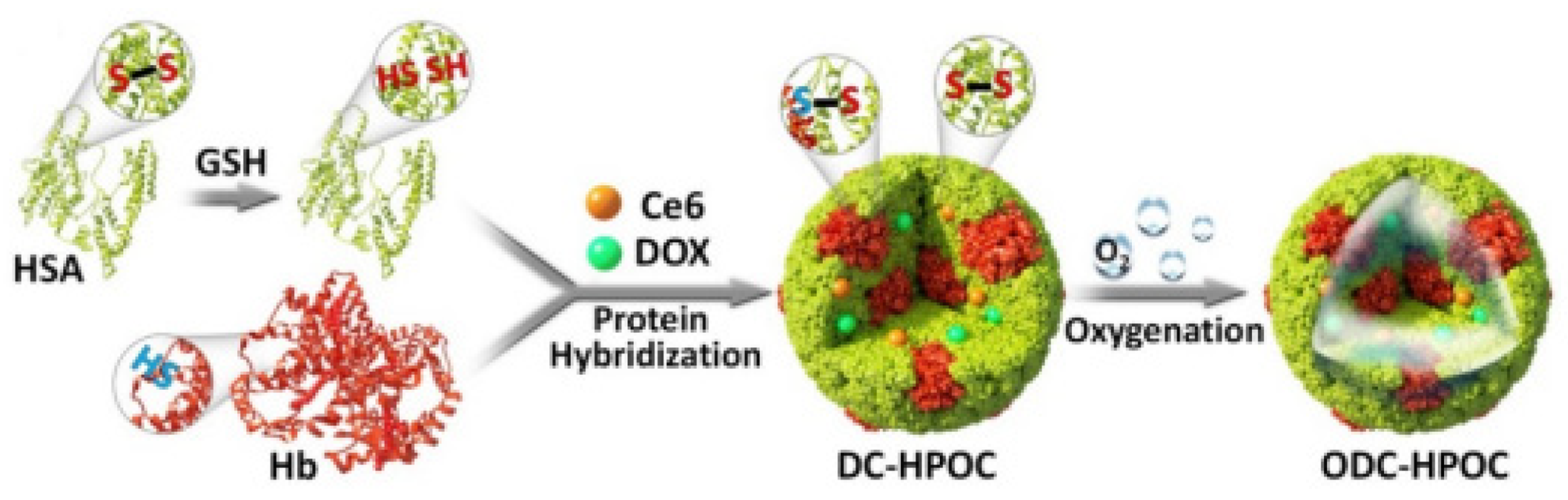
- Luo, Z.Y.; Tian, H.; Liu, L.L.; Chen, Z.K.; Liang, R.J.; Chen, Z.; Wu, Z.H.; Ma, A.Q.; Zheng, M.B.; Cai, L.T. Tumor-targeted hybrid protein oxygen carrier to simultaneously enhance hypoxia-dampened chemotherapy and photodynamic therapy at a single dose. Theranostics 2018, 8, 3584–3596. [Google Scholar] [CrossRef]
- Xu, J.; Han, W.; Yang, P.; Jia, T.; Dong, S.; Bi, H.; Gulzar, A.; Yang, D.; Gai, S.; He, F.; et al. Tumor microenvironment-responsive mesoporous MnO2-coated upconversion nanoplatform for self-enhanced tumor theranostics. Adv. Funct. Mater. 2018, 28, 1803804. [Google Scholar] [CrossRef]
- Xie, Z.; Cai, X.; Sun, C.; Liang, S.; Shao, S.; Huang, S.; Cheng, Z.; Pang, M.; Xing, B.; Kheraif, A.A.A.; et al. O2-Loaded pH-responsive multifunctional nanodrug carrier for overcoming hypoxia and highly efficient chemo-photodynamic cancer therapy. Chem. Mater. 2019, 31, 483–490. [Google Scholar] [CrossRef]
- He, H.; Zhu, R.Y.; Sun, W.; Cai, K.M.; Chen, Y.B.; Yin, L.C. Selective cancer treatment via photodynamic sensitization of hypoxia-responsive drug delivery. Nanoscale 2018, 10, 2856–2865. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, C.; Tian, C.; Guo, H.L.; Shen, Y.H.; Zhu, M.Z. Fe3O4@MnO2@PPy nanocomposites overcome hypoxia: Magnetic-targeting-assisted controlled chemotherapy and enhanced photodynamic/photothermal therapy. J. Mater. Chem. B 2018, 6, 6848–6857. [Google Scholar] [CrossRef]
- Deng, J.; Liu, F.; Wang, L.N.; An, Y.; Gao, M.; Wang, Z.; Zhao, Y.J. Hypoxia- and singlet oxygen-responsive chemo-photodynamic micelles featured with glutathione depletion and aldehyde production. Biomater. Sci. 2019, 7, 429–441. [Google Scholar] [CrossRef]
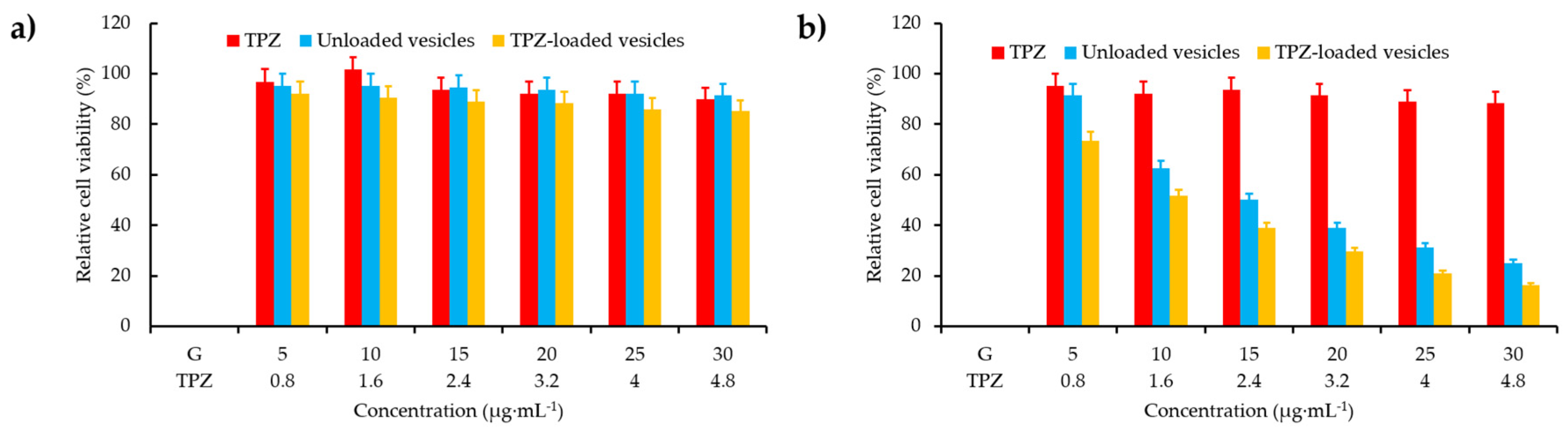
- Feng, L.; Cheng, L.; Dong, Z.; Tao, D.; Barnhart, T.E.; Cai, W.; Chen, M.; Liu, Z. Theranostic liposomes with hypoxia-activated prodrug to effectively destruct hypoxic tumors post-photodynamic therapy. ACS Nano 2017, 11, 927–937. [Google Scholar] [CrossRef]
- Luan, X.; Guan, Y.Y.; Liu, H.J.; Lu, Q.; Zhao, M.; Sun, D.X.; Lovell, J.F.; Sun, P.; Chen, H.Z.; Fang, C. A tumor vascular-targeted interlocking trimodal nanosystem that induces and exploits hypoxia. Adv. Sci. 2018, 5, 1800034. [Google Scholar] [CrossRef]
- He, Z.; Dai, Y.; Li, X.; Guo, D.; Liu, Y.; Huang, X.; Jiang, J.; Wang, S.; Zhu, G.; Zhang, F.; et al. Hybrid nanomedicine fabricated from photosensitizer-terminated metal–organic framework nanoparticles for photodynamic therapy and hypoxia-activated cascade chemotherapy. Small 2019, 15, 1804131. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.B.; Xu, S.T.; Huang, Y.; Jiang, H.Y.; Yasen, W.; Wang, N.; Su, Y.; Qian, J.W.; Li, J.; Zhang, C.; et al. Platinum (IV) complex-based two-in-one polyprodrug for a combinatorial chemo-photodynamic therapy. Biomaterials 2018, 177, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Xu, S.; Zhu, X.; Zhang, C.; Huang, W.; Zhou, Y.; Yan, D. Oxygen and Pt (II) self-generating conjugate for synergistic photo-chemo therapy of hypoxic tumor. Nat. Commun. 2018, 9, 2053. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.R.; Zhu, W.W.; Feng, L.Z.; Chen, Q.; Chao, Y.; Dong, Z.L.; Liu, Z. Nanoscale covalent organic polymers as a biodegradable nanomedicine for chemotherapy-enhanced photodynamic therapy of cancer. Nano Res. 2018, 11, 3244–3257. [Google Scholar] [CrossRef]
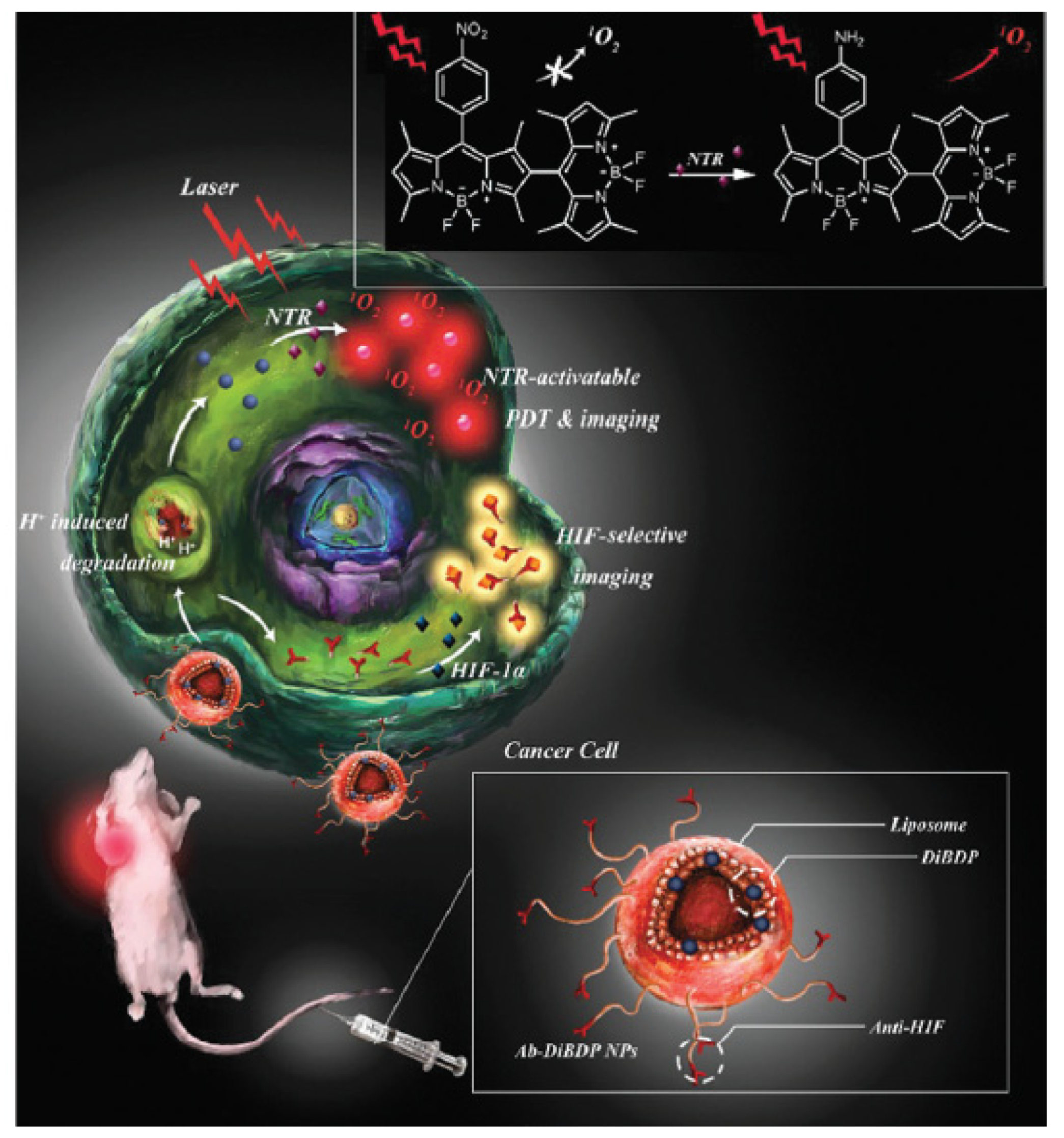
- Chen, W.H.; Lecaros, R.L.G.; Tseng, Y.C.; Huang, L.; Hsu, Y.C. Nanoparticle delivery of HIF1 alpha siRNA combined with photodynamic therapy as a potential treatment strategy for head-and-neck cancer. Cancer Lett. 2015, 359, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Sun, S.J.; Xu, Y.X.; Fu, P.; Chen, M.; Sun, S.H.; Zhao, R.R.; Wang, J.R.; Liang, X.L.; Wang, S.M. Ultrasound-targeted photodynamic and gene dual therapy for effectively inhibiting triple negative breast cancer by cationic porphyrin lipid microbubbles loaded with HIF1 alpha-siRNA. Nanoscale 2018, 10, 19945–19956. [Google Scholar] [CrossRef]
- Broekgaarden, M.; Weijer, R.; Krekorian, M.; van den Ijssel, B.; Kos, M.; Alles, L.K.; van Wijk, A.C.; Bikadi, Z.; Hazai, E.; van Gulik, T.M.; et al. Inhibition of hypoxia-inducible factor 1 with acriflavine sensitizes hypoxic tumor cells to photodynamic therapy with zinc phthalocyanine-encapsulating cationic liposomes. Nano Res. 2016, 9, 1639–1662. [Google Scholar] [CrossRef]
- Weijer, R.; Broekgaarden, M.; Krekorian, M.; Alles, L.K.; van Wijk, A.C.; Mackaaij, C.; Verheij, J.; van der Wal, A.C.; van Gulik, T.M.; Storm, G.; et al. Inhibition of hypoxia inducible factor 1 and topoisomerase with acriflavine sensitizes perihilar cholangiocarcinomas to photodynamic therapy. Oncotarget 2016, 7, 3331–3346. [Google Scholar] [CrossRef]
- Ferrario, A.; von Tiehl, K.F.; Rucker, N.; Schwarz, M.A.; Gill, P.S.; Gomer, C.J. Antiangiogenic treatment enhances photodynamic therapy responsiveness in a mouse mammary carcinoma. Cancer Res. 2000, 60, 4066–4069. [Google Scholar]
- Zhou, Q.Y.; Olivo, M.; Lye, K.Y.K.; Moore, S.; Sharma, A.; Chowbay, B. Enhancing the therapeutic responsiveness of photodynamic therapy with the antiangiogenic agents SU5416 and SU6668 in murine nasopharyngeal carcinoma models. Cancer Chemother. Pharmacol. 2005, 56, 569–577. [Google Scholar] [CrossRef]
- Weiss, A.; van Beijnum, J.R.; Bonvin, D.; Jichlinski, P.; Dyson, P.J.; Griffioen, A.W.; Nowak-Sliwinska, P. Low-dose angiostatic tyrosine kinase inhibitors improve photodynamic therapy for cancer: Lack of vascular normalization. J. Cell. Mol. Med. 2014, 18, 480–491. [Google Scholar] [CrossRef] [PubMed]
- Lecaros, R.L.G.; Huang, L.; Lee, T.C.; Hsu, Y.C. Nanoparticle delivered VEGF-A siRNA enhances photodynamic therapy for head and neck cancer treatment. Mol. Ther. 2016, 24, 106–116. [Google Scholar] [CrossRef] [PubMed]
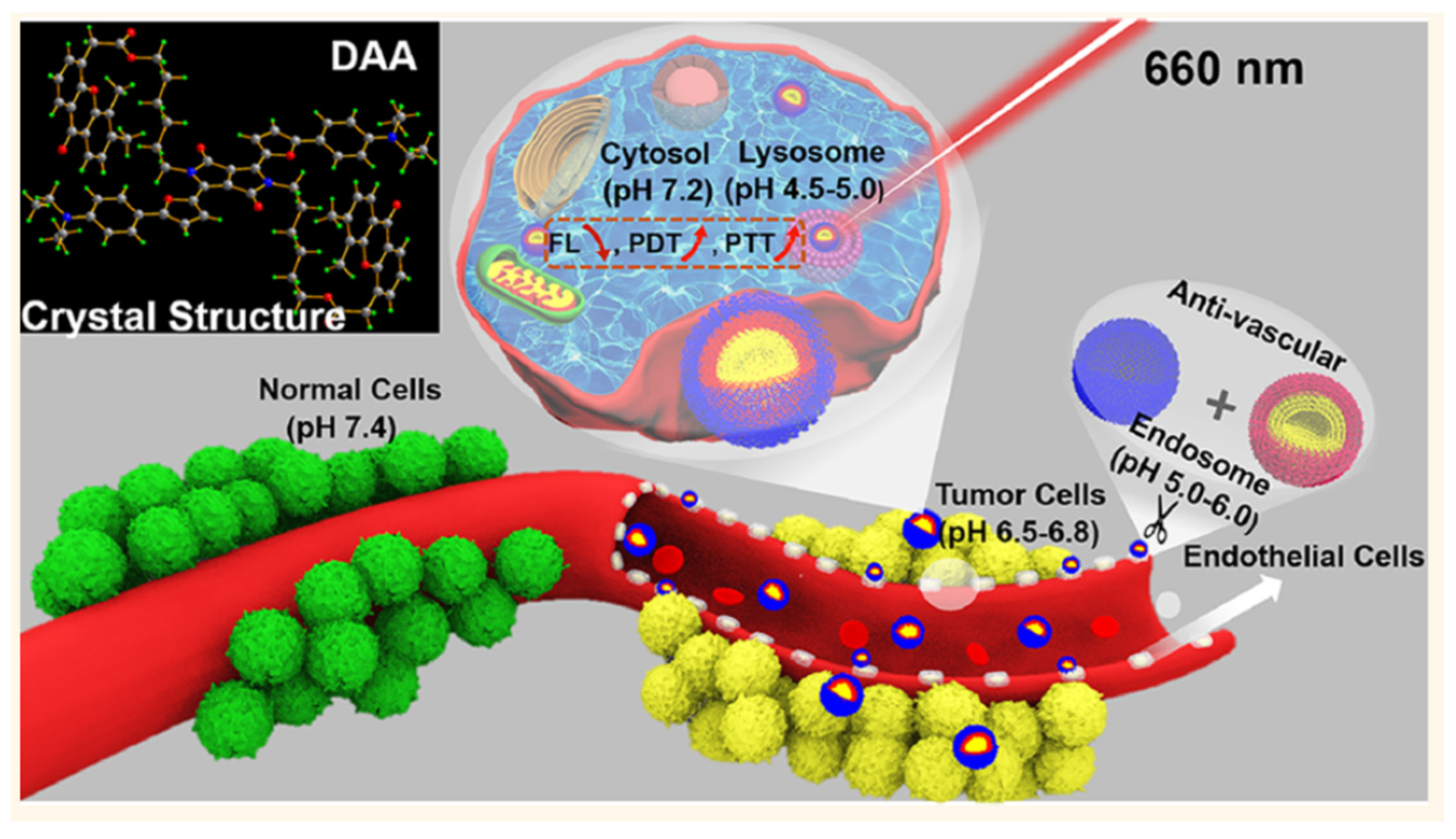
- Liang, P.P.; Huang, X.Y.; Wang, Y.; Chen, D.P.; Ou, C.J.; Zhang, Q.; Shao, J.J.; Huang, W.; Dong, X.C. Tumor-microenvironment-responsive nanoconjugate for synergistic antivascular activity and phototherapy. ACS Nano 2018, 12, 11446–11457. [Google Scholar] [CrossRef] [PubMed]
- Ferrario, A.; Fisher, A.M.; Rucker, N.; Gomer, C.J. Celecoxib and NS-398 enhance photodynamic therapy by increasing in vitro apoptosis and decreasing In vivo inflammatory and angiogenic factors. Cancer Res. 2005, 65, 9473–9478. [Google Scholar] [CrossRef] [PubMed]
