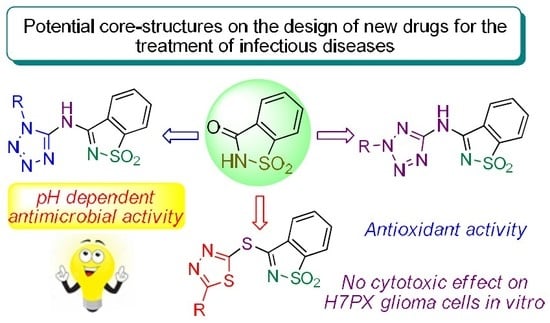
In Vitro Assessment of Antimicrobial, Antioxidant, and Cytotoxic Properties of Saccharin–Tetrazolyl and –Thiadiazolyl Derivatives: The Simple Dependence of the pH Value on Antimicrobial Activity
Abstract
:1. Introduction
2. Results and Discussion
2.1. Chemistry
2.2. Biological Assays
2.2.1. Antimicrobial Activity
2.2.2. Antioxidant Activity
2.2.3. Brine Shrimp Lethality Bioassay (General Toxicity)
2.2.4. Cell Viability after Treatment with MTSB, TS, TSMS, and 2MTS in H7PX Glioma Cells (IV Grade)
3. Experimental Section
3.1. Chemistry
3.1.1. General
3.1.2. Synthetic Protocols
3.2. Biologic Activities
3.2.1. Antioxidant Activity (DPPH Method)
3.2.2. Brine Shrimp Lethality Bioassay (General Toxicity)
3.2.3. Cell Culture
3.2.4. In Vitro Cell Viability by MTT Assay
3.2.5. Antimicrobial Activity
3.2.6. Statistical Analysis
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Schulze, B.; Illgen, K. Isothiazol-1,1-dioxide—Vom Süßstoff zum chiralen Auxiliar in der stereoselektiven Synthese. J. Prakt. Chem. 1997, 339, 1–14. [Google Scholar] [CrossRef]
- Ellis, J.W.J. Overview of sweeteners. Chem. Educ. 1995, 72, 671–675. [Google Scholar] [CrossRef]
- Remsen, I.; Fahlberg, C. On the oxidation of substitution products of aromatic hydrocarbons. IV. On the oxidation of orthotoluenesulphonamide. J. Am. Chem. Soc. 1879, 1, 426–438. [Google Scholar] [CrossRef]
- Price, M.J.; Biava, G.C.; Oser, L.B.; Vogin, E.E.; Steinfeld, J.; Ley, L.H. Bladder tumors in rats fed cyclohexylamine or high doses of a mixture of cyclamate and saccharin. Science 1970, 167, 1131–1