- Tuncel, S.; Trivella, A.; Atilla, D.; Bennis, K.; Savoie, H.; Albrieux, F.; Delort, L.; Billard, H.; Dubois, V.; Ahsen, V.; et al. Assessing the dual activity of a chalcone-phthalocyanine conjugate: Design, synthesis, and antivascular and photodynamic properties. Mol. Pharm. 2013, 10, 3706–3716. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.J.; Sui, H.; Jiang, C.L.; Li, S.M.; Han, Y.; Huang, P.; Du, X.X.; Du, J.W.; Bai, Y.X. Dihydroartemisinin increases the sensitivity of photodynamic therapy via NF-kappa B/HIF-1 alpha/VEGF pathway in esophageal cancer cell in vitro and In vivo. Cell. Physiol. Biochem. 2018, 48, 2035–2045. [Google Scholar] [CrossRef]
- Li, Y.J.; Zhou, J.H.; Du, X.X.; Jia, D.X.; Wu, C.L.; Huang, P.; Han, Y.; Sui, H.; Wei, X.L.; Liu, L.; et al. Dihydroartemisinin accentuates the anti-tumor effects of photodynamic therapy via inactivation of NF-kappa B in Eca109 and Ec9706 esophageal cancer cells. Cell. Physiol. Biochem. 2014, 33, 1527–1536. [Google Scholar] [CrossRef]
- Jung, H.S.; Han, J.; Shi, H.; Koo, S.; Singh, H.; Kim, H.J.; Sessler, J.L.; Lee, J.Y.; Kim, J.H.; Kim, J.S. Overcoming the limits of hypoxia in photodynamic therapy: A carbonic anhydrase IX-targeted approach. J. Am. Chem. Soc. 2017, 139, 7595–7602. [Google Scholar] [CrossRef]
- Jin, C.S.; Lovell, J.F.; Chen, J.; Zheng, G. Ablation of hypoxic tumors with dose-equivalent photothermal, but not photodynamic, therapy using a nanostructured porphyrin assembly. ACS Nano 2013, 7, 2541–2550. [Google Scholar] [CrossRef]
- Zhu, W.; Liu, Y.; Yang, Z.; Zhang, L.; Xiao, L.J.; Liu, P.; Wang, J.; Yi, C.F.; Xu, Z.S.; Ren, J.H. Albumin/sulfonamide stabilized iron porphyrin metal organic framework nanocomposites: Targeting tumor hypoxia by carbonic anhydrase IX inhibition and T-1-T-2 dual mode MRI guided photodynamic/photothermal therapy. J. Mater. Chem. B 2018, 6, 265–276. [Google Scholar] [CrossRef]
- Hu, D.H.; Sheng, Z.H.; Gao, G.H.; Siu, F.M.; Liu, C.B.; Wan, Q.; Gong, P.; Zheng, H.R.; Ma, Y.F.; Cai, L.T. Activatable albumin-photosensitizer nanoassemblies for triple-modal imaging and thermal-modulated photodynamic therapy of cancer. Biomaterials 2016, 93, 10–19. [Google Scholar] [CrossRef] [PubMed]
- Feng, L.Z.; Tao, D.L.; Dong, Z.L.; Chen, Q.; Chao, Y.; Liu, Z.; Chen, M.W. Near-infrared light activation of quenched liposomal Ce6 for synergistic cancer phototherapy with effective skin protection. Biomaterials 2017, 127, 13–24. [Google Scholar] [CrossRef] [PubMed]
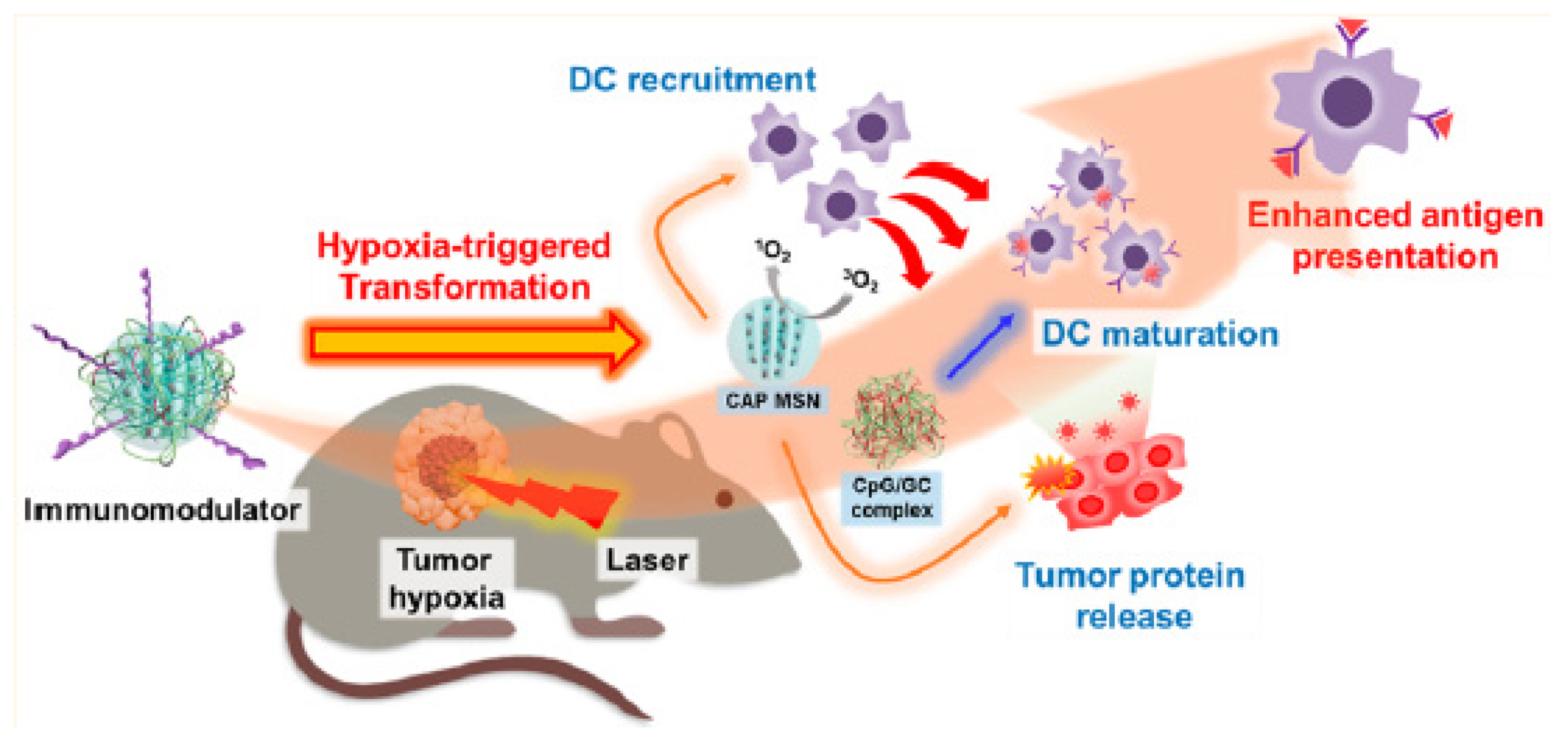
- Im, S.; Lee, J.; Park, D.; Park, A.; Kim, Y.M.; Kim, W.J. Hypoxia-triggered transforming immunomodulator for cancer immunotherapy via photodynamically enhanced antigen presentation of dendritic cell. ACS Nano 2018, 13, 476–488. [Google Scholar] [CrossRef]
- Zhou, Z.; Song, J.; Nie, L.; Chen, X. Reactive oxygen species generating systems meeting challenges of photodynamic cancer therapy. Chem. Soc. Rev. 2016, 45, 6597–6626. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, H.Y.; Yu, H.J.; Dong, Y.; Tian, R.H.; Huang, G.; Boothman, D.A.; Sumer, B.D.; Gao, J.M. Photoactivation switch from type II to type I reactions by electron-rich micelles for improved photodynamic therapy of cancer cells under hypoxia. J. Control. Release 2011, 156, 276–280. [Google Scholar] [CrossRef] [Green Version]
- Li, M.L.; Xia, J.; Tian, R.S.; Wang, J.Y.; Fan, J.L.; Du, J.J.; Long, S.; Song, X.Z.; Foley, J.W.; Peng, X.J. Near-infrared light-initiated molecular superoxide radical generator: Rejuvenating photodynamic therapy against hypoxic tumors. J. Am. Chem. Soc. 2018, 140, 14851–14859. [Google Scholar] [CrossRef]
- Liu, Y.; Zhen, W.Y.; Jin, L.H.; Zhang, S.T.; Sun, G.Y.; Zhang, T.Q.; Xu, X.; Song, S.Y.; Wang, Y.H.; Liu, J.H.; et al. All-in-one theranostic nanoagent with enhanced reactive oxygen species generation and modulating tumor microenvironment ability for effective tumor eradication. ACS Nano 2018, 12, 4886–4893. [Google Scholar] [CrossRef]
- Lv, Z.; Wei, H.J.; Li, Q.; Su, X.L.; Liu, S.J.; Zhang, K.Y.; Lv, W.; Zhao, Q.; Li, X.H.; Huang, W. Achieving efficient photodynamic therapy under both normoxia and hypoxia using cyclometalated Ru(II) photosensitizer through type I photochemical process. Chem. Sci. 2018, 9, 502–512. [Google Scholar] [CrossRef]
- Wang, X.X.; Xu, J.T.; Yang, D.; Sun, C.Q.; Sun, Q.Q.; He, F.; Gai, S.L.; Zhong, C.N.; Li, C.X.; Yang, P.P. Fe3O4@MIL-100(Fe)-UCNPs heterojunction photosensitizer: Rational design and application in near infrared light mediated hypoxic tumor therapy. Chem. Eng. J. 2018, 354, 1141–1152. [Google Scholar] [CrossRef]
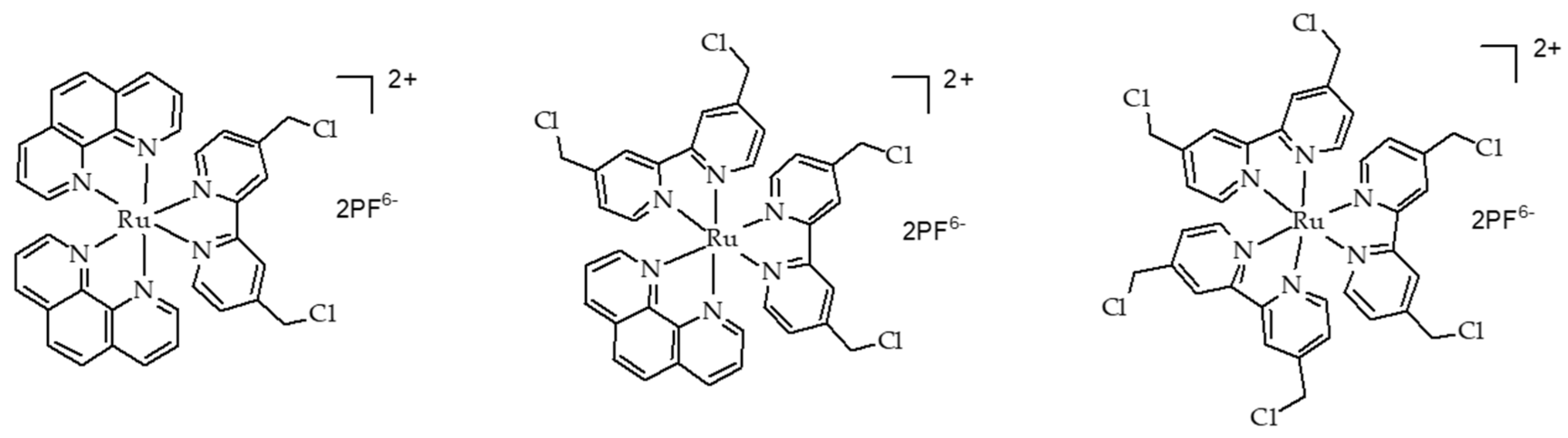
- Tian, N.; Sun, W.Z.; Guo, X.S.; Lu, J.; Li, C.; Hou, Y.J.; Wang, X.S.; Zhou, Q.X. Mitochondria targeted and NADH triggered photodynamic activity of chloromethyl modified Ru(ii) complexes under hypoxic conditions. Chem. Commun. 2019, 55, 2676–2679. [Google Scholar] [CrossRef]
- Lazic, S.; Kaspler, P.; Shi, G.; Monro, S.; Sainuddin, T.; Forward, S.; Kasimova, K.; Hennigar, R.; Mandel, A.; McFarland, S.; et al. Novel osmium-based coordination complexes as photosensitizers for panchromatic photodynamic therapy. Photochem. Photobiol. 2017, 93, 1248–1258. [Google Scholar] [CrossRef] [PubMed]
- Babii, O.; Afonin, S.; Garmanchuk, L.V.; Nikulina, V.V.; Nikolaienko, T.V.; Storozhuk, O.V.; Shelest, D.V.; Dasyukevich, O.I.; Ostapchenko, L.I.; Iurchenko, V.; et al. Direct photocontrol of peptidomimetics: An aternative to oxygen-dependent photodynamic cancer therapy. Angew. Chem. Int. Ed. 2016, 55, 5493–5496. [Google Scholar] [CrossRef] [PubMed]
- Fadhel, A.A.; Yue, X.L.; Zadeh, E.H.G.; Bondar, M.V.; Belfield, K.D. Pegylated and nanoparticle-conjugated sulfonium salt photo triggers necrotic cell death. Int. J. Nanomed. 2016, 11, 6161–6168. [Google Scholar] [CrossRef] [PubMed]
- Yue, X.L.; Yanez, C.O.; Yao, S.; Belfield, K.D. Selective cell death by photochemically induced pH imbalance in cancer cells. J. Am. Chem. Soc. 2013, 135, 2112–2115. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.Y.; Liu, T.; Zhang, M.K.; Wang, Z.X.; Ye, J.J.; Li, C.X.; Liu, W.L.; Li, R.Q.; Feng, J.; Zhang, X.Z. O2 economizer for inhibiting cell respiration to combat the hypoxia obstacle in tumor treatments. ACS Nano 2019, 13, 1784–1794. [Google Scholar] [CrossRef]
- Heinrich, T.A.; Tedesco, A.C.; Fukuto, J.M.; da Silva, R.S. Production of reactive oxygen and nitrogen species by light irradiation of a nitrosyl phthalocyanine ruthenium complex as a strategy for cancer treatment. Dalton Trans. 2014, 43, 4021–4025. [Google Scholar] [CrossRef]
- Wan, S.S.; Zeng, J.Y.; Cheng, H.; Zhang, X.Z. ROS-induced NO generation for gas therapy and sensitizing photodynamic therapy of tumor. Biomaterials 2018, 185, 51–62. [Google Scholar] [CrossRef]
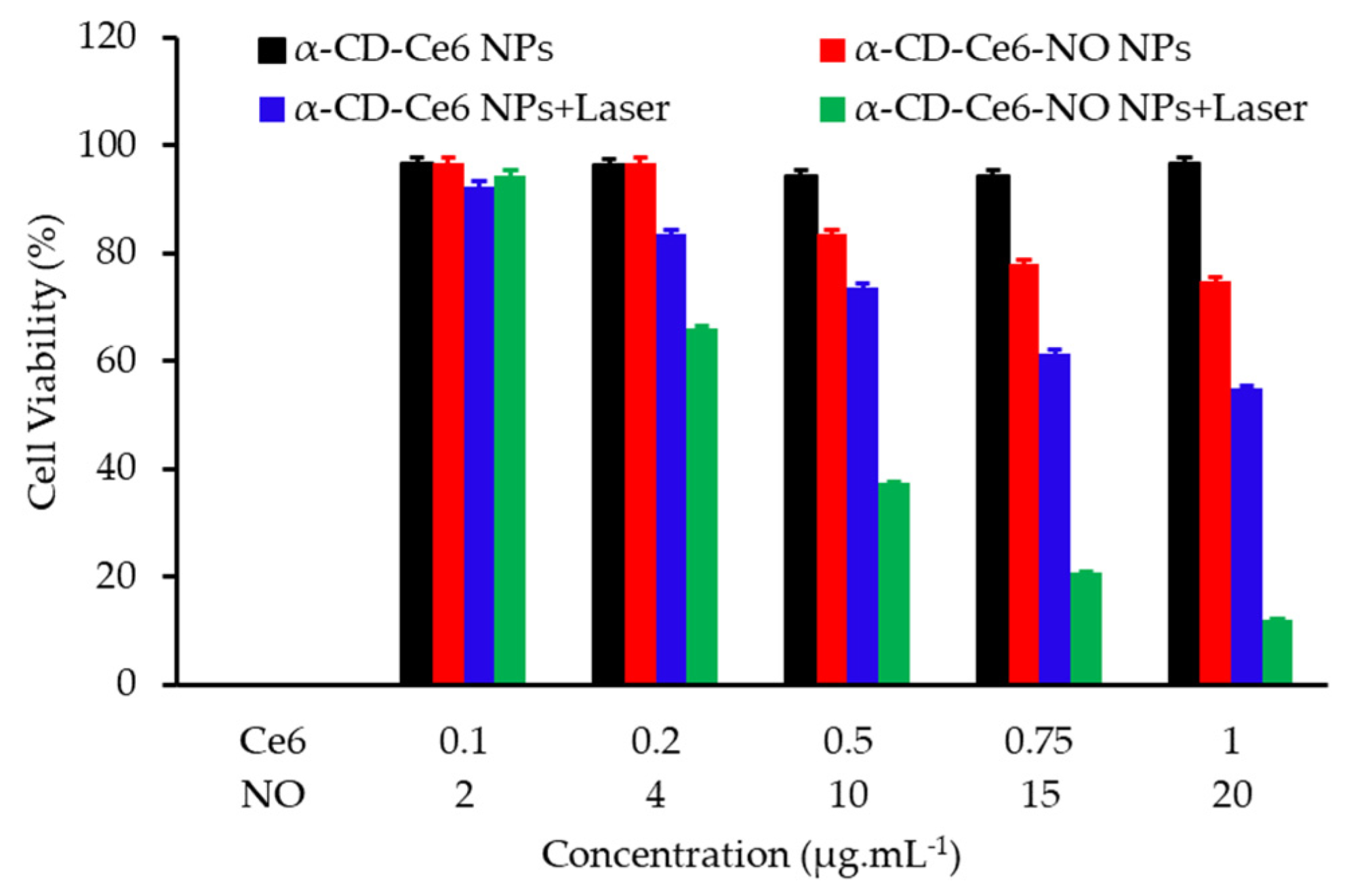
- Deng, Y.Y.; Jia, F.; Chen, S.Y.; Shen, Z.D.; Jin, Q.; Fu, G.S.; Ji, J. Nitric oxide as an all-rounder for enhanced photodynamic therapy: Hypoxia relief, glutathione depletion and reactive nitrogen species generation. Biomaterials 2018, 187, 55–65. [Google Scholar] [CrossRef]
- Yuan, Z.; Yu, S.; Cao, F.Y.; Mao, Z.W.; Gao, C.Y.; Ling, J. Near-infrared light triggered photothermal and photodynamic therapy with an oxygen-shuttle endoperoxide of anthracene against tumor hypoxia. Polym. Chem. 2018, 9, 2124–2133. [Google Scholar] [CrossRef]
- Han, Y.; Chen, Z.P.; Zhao, H.; Zha, Z.S.; Ke, W.D.; Wang, Y.H.; Ge, Z.S. Oxygen-independent combined photothermal/photodynamic therapy delivered by tumor acidity-responsive polymeric micelles. J. Control. Release 2018, 284, 15–25. [Google Scholar] [CrossRef]
- Lv, Z.; Zou, L.; Wei, H.J.; Liu, S.J.; Huang, W.; Zhao, Q. Phosphorescent starburst Pt(II) porphyrins as bifunctional therapeutic agents for tumor hypoxia imaging and photodynamic therapy. ACS Appl. Mater. Interfaces 2018, 10, 19523–19533. [Google Scholar] [CrossRef] [PubMed]
- Pinto, A.; Mace, Y.; Drouet, F.; Bony, E.; Boidot, R.; Draoui, N.; Lobysheva, I.; Corbet, C.; Polet, F.; Martherus, R.; et al. A new ER-specific photosensitizer unravels 1O2-driven protein oxidation and inhibition of deubiquitinases as a generic mechanism for cancer PDT. Oncogene 2016, 35, 3976–3985. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Li, X.; Du, K.; Feng, F.D. A water soluble donor-acceptor-donor conjugated oligomer as a photosensitizer for mitochondria-targeted photodynamic therapy. Chem. Commun. 2018, 54, 9194–9197. [Google Scholar] [CrossRef] [PubMed]
- Wieczorek, E.; Mlynarczyk, D.T.; Kucinska, M.; Dlugaszewska, J.; Piskorz, J.; Popenda, L.; Szczolko, W.; Jurga, S.; Murias, M.; Mielcarek, J.; et al. Photophysical properties and photocytotoxicity of free and liposome-entrapped diazepinoporphyrazines on LNCaP cells under normoxic and hypoxic conditions. Eur. J. Med. Chem. 2018, 150, 64–73. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.L.; Wu, M.; Li, J.; Lan, S.Y.; Zeng, Y.Y.; Liu, X.L.; Liu, J.F. Light-enhanced hypoxia-response of conjugated polymer nanocarrier for successive synergistic photodynamic and chemo-therapy. ACS Appl. Mater. Interfaces 2018, 10, 21909–21919. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.X.; Zheng, J.; Ma, D.D.; Liu, N.; Zhu, C.; Li, J.S.; Yang, R.H. Hypoxia-triggered gene therapy: A new drug delivery system to utilize photodynamic-induced hypoxia for synergistic cancer therapy. J. Mater. Chem. B 2018, 6, 6424–6430. [Google Scholar] [CrossRef]
- Piao, W.; Hanaoka, K.; Fujisawa, T.; Takeuchi, S.; Komatsu, T.; Ueno, T.; Terai, T.; Tahara, T.; Nagano, T.; Urano, Y. Development of an azo-based photosensitizer activated under mild hypoxia for photodynamic therapy. J. Am. Chem. Soc. 2017, 139, 13713–13719. [Google Scholar] [CrossRef]
- Li, J.J.; Meng, X.; Deng, J.; Lu, D.; Zhang, X.; Chen, Y.R.; Zhu, J.D.; Fan, A.P.; Ding, D.; Kong, D.L.; et al. Multifunctional micelles dually responsive to hypoxia and singlet oxygen: Enhanced photodynamic therapy via interactively triggered photosensitizer delivery. ACS Appl. Mater. Interfaces 2018, 10, 17117–17128. [Google Scholar] [CrossRef]
- Li, Z.B.; Wu, M.; Bai, H.Z.; Liu, X.G.; Tang, G.P. Light-enhanced hypoxia-responsive nanoparticles for deep tumor penetration and combined chemo-photodynamic therapy. Chem. Commun. 2018, 54, 13127–13130. [Google Scholar] [CrossRef]
- Wang, W.L.; Lin, L.; Ma, X.J.; Wang, B.; Liu, S.R.; Yan, X.X.; Li, S.R.; Tian, H.Y.; Yu, X.F. Light-induced hypoxia-triggered living nanocarriers for synergistic cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 19398–19407. [Google Scholar] [CrossRef]
- Chen, H.C.; Bi, Q.R.; Yao, Y.R.; Tan, N.H. Dimeric BODIPY-loaded liposomes for dual hypoxia marker imaging and activatable photodynamic therapy against tumors. J. Mater. Chem. B 2018, 6, 4351–4359. [Google Scholar] [CrossRef]
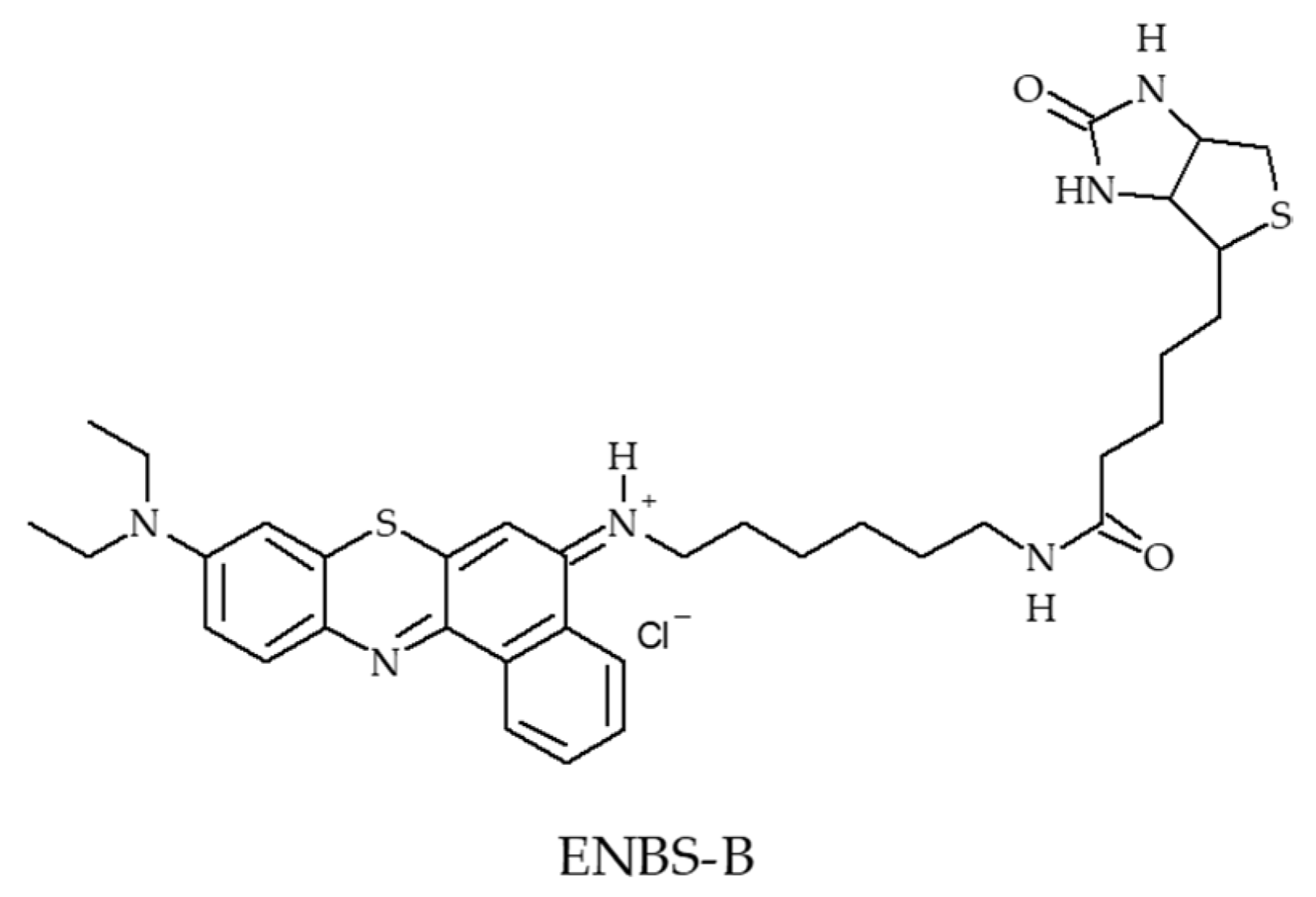
- Evans, C.L.; Abu-Yousif, A.O.; Park, Y.J.; Klein, O.J.; Celli, J.P.; Rizvi, I.; Zheng, X.; Hasan, T. Killing hypoxic cell populations in a 3D tumor model with EtNBS-PDT. PLoS ONE 2011, 6, e23434. [Google Scholar] [CrossRef] [PubMed]
- Guan, S.Y.; Yang, D.; Weng, Y.Z.; Lu, H.; Meng, X.M.; Qu, X.Z.; Zhou, S.Y. Excitation-dependent theranostic nanosheet for cancer treatment. Adv. Healthc. Mater. 2018, 7, 1701123. [Google Scholar] [CrossRef] [PubMed]
- Guan, M.R.; Qin, T.X.; Ge, J.C.; Zhen, M.M.; Xu, W.; Chen, D.Q.; Li, S.M.; Wang, C.R.; Su, H.M.; Shu, C.Y. Amphiphilic trismethylpyridylporphyrin-fullerene (C-70) dyad: An efficient photosensitizer under hypoxia conditions. J. Mater. Chem. B 2015, 3, 776–783. [Google Scholar] [CrossRef]
- Vangeel, I.P.J.; Oppelaar, H.; Marijnissen, J.P.A.; Stewart, F.A. Influence of fractionation and fluence rate in photodynamic therapy with photofrin or mTHPC. Radiat. Res. 1996, 145, 602–609. [Google Scholar] [CrossRef]
- Klimenko, V.V.; Bogdanov, A.A.; Knyazev, N.A.; Dubina, M.V. Pulse mode irradiation at Radachlorin PDT shifted cell death to apoptosis in vitro. In Proceedings of the European Conferences on Biomedical Optics, Munich, Germany, 15 July 2015; Lilge, L.D., Sroka, R., Eds.; Volume 9542, p. 95421. [Google Scholar]
- Klimenko, V.V.; Knyazev, N.A.; Moiseenko, F.V.; Rusanov, A.A.; Bogdanov, A.A.; Dubina, M.V. Pulse mode of laser photodynamic treatment induced cell apoptosis. Photodiagn. Photodyn. Ther. 2016, 13, 101–107. [Google Scholar] [CrossRef]
- Kawauchi, S.; Morimoto, Y.; Sato, S.; Arai, T.; Seguchi, K.; Asanuma, H.; Kikuchi, M. Differences between cytotoxicity in photodynamic therapy using a pulsed laser and a continuous wave laser: Study of oxygen consumption and photobleaching. Lasers Med. Sci. 2004, 18, 179–183. [Google Scholar] [CrossRef]
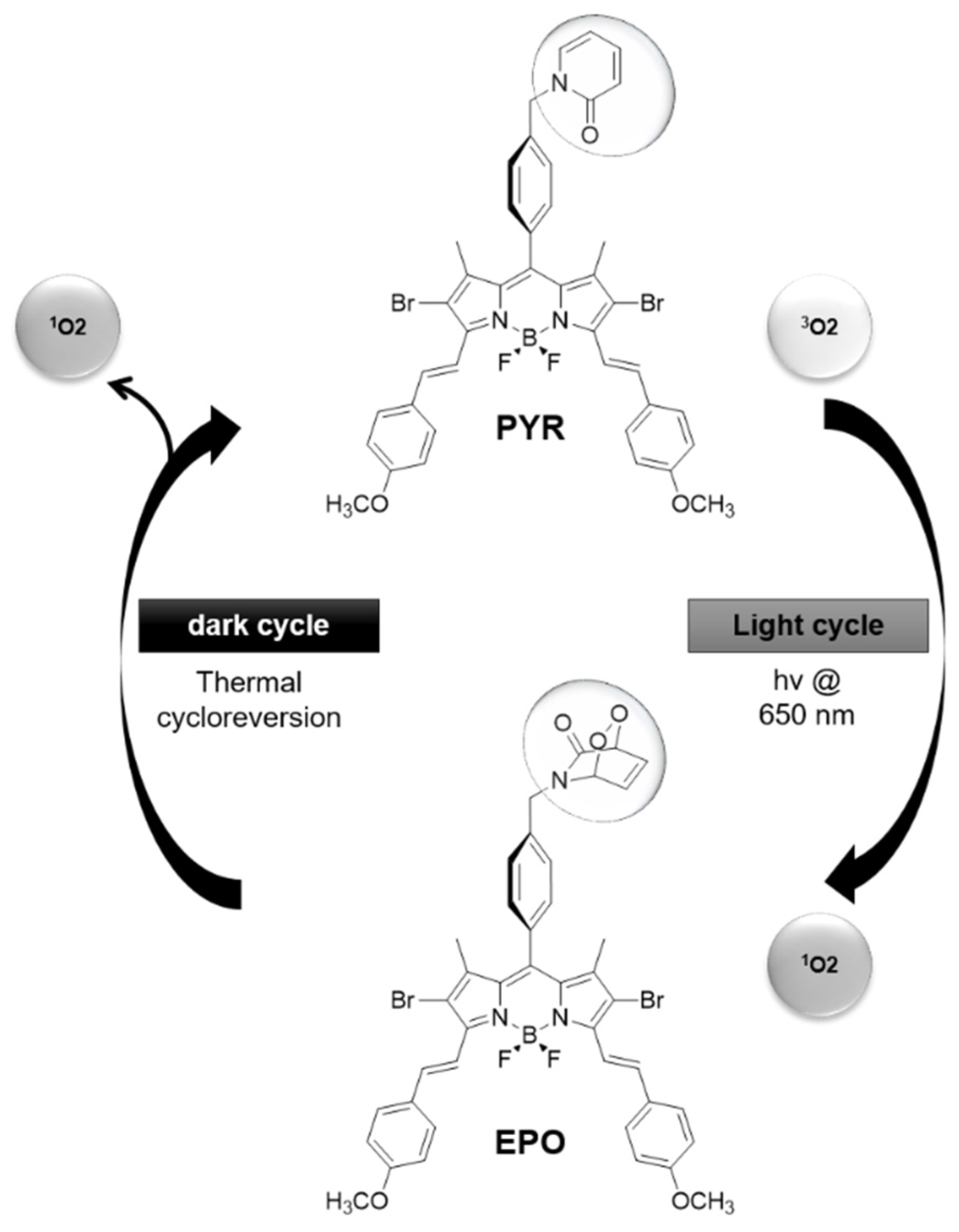
- Turan, I.S.; Yildiz, D.; Turksoy, A.; Gunaydin, G.; Akkaya, E.U. A bifunctional photosensitizer for enhanced fractional photodynamic therapy: Singlet oxygen generation in the presence and absence of light. Angew. Chem. Int. Ed. 2016, 55, 2875–2878. [Google Scholar] [CrossRef]






















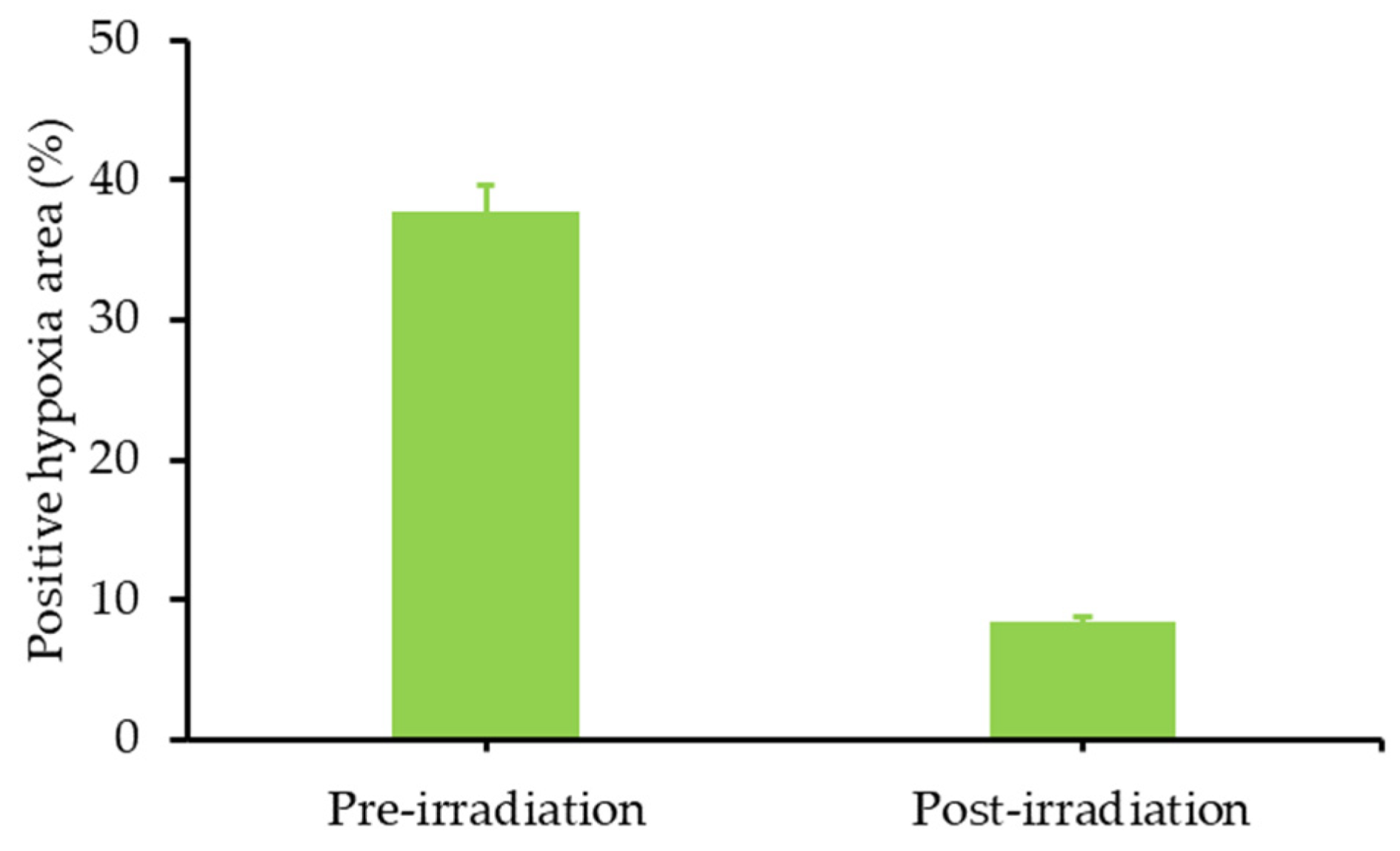
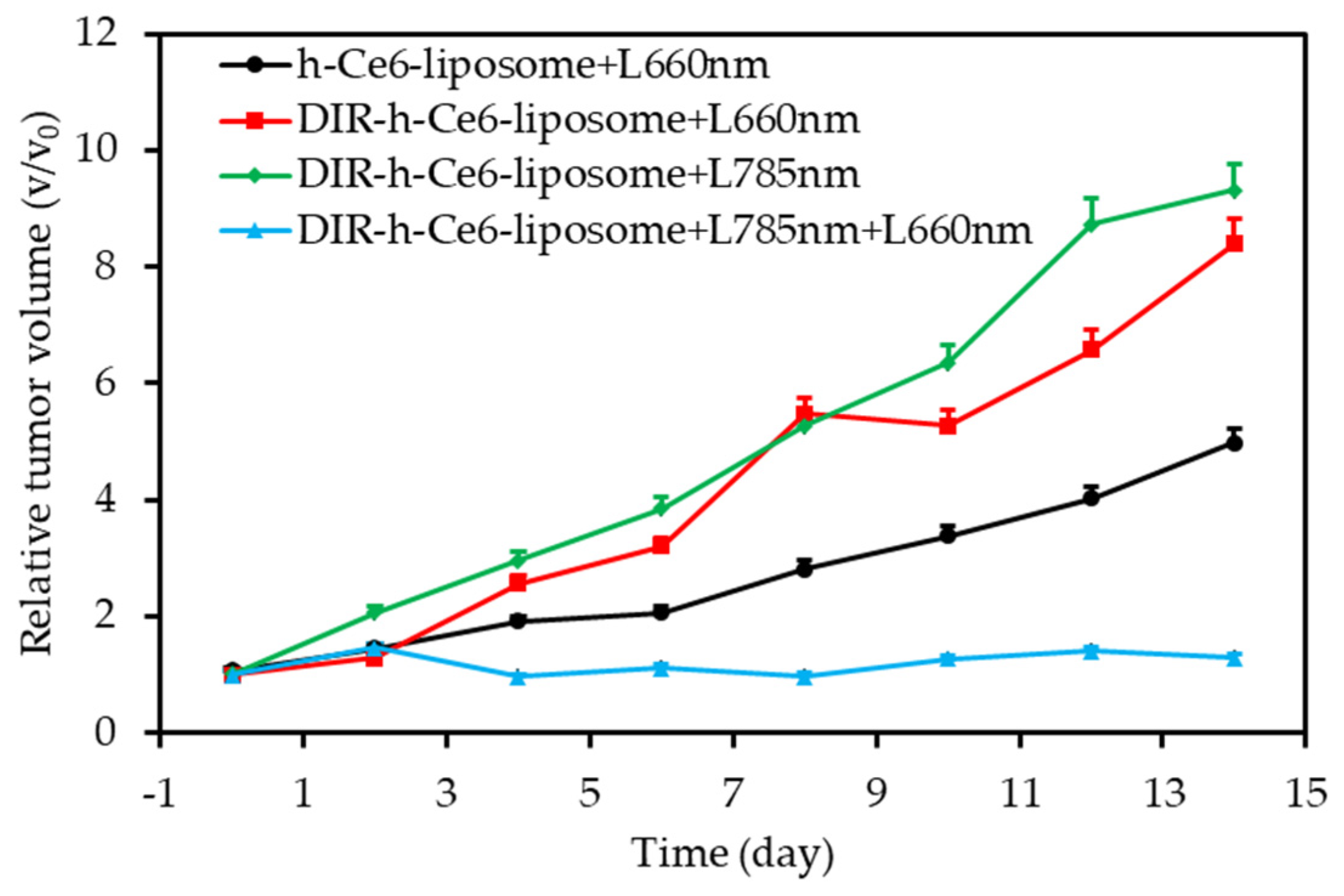















| 0 h | 4 h | 18 h | 24 h | |
|---|---|---|---|---|
| RA | 0.495 | 0.381 | 0.043 | 0.0045 |
| NBO | 0.185 | 0.120 | 0.006 | 0.00035 |
| Ref | Application | O2 Source | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|
| [20] | PDT | Hyperbaric chamber with 100% O2 (21, 100, 200, 300, or 400 kPa) | 5-ALA | 435 nm, 12.9 mW∙cm−2, 0.5 min and 30 min | 1O2 | AY27, WiDr and SW840 cell line | No |
| [21] | PDT | HBO (0.25 MPa; 98% O2 + 2% CO2) | 5-ALA | 630 nm, 5 J∙cm−2, 8 h per days | ROS | A431 cancer cell line | No |
| [22,23,24,25] | PDT | HBO or NBO 100% O2 (hyperbaric chamber: 3 atp) | Photofrin | 630 nm, “Fractional light” group: 150 mW∙cm−2, 200 J∙cm−2, 30 s light and dark intervals “Reduced light dose rate” group: 75 mW∙cm−2, 200 J∙cm−2 | 1O2 | No | MCa tumour-bearing mice |
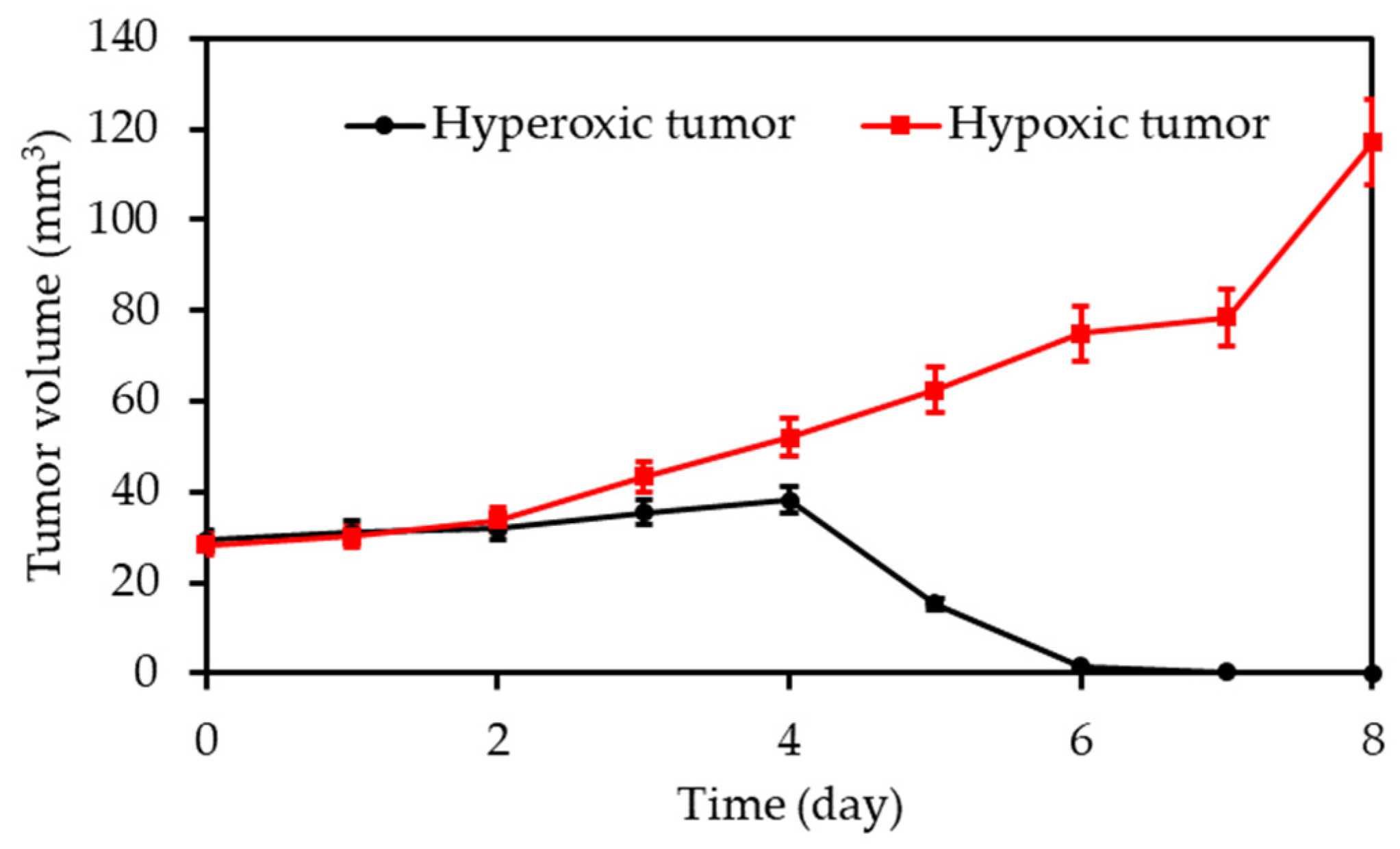
| [26] | PDT | HBO (hyperbaric chamber: 2.5 atm) | RB | 808 nm 16 min, 0.75 W∙cm−2 | ROS | 4T1 cell line | 4T1 tumour bearing mice |
| [27,28,29,30,31,32,33] | PDT | HBO (hyperbaric chamber: 2 atm O2) | HpD (Photosan) or 5-ALA | 630 nm, 300 J∙cm−2 | nd | No | Clinical study on patients with oesophageal carcinoma |
| [34] | PDT | Nicotinamide and carbogen (95% oxygen with 5% carbodioxide) | m-THPC | 652 nm, 20, 100 and 200 mW∙cm−2, 5 min | nd | No | H-MESO1 tumour-bearing mice |
| Ref | Application | Vehicle | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|
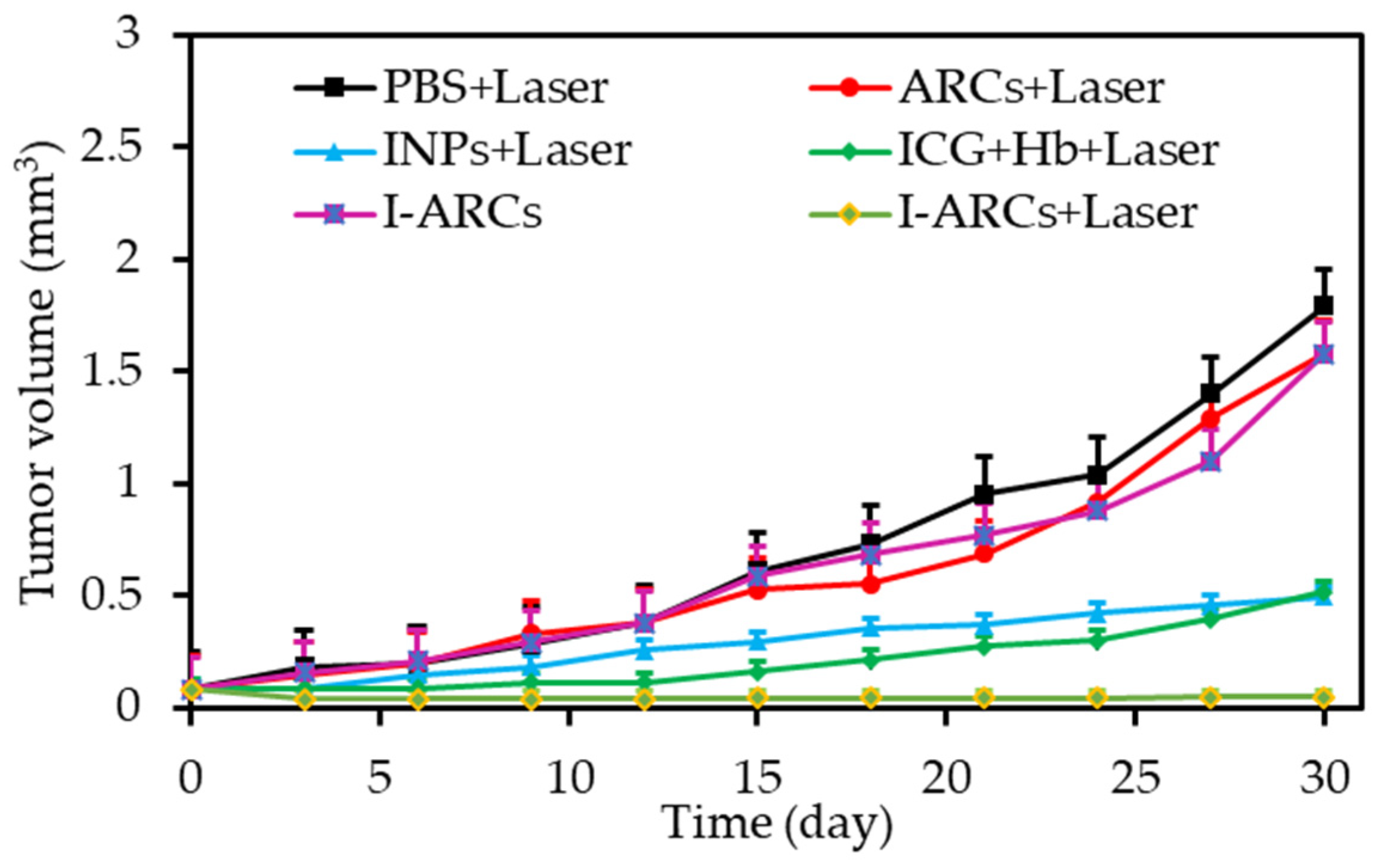
| [36] | PDT | Artificial red cells (ARCs) containing Hb | IGC | In vitro: 808 nm, 100 mW∙cm−2, 5 min In vivo: 808 nm, 100 mW∙cm−2, 30 min | ROS | MCF-7 cell line | MCF-7 tumour-bearing mice |
| [37] | PDT | Hb | Ce6 | In vitro: 660 nm, 0.1 W∙cm−2, 2 min In vivo: 660 nm, 0.1 W∙cm−2, 30 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [38] | PDT | RBCs | ZnF16Pc | In vitro: 671 nm, 100 mW∙cm−2, 0–60 min In vivo: 671 nm, 100 mW∙cm−2, 30 min | 1O2 | U87MG cell line | U87MG tumour bearing mice |
| [39] | PDT | RBCs | RB | 980 nm + 808 nm, 1.5 W∙cm−2, 15 min | 1O2 | U87MG cell line | U87MG tumour-bearing mice |
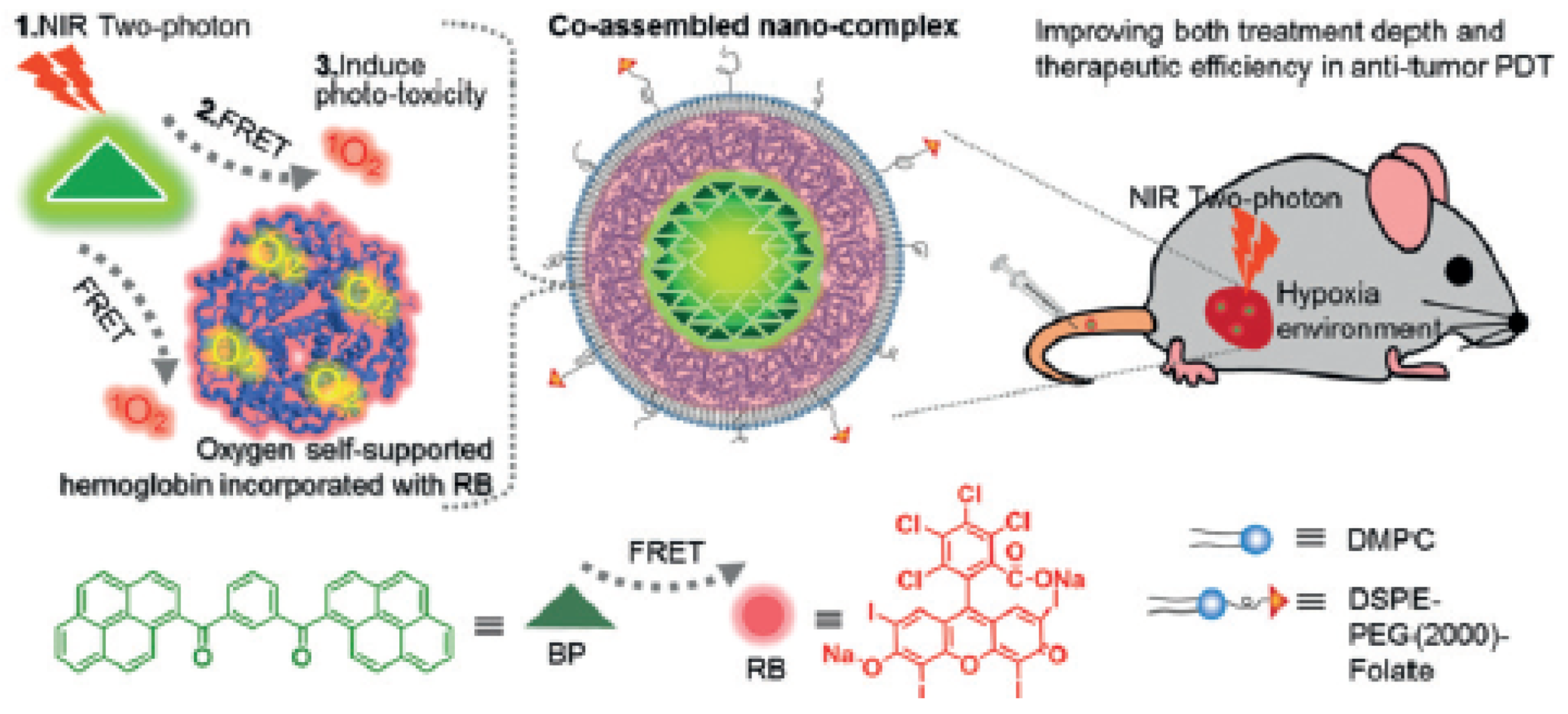
| [40] | PDT | Hb | RB | In vitro: two-photon 808 nm, 390 mW∙cm−2, 20 min In vivo: two-photon 808 nm, 390 or 250 mW∙cm−2, 8 or 15 min | 1O2 | MCF-7 cell line | MCF-7 tumour-bearing mice |
| [41] | PDT | Hb | ICG | 808 nm, 1 W∙cm−2, 1min | 1O2 | CT-26 cell line | S180 and CT26 tumour-bearing mice |
| [42] | PDT | Hb | BODIPY-Br2 | 635 nm, 25 mW∙cm−2, 10 min | 1O2 | HepG2 cell line | No |
| [43] | PDT | Hb | TCPP | 600 nm, 70 mW∙cm−2, 2min | 1O2 | 4T1 cell line | No |
| [44] | PDT | RBC membranes encapsulating Hb | MB | In vitro: 660 nm, 30 mW∙cm−2, 5 min In vivo: 660 nm, 220 mW∙cm−2, 5 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [45] | PDT | Hb-RBC | ICG | 808 nm 60 mW∙cm−2 5 min | 1O2 | Macrophages (RAW264.7 cell line) + multicellular tumour spheroids | MCF-7 tumour bearing mice |
| Ref | Application | O2 Source | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|
| [48] | PDT | PFH | IR780 | 808 nm, 2 W∙cm−2, 20 s | 1O2 | MCF-7 and CT26 adenocarcinoma cell lines | CT26 tumour-bearing mice |
| [49] | PDT | PFC | Fluorous porphyrin | 420 nm, 8.5 mW∙cm−2, 30 min | 1O2 | A375 cell line | No |
| [50] | PDT | PFH | Plasmon resonance | 670 nm, 0.48 mW∙cm−2, 10 min | 1O2 | MCF-7 cell line | MCF-7 tumour-bearing mice |
| [51] | PDT | Pentafluorophenyl | 5,10,15,20-tetrakis(4-aminophenyl) porphyrin | In vitro: 655 nm, 1.52 mW∙cm−2, 15 min In vivo: 655 nm, 1.52 mW∙cm−2, 60 min | 1O2 | SMMC-7721 cell line | No |
| [52] | PDT | PFOB | ICG | In vitro: 808 nm, 1 W∙cm−2, 3 min In vivo: 808 nm, 1 W∙cm−2, 10 min | 1O2 + ROS | MDA-MB-231 cell line | MDA-MB-231 tumour-bearing mice |
| [53] | Ultrasound triggered PDT and RT | PFC | Ce6 | 671 nm, 1.2 W∙cm−2, 5 min | 1O2 | 4T1 and CT26 cell line | 4T1 and CT26 tumour-bearing mice |
| [54] | PDT | PFC | ZnF16Pc | In vitro: 671 nm, 100 mW∙cm−2, 200 s In vivo: 671 nm, 500 mW∙cm−2, 10 min | 1O2 | U87MG cell line | U87MG tumour-bearing mice |
| [55] | PDT | PFH | IR780 | 808 nm, 2 W∙cm−2, 20 s | 1O2 | CT26 cell line | CT26 tumour-bearing mice |
| [56] | PDT | PFSEA | THPP | In vitro: 660 nm, 10 mW∙cm−2, 30 min In vivo: 660 nm, 50 mW∙cm−2, 60 min | 1O2 | 4T1 cell line | 4T1-tumour-bearing mice |
| [57] | PDT + PTT | PFH | AlPc | 670 nm, 1 W∙cm−2, 5 min | 1O2 | 4T1 cell line | 4T1-tumour-bearing mice |
| [58] | PDT | Perfluorooctanoic acid | Ce6 | In vitro: 630 nm, 30 mW∙cm−2, 3 min In vivo: 630 nm, 500 mW∙cm−2, 20 min | 1O2 | C6, HepG2 and HeLa cell lines | C6 glioma tumour-bearing mice |
| [59] | PDT | PFH | Ce6 | 670 nm, 1 W∙cm−2, 1 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [60] | PDT | Fluorinated polypeptide NPs | BODIPY-Br2 | In vitro: 635 nm, 34 mW∙cm−2, 10 min In vivo: 635 nm, 100 mW∙cm−2, 10 min | 1O2 | HepG2 cell line | 4T1 tumour-bearing mice |
| [61] | PDT + PTT | Perfluorooctyl bromide | IR780 | In vitro: 808 nm, 1 W∙cm−2, 5 min In vivo: 808 nm, 1 W∙cm−2, on 30 s, off to room temperature, 20 cycles | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [62] | PDT | Perfluorooctyl bromide | IR780 | 808 nm, 2 W∙cm−2, 20 s | 1O2 | MDA-MB-231 and MCF-7 cell lines | MDA-MB-231 tumour-bearing mice |
| [63] | PDT | PFH | Ce6 | In vitro: 660 nm, 100 mW∙cm−2, 5 min In vivo: 660 nm, 100 mW∙cm−2, 10 min | 1O2 + ROS | MDA-MB-231 cell line | MDA-MB-231 tumour-bearing mice |
| [64] | PDT | PFC | Hypocrellin B | 630 nm, 20 mW∙cm−2, 300 s, 6 J per well | 1O2 | H1299 cell line | No |
| [65] | PDT | NPs with fluorocarbon chains | IR780 | In vitro: 808 nm, 2 W∙cm−2, 20 s In vivo: 808 nm, 2 W∙cm−2, 5 min | 1O2 | 4T1 tumour spheroids | 4T1 tumour-bearing mice |
| Ref | Application | O2 Source | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|
| [67] | Hyperthermia/PDT | O2-loaded polymer bubbles | Ce6 | 660 nm, 100 mW∙cm−2, 10 min | 1O2 | RAW 264.7 cell line | Tramp-C1 tumour-bearing C57BL/6JNarl mice |
| [68] | PDT | O2 nanobubbles | Ce6 | 670 nm, 300 mW∙cm−2, 30 min | 1O2 | C6 glioma cell line | C6 glioma tumour-bearing mice |
| Ref | Application | Catalyst | O2 Source | PS | Energy of Excitation | Type of ROS | IN VITRO | In Vivo |
|---|---|---|---|---|---|---|---|---|
| [75] | PDT | Mn (II) complex | H2O2 | BODIPY derivatives | 500–600 nm, 15 min | 1O2, •OH and others ROS | HepG-2 cell line | |
| [76] | PDT | MnO2 | H2O2 | Ce6 | In vitro: 661 nm, 5 mW∙cm−2, 30 min In vivo: 661 nm, 5 mW∙cm−2, 1 h | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
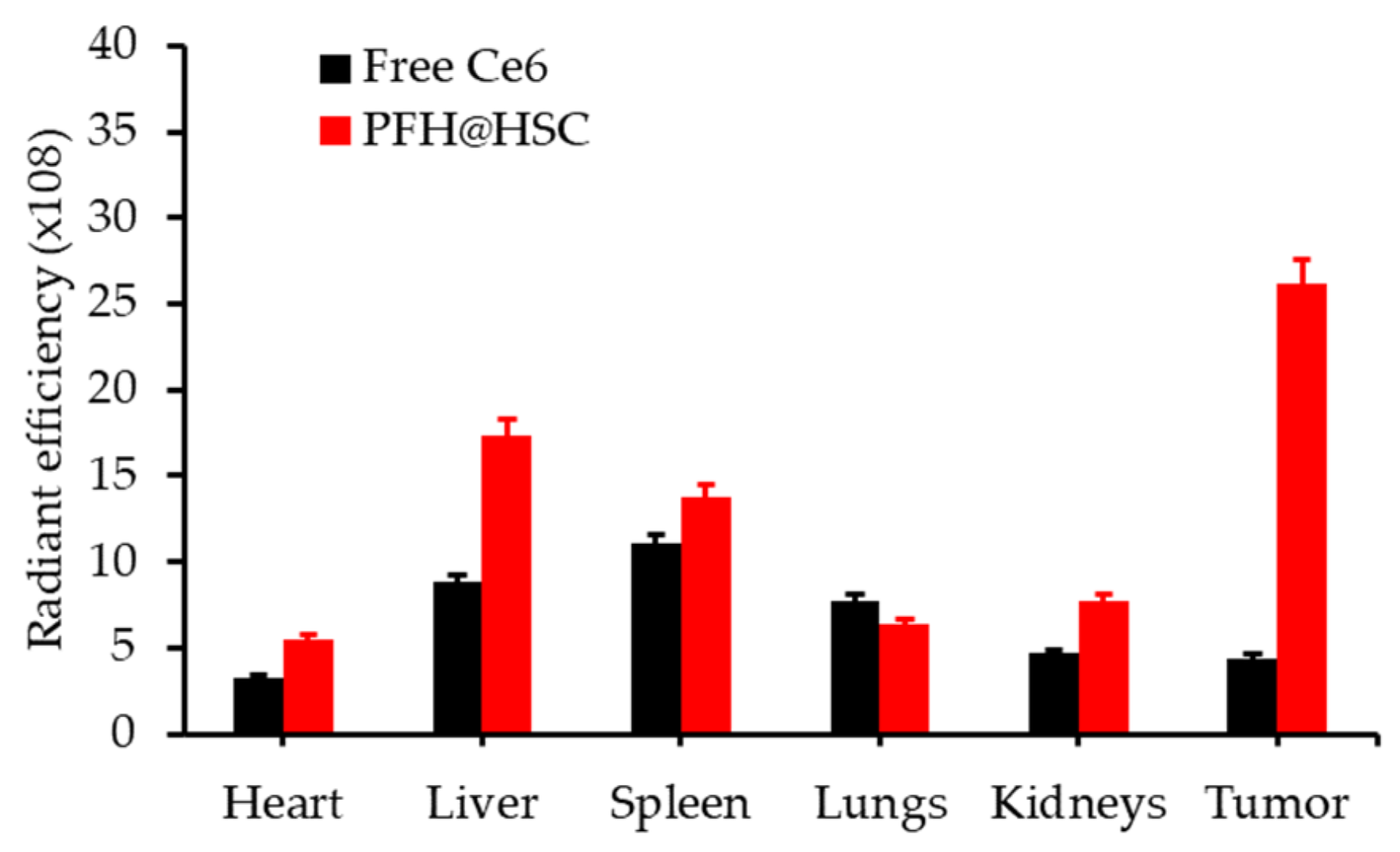
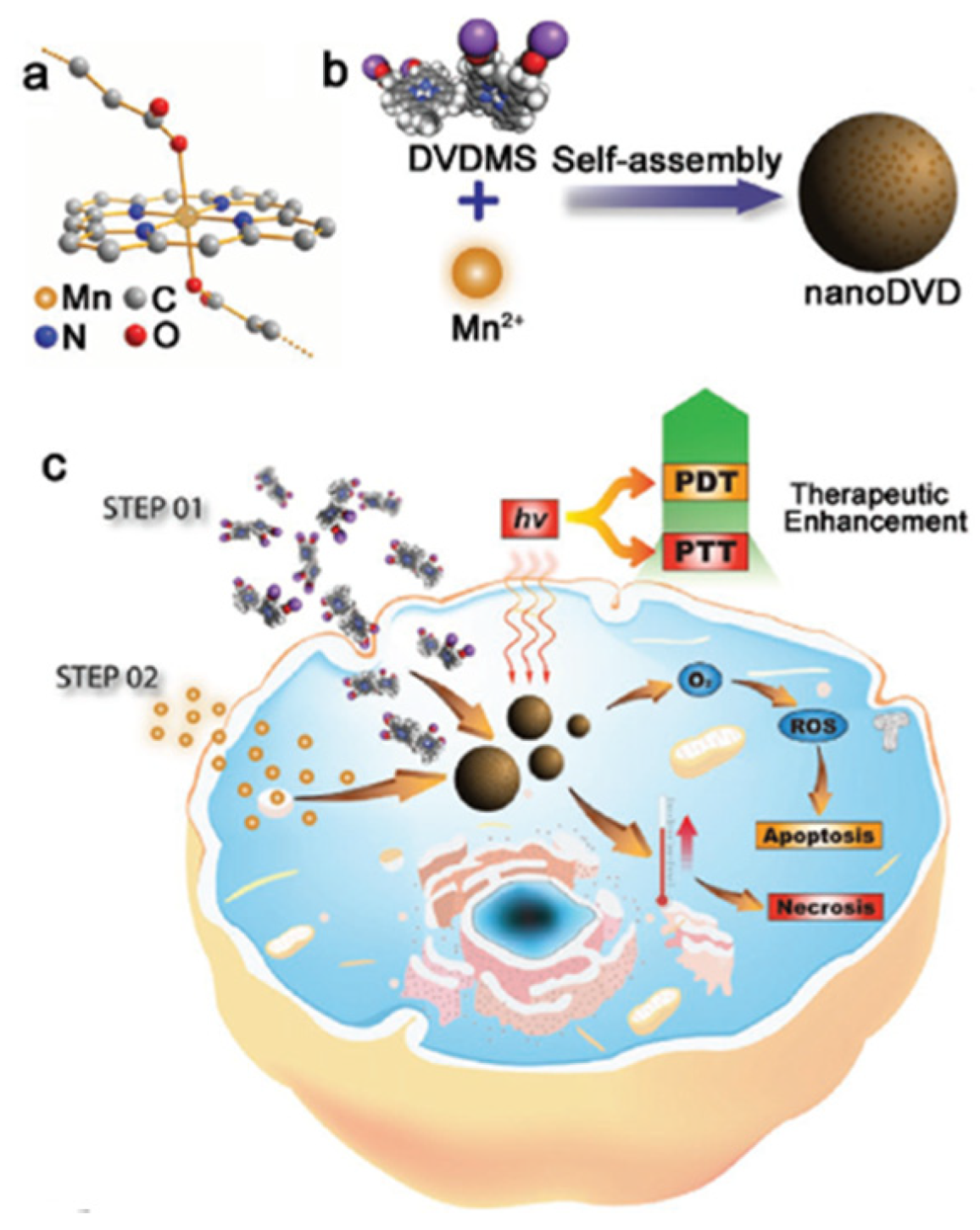
| [77] | PDT | MnO2 | H2O2 | DVDMS | In vitro: 630 nm, 75 mW∙cm−2, 5 min In vivo: 630 nm, 300 mW∙cm−2, 8 min | 1O2 | MCF-7 cell line | MCF-7 tumour-bearing mice |
| [78] | PDT | MnO2 | H2O2 | ICG | In vitro: 808 nm, 0.5 W∙cm−2, 10 min In vivo: 808 nm, 0.8 W∙cm−2, 10 min | 1O2 | SCC-7 cell line | SCC7 tumour-bearing mice |
| [79] | PDT | MnO2 | H2O2 | HMME | In vitro: 532 nm, 1.5 W∙cm−2, 2 min In vivo: 808 nm, 1.5 W∙cm−2, 1.5 min | 1O2 | MCF-7 cells line | S180 tumour model |
| [80] | PDT | manganese ferrite NPs | H2O2 | Ce6 | In vitro: 670 nm, 0.5 W∙cm−2, 30 s In vivo: 670 nm, 0.8 W∙cm−2, 5 min | 1O2 | U87 MG cell line | U87 tumour-bearing mice |
| [81] | PDT | MnO2 | H2O2 | HPPH | In vitro: 10 mW∙cm−2, 1 min In vivo: 630 nm, 10 mW∙cm−2, 10 min | U87MG cell line | U87MG tumour-bearing mice | |
| [82] | PDT | MnO2 | H2O2 and H+ | MB | 650 nm, 100 mW∙cm−2, 15 min | 1O2 | HeLa cells line | U14 tumour-bearing mice |
| [83] | PDT | MnO2 nanosheets | H2O2 | Ce6 | 808 nm, 0.4 W∙cm−2, 60 min | 1O2 | B16F10 cell line | No |
| [84] | PDT | MnO2 | H2O2 | Ce6 | In vitro: 980 nm, 0.5 W∙cm−2, 10 min In vivo: 660 nm, 0.5 W∙cm−2, 30 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [85] | PDT | MnO2 | H2O2 | CD | In vitro: 635 nm, 100 mW∙cm−2, 30 min In vivo: 635 nm, 100 mW∙cm−2, 10 min | 1O2 | HeLa cell line | 4T1-luc tumour-bearing mice |
| [86] | PDT | MnO2 | H2O2 | Ce6 | 660 nm, 100 mW∙cm−2, 10 min | 1O2 | HeLa cells line | No |
| [87] | PDT | MnO2 | H2O2 | PEGylated p–n heterojunction nanosheets | 980 nm, 0.4 W∙cm−2, 5 min | 1O2 | HeLa cells and HEK 293 cell lines | |
| [88] | PDT | MnO2 | H2O2 | Gold nano-cage (AuNC) | 808 nm, 0.8 W∙cm−2, 3 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
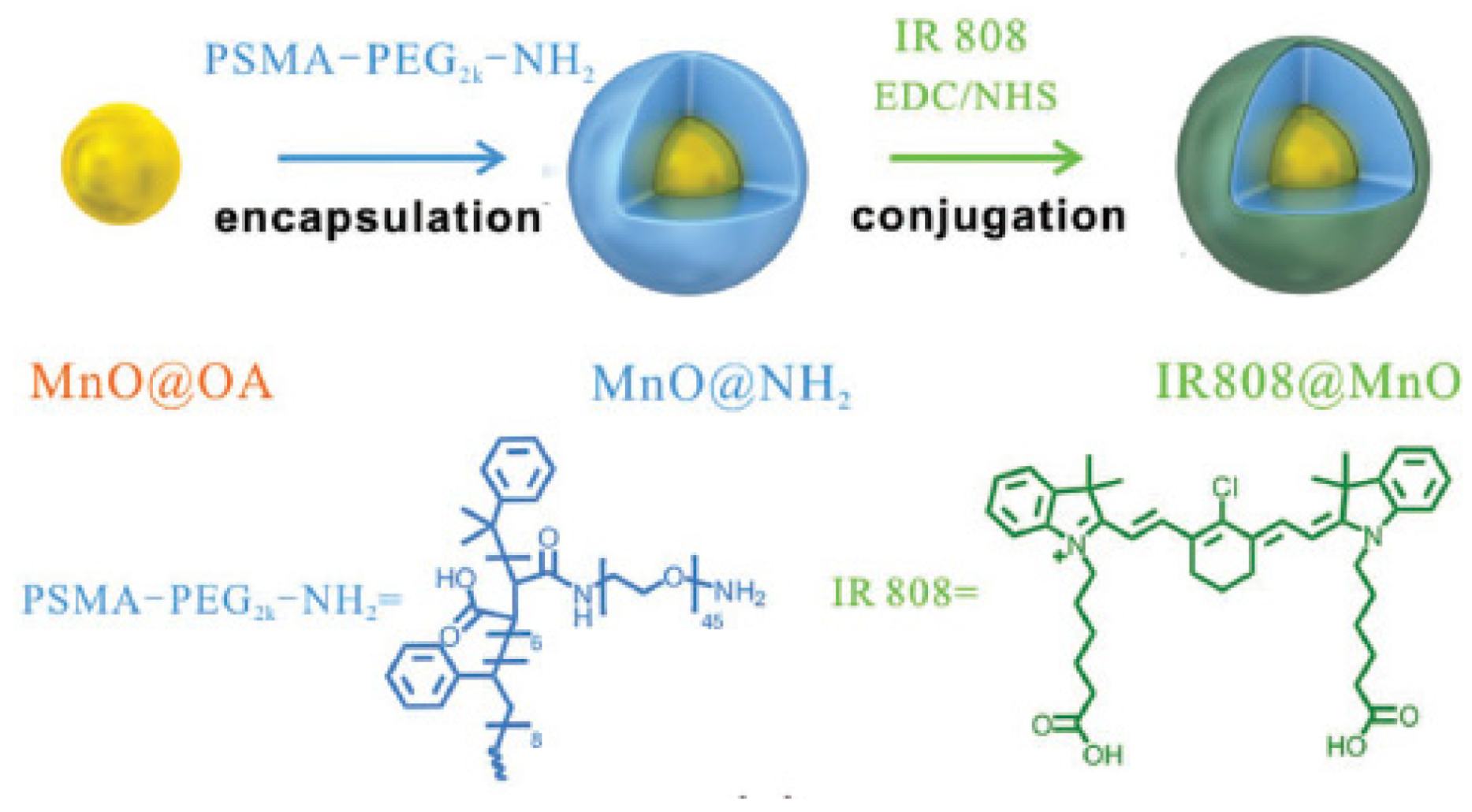
| [89] | PDT | MnO NP | H2O2 | IR808 | 808 nm, 0.5 W∙cm−2, 5 min | 1O2 | MCF-7 cancer cells | MCF-7 tumour-bearing mice |
| [90] | PDT | MnO2 | H2O2 | Ce6 | In vitro: 660 nm, 5 mW∙cm−2, 30 min In vivo: 660 nm, 200 mW∙cm−2, 15 min | 1O2 | MB-49 cells line | Mice with orthotopic bladder cells |
| [91] | PDT | MnO2 | H2O2 | Ce6 | In vitro: 638 nm, 5 mW∙cm−2, 30 min In vivo: 638 nm, 5 mW∙cm−2, 5 min | 1O2 | HeLa and HepG-2 cell lines | HeLa tumour-bearing mice |
| [92] | PDT | MnO2 | H2O2 and H+ | Poly(cyclopentadithiophene-alt-benzothiadiazole) (PCPDTBT) | In vitro: 808 nm, 0.44 W∙cm−2, 5 min In vivo: 808 nm, 0.30 W∙cm−2, 5 min | 1O2 | 4T1 cells line | 4T1 tumour-bearing mice |
| [93] | PDT | MnO2 | H2O2 | NMOFs composed by TCPP and Fe3+ | 660 nm, 50 mW∙cm−2, 15 min | .OH | 4T1 cells line | 4T1 tumour-bearing mice |
| [94] | PDT | MnO2 | H2O2 and H+ | FBP | In vitro: 660 nm, 150 mW∙cm−2, 3 min In vivo: 660 nm, 150 mW∙cm−2, 10 min | 1O2 | HeLa cell line | HeLa tumour-bearing mice |
| [95] | PTT/PDT | MnO2 | H2O2 | Cu2-xS | 980 nm, 0.72 W∙cm−2, 5 min | 1O2 | MCF-7 and A549 cell lines | B16 tumour-bearing mice |
| [96] | PTT/PDT | MnO2 | H2O2 and H+ | Ce6 | 808 + 660 nm, 1.0 W∙cm−2, 10 min | 1O2 | HeLa cell line | HeLa tumour-bearing mice |
| [97] | PTT/PDT | MnO2 | H2O2 and H+ | IR780 | 785 nm PDT: 50 mW∙cm−2 PTT/PDT: 0.3 to 1.0 W∙cm−2 | 1O2 | HepG2 and 3T3 cell lines | HepG2 tumour-bearing mice |
| [98] | Chemo-PDT | MnO2 | H2O2 | Ce6 | In vitro: 660 nm, 5 mW∙cm−2, 30 min In vivo: 660 nm, 5 mW∙cm−2, 1 h | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
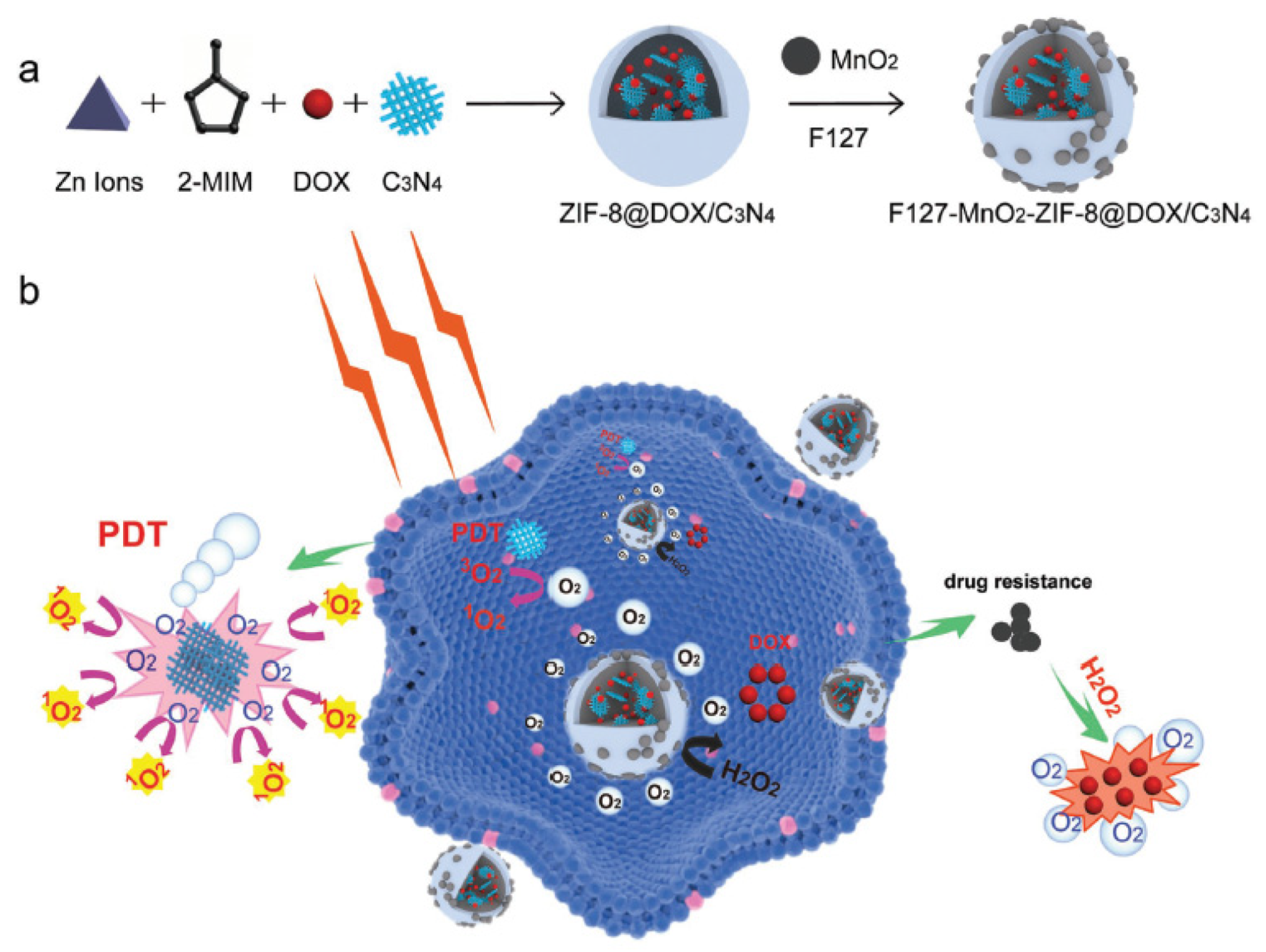
| [99] | Chemo-PDT | MnO2 | H2O2 and H+ | g-C3N4 | 660 nm, 5 mW∙cm−2, 30 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [100] | PDT | MnO2 nanosheets | H2O2, H+ | Silicon phthalocyanine dihydroxide (SPCD) | In vitro: NIR, 1.5 W∙cm−2, 5 min In vivo: NIR, 2 W∙cm−2, 10 min | 1O2 | hc-4T1 cell line | 4T 1 tumour-bearing mice |
| [101] | PDT and gene-silencing therapy | O2/Mn2+-evolving nanocomposite | H2O2 | Ce6 | In vitro: 635 nm, 30 mW∙cm−2, 5 min In vivo: 635 nm, 100 W∙cm−2, 5 min | 1O2 | MCF-7 cell line | MCF-7 tumour-bearing mice |
| [102] | PDT | Mn-Carbon dot | H2O2 | Mn-Pc | 635 nm, 50 mW∙cm−2, 10 min | 1O2 | HeLa cell line | 4T1 tumour-bearing mice |
| Ref | Application | Catalyst | O2 Source | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|---|
| [103] | PDT | Catalase | H2O2 | MB | 635 nm, 100 mW∙cm−2, 5 min | 1O2 | HaCaT, U87-MG, MCF-7 and SKOV-3 cell lines | U87-MG tumour bearing mice |
| [104] | PDT | Catalase | H2O2 | MB | 808 nm, 1 W∙cm−2, 5 min | 1O2 | PC-3 and bMSCs cell lines | No |
| [105] | PDT | Catalase | H2O2 | MB | In vivo: 635 nm, 50 mW∙cm−2, 10 min | 1O2 | SW1990 and 293 T cell lines | SW1990 tumour-bearing mice |
| [106] | PDT | Catalase | H2O2 | AlPcS4 | In vitro: 660 nm, 30 mW∙cm−2, 1 min In vivo: 660 nm, 220 mW∙cm−2, 5 min | 1O2 | HeLa, COS7, SCC-7 and 4T1 cell lines | HeLa tumour-bearing mice |
| [72] | PDT | Catalase | H2O2 | Ce6 | In vitro: 660 nm, 5 mW∙cm−2, 30 min In vivo: 660 nm, 5 mW∙cm−2, 60 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [107] | PDT | Catalase | H2O2 | Ce6 | 660 nm, 5 mW∙cm−2, 60 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [108] | PDT | Catalase | H2O2 | Ce6 | In vitro: 650 nm, 100 mW∙cm−2, 10 min, 25 J∙cm−2 In vivo: 650 nm, 100 mW∙cm−2, 10 min | 1O2 | CAL-27, HeLa, L929 cell lines | CAL-27 tumour-bearing mice |
| [109] | PDT | Catalase | H2O2 | HMME | LED, 3000 mW∙cm−2 for 2 min | 1O2 | B16-F10 cell line | Female C57 mice |
| [111] | PDT | Thiolate catalase (CAT) | H2O2 | RB | Green LED array, 40 mW∙cm−2, 10 min | 1O2 | RAW 264.7 cell line | No |
| [112] | PDT | Biomimetic nanothylakoid | H2O2 | chlorophyll | 660 nm 1 W∙cm−2, 10 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [114] | PDT | Catalase | H2O2 | THPP | In vitro: 660 nm, 5 mW∙cm−2, 30 min In vivo: 660 nm, 5 mW∙cm−2, 60 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [115] | PDT/lung cancer | AuNCs-NH2 | H2O2 | PpIX | 532 nm, 200 mW∙cm−2, 5 J per well | 1O2 | H 460 cell line | No |
| [116] | PDT | G-quadruplex-hemin DNAzyme | H2O2 | Ce6 | In vitro: 660 nm, 5 mW∙cm−2, 30 min In vivo: 660 nm, 5 mW∙cm−2, 60 min | 4T1 cell line | 4T1 tumour-bearing mice | |
| [117] | PDT | MnFe-LDH | H2O2 | MB | In vitro: 650 nm, 100 mW∙cm−2, 10 min In vivo: 650 nm, 100 mW∙cm−2, 15 min | 1O2 | HeLa cell line | U14 tumour-bearing mice |
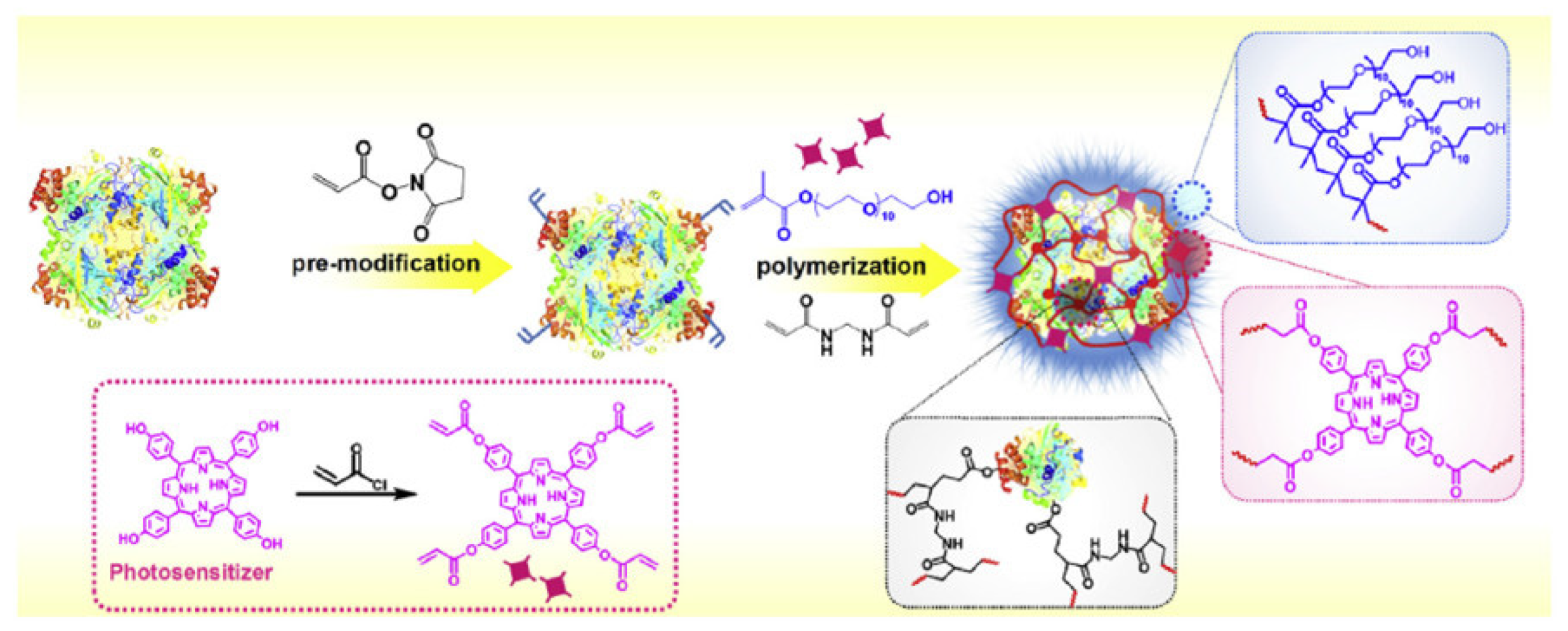
| [118] | PDT | PB NPs | H2O2 | ZnPc | 671 nm, 400 mW∙cm−2, 5 min | 1O2 | HeLa, A549 and 4T1 cell lines | 4T1 tumour-bearing mice |
| [119] | PDT | PB NPs | H2O2 | Ce6 | In vitro: 660 nm, 1.0 W∙cm−2, 5 min In vivo: 660 nm, 1.0 W∙cm−2, 2 min | 1O2 | U87MG and HUVEC cell lines | U87MG tumour-bearing mice |
| [120] | PDT | Fe(III) | H2O2 | MB | 650 nm, 100 mW∙cm−2, 15 min | 1O2 | HeLa cell line | U14 tumour-bearing mice |
| [121] | PDT | Fe(III) | H2O2 | MB | In vitro: 650 nm, 100 mW∙cm−2, 10 min In vivo: 650 nm, 100 mW∙cm−2, 15 min | 1O2 | HeLa cell line | - |
| [122] | PDT | Fe(III) | H2O2 | Ce6, TPEDC, TPETCF | In vitro: white light excitation, 50 mW∙cm−2 5 min In vivo: white light excitation, 50 mW∙cm−2, 10 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [123] | Immuno-PDT | Fe (III) | H2O2 | TBP | In vitro: 650 nm, 20 mW∙cm−2, 15 min In vivo: 650 nm, 100 mW∙cm−2, 7.5 min | 1O2 | CT26 cell line | CT26 tumour-bearing mice |
| [124] | PDT | Cy5- aptamer-heme (H1) and 3’-heme labelled oligonucleotide (H2) | H2O2 | BPNS | In vitro: 660 nm, 150 mW∙cm−2, 3 min In vivo: 660 nm, 150 mW∙cm−2, 10 min | 1O2 | HeLa cell line | 4T1 tumour-bearing mice |
| Ref | Application | Catalyst | O2 Source | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|---|
| [125] | PDT | Pt NP | H2O2 | TCPP | In vitro: 638 nm, 1.0 W∙cm−2, 10 min In vivo: 638 nm, 1.0 W∙cm−2, 8 min | 1O2 | HeLa and 4T1 cell lines | H22 tumour-bearing mice |
| [126] | PDT | Pt NP | H2O2 | Ce6 | 660 nm, 150 mW∙cm−2, 5 min + 808 nm, 500 mW∙cm−2, 5 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [127] | PDT | Pt NP | H2O2 | zirconium-TCPP | In vitro: 660 nm, 30 mW∙cm−2, 3 min In vivo: 660 nm, 200 mW∙cm−2, 5 min | 1O2 | CT26 cell line | CT26 tumour-bearing mice |
| [128] | PDT | Pt NP | H2O2 | Ce6 | 660 nm, 50 mW∙cm−2; 5 min | 1O2 | A549 cell line | No |
| [129] | PDT | Pt NP | H2O2 | BPNS | 660 nm, 1.0 W∙cm−2, 10 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| Ref | Application | Catalyst | O2 Source | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|---|
| [130] | PDT | Light-triggered polymeric vesicle coupled to Ce6 and encapsulating H2O2. | H2O2 | Ce6 | 805 nm, 1000 mW∙cm−2, 3 min - 660 nm, 100 mW∙cm−2, 10 min | 1O2 | BxPC-3 cell line | BxPC-3 tumour-bearing mice |
| [131] | PTT/PDT | Hydrophilic H2O2/poly(vinylpyrrolidone) complex | H2O2 | IR780 | 808 nm, 0.5 W∙cm−2, 3 min | ROS | HepG2 cell line | HepG2 tumour-bearing mice |
| [132] | PDT | Cream | H2O2 | 5-ALA methyl ester (MAL, Metvix®) | 570–670 nm, 105 mW∙cm−2, 16 min, in vvo | nd | Erithema cell line | 40 healthy volunteers |
| [133] | PDT | BiOI/BiOIO3 nanocomposite | H2O2 | BiOI/BiOIO3 | 650 nm, 500 mW∙cm−2, 15 min | 1O2 and ●OH | HeLa cell line | 4T1 tumour-bearing mice |
| [134] | Chemo-PDT | Fe(OH)-modified UCNP | H2O2 | Ce6 | In vitro: 808 nm, 1 W∙cm−2, 10 min In vivo: 808 nm, 1 W∙cm−2, 30 min | 1O2, ●OOH and ●OH | 4T1 cell line | 4T1 tumour-bearing mice |
| Ref | Application | Catalyst | O2 Source | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|---|
| [136] | PDT | CaO2 | Water splitting | RB | 980 nm, 2 W∙cm−2, 5 s | 1O2 | HT29 cell line | HT29 tumour-bearing mice |
| [137] | PDT | CaO2 | Water splitting | MB | In vitro: 660 nm, 30 mW∙cm−2, 30 + 60 s In vivo: 658 nm, 280 mW∙cm−2, 2 + 8 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [138] | PDT | CaO2 | Water splitting | RB | White light, 205 J∙cm−2, 5 min | 1O2 | BxPC-3 cell line | MIAPaCa-2 tumour-bearing mice |
| [139] | PDT | C3N4 NPs | Water splitting | PpIX | 630 nm, 80 mW∙cm−2, 10 min | nd | 4T1 cell line | 4T1 tumour-bearing mice |
| [140] | PDT | Fe-C3N4 NPs | Water splitting | Ru(bpy)32+ | In vitro: 800 nm - 2 photons, 2.7 W, 3 or 5 min In vivo: 800 nm - 2 photons, 2.7 W, 5 min | 1O2 | 4T1 cell line | 4T1 tumour bearing Balb/ c mice |
| [141] | PDT | C3N4 nanosheets | Water splitting | CD | In vitro: 980 nm, 500 mW∙cm−2, 5 min In vivo: 980 nm, 500 mW∙cm−2, 10 min | ●OH O2−● | HeLa cell line | U14 tumour-bearing mice |
| [142] | PDT | Thylakoid membrane from plants | Water splitting | Ag, SiO2 or ZnO NPs | 660 nm, 155 mW∙cm−2, 2 min | ROS | CT26 cell line | CT26 tumour-bearing mice |
| [144] | PTT/PDT | Bi2WO6 NPs | Water splitting | Bi2WO6 NPs | 808 nm, 1 W∙cm−2, 5 to 20 min | ●OH | HeLa cell line | HeLa tumour-bearing mice |
| [145] | PDT | Au-semiconductor nanocomposite | Water splitting | In vitro: 450 nm, 500 mW∙cm−2, 10 min In vivo: 450 nm, 500 mW∙cm−2, 30 min | nd | 4T1 cell line | 4T1 tumour-bearing mice |
| Ref | Application | Target | Additional Compound | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|---|
| [146] | PDT | Hyaluronan of ECM | Hyaluronidase | Ce6 | 660 nm, 2 mW∙cm−2 60 min | 1O2 and others ROS | No | 4T1 tumour-bearing mice |
| [147] | PDT | Collagen of ECM | Collagenase | Ce6 | 660 nm, 5 mW∙cm−2, 60 min | nd | No | 4T1 tumour-bearing mice |
| Ref | Application | Additional Compound | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|
| [148] | PDT | Met | HCe6 | In vitro: 660 nm, 10 min In vivo: 660 nm, 30 min, 0.035 W∙cm−2 | nd | 4T1 cell line | 4T1 tumour-bearing mice |
| [149] | PDT | Met | W18O49 | 808 nm, 1 W∙cm−2, 10 min | 1O2 | Raji cell line | Raji lymphoma-bearing mice |
| [150] | PDT | TAM | Ce6 | 660 nm, 5 mW∙cm−2, 30 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [151] | PDT | Ato | ICG-BSA nanocomplex | 808 nm, 1 W∙cm−2, 5 min | ROS | HeLa cell line | HeLa tumour-bearing mice |
| Ref | Application | Supplementary Therapy Used | Chemo-Drug | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|---|
| [156] | Chemo-PDT | Chemotherapy | TPZ | SPCD | In vitro: 980 nm, 0.7 W∙cm−2, 5 min In vivo: 980 nm, 1.4 W∙cm−2, 15 min | 1O2 and others ROS | HeLa cell line | HeLa tumour-bearing mice |
| [157] | Chemo-PDT | Chemotherapy | TPZ | TPC | 650 nm, 1.2 W∙cm−2,10 min | 1O2 | MCF-7 cell line | MCF-7 tumour-bearing mice |
| [158] | Chemo-PDT | Chemotherapy | TPZ | PCN-224 | In vitro: 660 nm, 30 mW∙cm −2, 5 min In vivo: 660 nm, 220 mW∙cm −2, 10 min | 1O2 | 4T1 and COS7 cell line | 4T1 tumour-bearing mice |
| [159] | Chemo-PDT | Chemotherapy | TPZ | ICG | In vitro: 808 nm, 2 W∙cm−2, 3 min In vivo: 808 nm, 2 W∙cm−2, 5 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [160] | Chemo-PDT | Chemotherapy | TPZ | ICG | 405 nm, 0.75 W∙cm−2, 5 min and 808 nm, 1.0 W∙cm−2, 10 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [161] | Chemo-PDT | Chemotherapy | TPZ | TCPP | In vitro: 635 nm, 12 mW∙cm−2, 9 min In vivo: 635 nm, 0.1 W∙cm−2, 30 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [162] | Chemo-PDT | Chemotherapy | TPZ | IR780 | 808 nm, 1 W∙cm−2, 3 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [163] | Chemo-PDT | Chemotherapy | TPZ | Ce6 | 670 nm, 0.48 W∙cm−2, 10 min | 1O2 | MCF-7 cell line | MCF-7 tumour-bearing mice |
| [164] | Chemo-PDT | Chemotherapy | TPZ | Diketopyrrolopyrrole (DPP)-based compound | 660 nm, 1.5 W∙cm2, 10 min | 1O2 | MCF-7 cell line | No |
| Ref | Application | Supplementary Therapy Used | Drug | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|---|
| [165] | Chemo-PDT | Chemotherapy | DOX | 2-nitroimidazole (NI) | In vitro: light, 0.1 W∙cm−2, 5 or 20 min In vivo: 532 nm, 0.1 W∙cm−2, 5 min or 635 nm, 0.1 W∙cm−2, 5 min | 1O2 | HeLa cell line | HeLa tumour-bearing mice |
| [166] | Chemo-PDT | Chemotherapy | DOX | Ce6 | In vitro: 660 nm, 100 mW∙cm−2, 5 min In vivo: 660 nm, 100 mW∙cm−2, 10 min | 1O2 | MCF-7 cell line | MCF-7 tumour-bearing mice |
| [167] | Chemo-PDT | Chemotherapy | DOX | Ce6 | In vitro: 660 nm, 100 mW∙cm−2, 2 min In vivo: 660 nm, 100 mW∙cm−2, 20 min | 1O2 | MCF-7 cell line | Female BALB/c nude mice |
| [168] | Chemo-PDT | Chemotherapy | DOX | Ce6 | 980 nm, 0.5 W∙cm−2, 5 min | ROS | HeLa cell line | U14 tumour-bearing mice |
| [169] | Chemo-PDT | Chemotherapy | DOX | RB | In vitro: 808 nm, 0.5 W∙cm−2, 10 min In vivo: 808 nm, 0.5 W∙cm−2, 5 min | 1O2 | L929 cell line | H22 tumour bearing mice |
| [170] | Chemo-PDT | Chemotherapy | DOX | Ce6 | In vitro: 660 nm, 2 mW∙cm−2, 30 min In vivo: 660 nm, 10 mW∙cm−2, 30 min | 1O2 | LLC cell line | LLC tumour bearing mice |
| [171] | Chemo-PDT | Chemotherapy | DOX | PPy | 638 nm, 1 W∙cm−2, 10 min | 1O2 | HepG2 cell line | No |
| [172] | Chemo-PDT | Chemotherapy | DOX | Ce6 | 660 nm, 100 mW∙cm−2, 10 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| Ref | Application | Supplementary Therapy Used | Drug | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|---|
| [173] | Chemo-PDT | Chemotherapy | AQ4N | Ce6 | In vitro: 660 nm, 2 mW∙cm−2, 30 min In vivo: 660 nm, 2 mW∙cm−2, 1 h | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [174] | Chemo-PDT | Chemotherapy | AQ4N | Verteporfin | In vitro: 690 nm, 30 mW∙cm−2, 10 min In vivo: 690 nm, 50 mW∙cm−2, 20 min | 1O2 | PC-3 cell line | PC-3 tumour-bearing mice |
| [175] | Chemo-PDT | Chemotherapy | AQ4N | Photochlor (HPPH) | In vitro: 671 nm, 100 mW∙cm−2, 6 min In vivo: 671 nm, 100 mW∙cm−2, 10 min | ROS | U87MG cell line | U87MG tumour-bearing mice |
| Ref | Application | Supplementary Therapy Used | Drug | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|---|
| [176] | Chemo-PDT | Chemotherapy | Pt (IV) complex | No | In vitro: 396 nm, 5 mW·cm−2, 5min In vivo: 396 nm, 0.4 W·cm−2, 10 min | ●OH and 1O2 | A549 cell line | A549 tumour- bearing mice |
| [177] | Chemo-PDT | Chemotherapy | Pt (IV) Up conversion NP | Ce6 | 980 nm near-infrared light convert into 365 nm and 660 nm emissions In vitro: 0.85 W∙cm−2, 5 min In vivo: 0.80 W∙cm−2, 10 min | 1O2 | L929 cell line | HeLa, B16, HCT116 or MDA-MB-231 tumour-bearing mice |
| [178] | Chemo-PDT | Chemotherapy | cis-Pt (IV) | THPP | In vitro: 660 nm, 5 mW·cm−2, 20 min In vivo: 660 nm, 5 mW·cm−2, 45 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| Ref | Application | Therapy Used | Drug | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|---|
| [179] | Antiangiogenic-PDT | Antiangiogenic | HIF-1α siRNA | Photosan | 640 nm, 320 mW∙cm−2, 100 J∙cm−2 | nd | SCC4 and SAS cell lines | SCC4 and SAS tumour bearing nude mice. |
| [180] | Antiangiogenic-PDT | Antiangiogenic | HIF-1α siRNA | Cationic porphyrin-grafed lipid | 650 nm, 200 mW, 10 min | 1O2 | MDA-MB-231 cell line | MDA-MB-231 tumour-bearing mice |
| [181] | Antiangiogenic-PDT | Antiangiogenic | Acriflavine | ZnPc | 671 nm, 500 mW, 15 J∙cm−2 | nd | A431 cell line | No |
| [182] | Antiangiogenic-PDT | Antiangiogenic | Acriflavine | ZnPc | 671 nm, 500 mW, 15 J∙cm−2 | ROS | SK-ChA-1 cell line | No |
| Ref | Application | Therapy Used | Drug | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|---|
| [183] | Antiangiogenic-PDT | Antiangiogenic | EMAP-II or IM862 | Photofrin | In vitro: 570–650 nm, 0.35 mW∙cm−2 In vivo: 630 nm, 75 mW∙cm−2, 200 J∙cm−2 | nd | BA cell line | BA tumour-bearing mice |
| [184] | Antiangiogenic-PDT | Antiangiogenic | SU5416 and SU6668 | hypericin | Halogen light with red acetate filter, 47.7 J∙cm−2, 60 mW∙cm−2 | nd | No | CNE2 tumour-bearing mice |
| [185] | Antiangiogenic-PDT | Antiangiogenic | sunitinib, sorafenib and axitinib/bevacizumab | Visudyne | 420 nm, 5 J∙cm−2, 35 mW∙cm−2 | nd | No | A2780 tumour-bearing mice |
| [186] | Antiangiogenic-PDT | Antiangiogenic | VEGF-A siRNA | Photosan | 640 nm In vitro: 10 J∙cm−2, 159 s In vivo: 320 mW∙cm−2, 100 J∙cm−2, 11 min | nd | SCC4 and SAS cell lines | SCC4 and SAS tumour bearing nude mice . |
| [187] | Antiangiogenic-PDT | Antiangiogenic | 5,6-dimethylxanthenone-4-acetic acid | DPP-4 | 660 nm, 0.8 W∙cm−2, 4 min | 1O2 and others ROS | HeLa and HUVEC cell lines | HeLa tumour-bearing mice |
| Ref | Application | Therapy Used | Drug | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|---|
| [188] | Antiangiogenic-PDT | Antiangiogenic | celecoxib or NS-398 | Photofrin | 570–650 nm, 0.35 mW∙cm−2, 0 to 525 J∙cm−2, 0 to 150 s | nd | BA cell line | BA tumour-bearing mice |
| [189] | Antiangiogenic-PDT | Antiangiogenic | Chalcone | phthalocyanine | red light (>600 nm), 3.6 J∙cm−2 | 1O2 | HT29 cell line | No |
| [190] | Antiangiogenic-PDT | Antiangiogenic | DHA | 5-ALA | 630 nm, 25 W∙cm−2 | nd | Eca109 cell line | Eca109 tumour-bearing mice |
| [191] | Antiangiogenic-PDT | Antiangiogenic | DHA | 5-ALA | 630 nm, 20 or 25 W∙cm−2 | nd | Eca109 and Ec9706 cell lines | No |
| [192] | Antiangiogenic-PDT | Antiangiogenic | acetazolamide | AZBPS | 660 nm, 2 W∙cm−2, 30 min | 1O2 | MDA-MB-231 and MCF7 cell line | MDA-MB-231 tumour-bearing nude mice |
| Ref | Application | Supplementary Therapy Used | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|
| [193] | PTT/PDT | PTT | Photofrin and Porphysome | Photofrin: 635 nm, 200 mW, 318 s Porphysome: 671 nm, 200 mW, 18 s; PTT: 671 nm, 750 mW, 85 s | nd | No | KB tumour-bearing mice |
| [194] | PTT/PDT | PTT | Iron porphyrin | 600 nm, PDT: 50 mW∙cm−2, 10 min PTT: 1.0 W∙cm−2 for 5 min, | ROS | 4T1 cell line | 4T1 tumour-bearing mice |
| [195] | PDT | External heating | Ce6 | In vitro: 660 nm, 50 mW∙cm−2, 5 min In vivo: 660 nm, 200 mW∙cm−2, 20 min | 1O2 | 4T1 cell line | 4T1 tumour-bearing mice |
| [196] | PTT/PDT | PTT | hCe6 | NIR irradiation: 785 nm, 1 W∙cm−2, 10 min Light irradiation: 660 nm, 2 mW∙cm−2, 30 min | nd | 4T1 cell line | 4T1 tumour-bearing mice |
| Ref | Application | Hypoxia-Independent Strategy | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|
| [199] | PDT | Modulation of the mechanism of photoactivation by micelles | mTHPP | 532 nm, 20 mW∙cm−2, 10 min | 1O2 and others ROS | H2009, A549 and PC-3 cell lines | No |
| [16] | PDT | Alginate formulation to switch photochemistry of PS from type II to type I | MB | 600 nm, 6 mW∙cm−2 | O2−● and others ROS | MDAMB231, 4T1, SKBR3, and MCF7 cell lines | |
| [200] | PDT | Superoxide radical generator | ENBS-B | 660 nm, 14.4 J∙cm−2, 15 min | O2−●, ●OH | HepG2 cell line | HepG2 tumour-bearing mice |
| [201] | PTT/PDT | Fenton reaction | CFNs | 650 nm, 0.49 W∙cm−2, 15 min and 808 nm 1.3 W∙cm−2, 10 min | ●OH, O2−● | HeLa cell line | U14 tumour-bearing mice |
| [202] | PDT | Type I PSs | Ru (II) complexes | In vitro: white light (400–800 nm) 30 mWcm−2, 10 min In vivo: xenon lamp, 250 mW∙cm−2, 15 min | 1O2 and others ROS | HeLa cell line | HeLa tumour-bearing mice |
| [203] | PDT | Fenton reaction | Fe3O4@MIL-100(Fe)-UCNP | In vitro: 980 nm laser 0.9 Wcm−2, 10 min In vivo: 980 nm laser 0.9 Wcm−2, 15 min | ●OH | HeLa cell line | U14 tumour-bearing mice |
| [204] | PDT | Carbon radical generator | Ru(III) complexes | 470 nl LED | Carbon radicals | SKOV-3 cell line | No |
| [205] | PDT | Type I reaction | Os(biq)2(phen)](PF6)2, Os(biq)2(IP)](PF6)2 and Os(biq)2(dppn)](PF6)2 | 625 nm (90 J∙cm−2 = 450 mW∙cm−2, 200 s) or 808 nm light (600 J∙cm−2 = 900 mW∙cm−2, 667 s) | 1O2 and oxygen-independent pathways | HT1376 and U87 cell lines | CT26.CL25 tumour-bearing mice |
| Ref | Application | Hypoxia-Independent Strategy | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|
| [206] | PDT | Photocontrollable cytotoxic peptidomimetic (photoisomerization) | Diaryletene-derived peptodomimetics | Visible light, 100 mW∙cm−2, 20 min | No | HeLa and COLO-205 cell lines | LLC tumour-bearing mice |
| [207,208] | PDT | Photoacid generators to induce an imbalance of the pH of tumour cells | PAG | In vitro: 377 nm, 5.4 mW∙cm−2 In vivo: two-photon (710 nm), 2.0 mW∙cm−2 | No | HCT-116 cell line | No |
| Ref | Application | Hypoxia-Independent Strategy | PS | Energy of Excitation | Type of ROS | Others Radicals | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|---|
| [209] | PDT | SNP: NO donor to reduce O2 consumption | Tetraphenylporphyrin | 660 nm, 20 mW. cm−2, 3 or 10 min | 1O2 | NO + others ROS | 4T1 cells | 4T1 tumour-bearing mice |
| [210] | PDT | Compound able to produce both NO and 1O2 under irradiation | Ru complex | 660 nm, 5 J∙cm−2 | 1O2 | NO | B16F10 cell line | No |
| [211] | PDT | NO-generation induced by ROS production (NO donor: L-arginine) | Porphyrinic metal-organic framework | In vitro: 660 nm, 30 mW∙cm−2, 8 min In vivo: 660 nm, 200 mW∙cm−2, 10 min | 1O2 | NO | 4T1 cell line | 4T1 tumour-bearing mice |
| [212] | PDT | Glutathione-sensitive supramolecular NO nanogenerator | Ce6 | In vitro: 660 nm laser, 0.2 W, 2 min In vivo: 660 nm laser, 0.5 W, 5 min | NO + Peroxynitrite anions | MCF-7 cell line | MCF-7 tumour-bearing mice |
| Ref | Application | Hypoxia-Independent Strategy | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|
| [213] | PTT/PDT | Use of 1O2 donor: endoperoxide of DPA | tetraphenylporphyrin | In vitro: 808 nm, 0.5 W∙cm−2, 1 min In vivo: 808 nm, 1.0 W∙cm−2, 0–5 min | 1O2 and others ROS | HepG2 cell line | HepG2 tumour-bearing mice |
| [214] | PTT/PDT | Use of 1O2 donor: DPAE | DPAE | 808 nm, 1.5 W∙cm−2, 5 min | ROS | 4T1 cell line | 4T1 tumour-bearing mice |
| Ref | Application | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|
| [215] | PDT | Pt (II) porphyrins linked to cationic oligofluorenes arms | In vitro: 520 nm, 10.0 mW∙cm−2, 10 min In vivo: 520 nm, 160 mW∙cm−2, 10 min | 1O2 | HeLa cell line | HeLa tumour-bearing mice |
| [216] | PDT | OR141 | White light, 15 min | 1O2 | Endothelial cells | Human colon carcinoma xenograft model |
| [217] | PDT | Mito-DAD | 470 nm, 16 mW∙cm−2, 3 min in normoxia and 5 min in hypoxia | 1O2 | HeLa cell line | No |
| [218] | PDT | Porphyrazines derivatives | 690 nm, 2 J∙cm−2 | 1O2 | LNCaP cell line | No |
| Ref | Application | Hypoxia-Dependent Strategy | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|---|
| [219] | Chemo-PDT | Hypoxia-cleaved Azobenzene | Ce6 | In vitro: 670 nm, 50 mW∙cm−2, 6 min In vivo: 670 nm, 50 mW∙cm−2, 10 min | 1O2 | HeLa and NIH3T3 cell lines | HeLa tumour-bearing mice |
| [220] | Chemo-PDT | Hypoxia-cleaved Azobenzene | TMPyP4 | 660 nm, 1 W∙cm−2, 30 min | ROS | HepG2 and L02 cell lines | No |
| [222] | PDT | Hypoxia-cleaved Azobenzene | Ce6 | In vitro: 660 nm, 100 mW∙cm−2, 10 and 5 min In vivo: 660 nm, 200 mW∙cm−2, 30 min | 1O2 | LLC cell line | LLC tumour-bearing mice |
| [221] | PDT | Hypoxia-cleaved Azobenzene | azoSeR | 535 nm, 28 mW∙cm−2, 3 min | 1O2 | A549 cell line | No |
| [223] | Chemo-PDT | Hypoxia-cleaved Azobenzene | Ce6 | In vitro: 660 nm, 8 mW∙cm−2, 6 min In vivo: 660nm, 8 mW∙cm−2, 30 min | 1O2 | MCF-7 cell line | MCF-7 tumour-bearing mice |
| [224] | Chemo-PDT | Hypoxia-cleaved Azobenzene | Ce6 | In vitro: 671 nm, 10 mW∙cm−2, 5 min In vivo: 671 nm, 150 mW∙cm−2, 10 min | 1O2 | HeLa cell line | HeLa tumour-bearing mice |
| [225] | PDT | Hypoxia-reducible compound by NTR | DiBDP substituted with a nitro group | 520 nm, 100 mW∙cm−2, 5 min | 1O2 | HeLa cell line | HeLa tumour-bearing mice |
| Ref | Application | PS | Energy of Excitation | Type of ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|
| [226] | PDT | EtNBS | EtNBS-PDT: 652 nm, 15 J∙cm−2, 25 to 300 mW∙cm−2 Hypoxia EtNBS-PDT: 670 nm, 100 mW∙cm−2, 5 to 20 J∙cm−2 | nd | 3D adherent OVCAR-5 human OvCa model | No |
| [227] | PDT | Ru(C-bpy)2/mLDH | 520 nm, 100 mW∙cm−2, 8 min | 1O2 | HeLa cell line | HeLa tumour-bearing mice |
| Ref | Application | PS | Excitation Wavelength | Type de ROS | In Vitro | In Vivo |
|---|---|---|---|---|---|---|
| [229] | Continuous vs. fractional PDT | Photofrin and mTHPC | Interstitial: 628±3 nm for Photofrin and 652±3 nm for mTHPC Superficial: 100 mW∙cm−2 | nd | No | RIF1 tumour-bearing mice |
| [230,231] | Continuous vs. fractional PDT | Radachlorin | 20 mW∙cm−2 Pulse mode: 200 ms – pulse duration, 700 ms - period | 1O2 | k562 cell line | No |
| [232] | Continuous vs. fractional PDT | PAD-S31 | Pulsed: 670 nm nanosecond pulsed Nd:YAG laser, peak fluence rate 1 MW∙cm−2, 30 Hz CW: 670 nm, 40 J∙cm−2, 180 mW∙cm−2 or 270 mW∙cm−2 | nd | Renca cell line | No |
| [233] | Fractional PDT | Pyridone | 655 nm, 324 µmol∙m−2∙s−1 photon flux for 10 min every 1h | 1O2 | HeLa cell line | No |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Larue, L.; Myrzakhmetov, B.; Ben-Mihoub, A.; Moussaron, A.; Thomas, N.; Arnoux, P.; Baros, F.; Vanderesse, R.; Acherar, S.; Frochot, C. Fighting Hypoxia to Improve PDT. Pharmaceuticals 2019, 12, 163. https://0-doi-org.brum.beds.ac.uk/10.3390/ph12040163
Larue L, Myrzakhmetov B, Ben-Mihoub A, Moussaron A, Thomas N, Arnoux P, Baros F, Vanderesse R, Acherar S, Frochot C. Fighting Hypoxia to Improve PDT. Pharmaceuticals. 2019; 12(4):163. https://0-doi-org.brum.beds.ac.uk/10.3390/ph12040163
Chicago/Turabian StyleLarue, Ludivine, Bauyrzhan Myrzakhmetov, Amina Ben-Mihoub, Albert Moussaron, Noémie Thomas, Philippe Arnoux, Francis Baros, Régis Vanderesse, Samir Acherar, and Céline Frochot. 2019. "Fighting Hypoxia to Improve PDT" Pharmaceuticals 12, no. 4: 163. https://0-doi-org.brum.beds.ac.uk/10.3390/ph12040163





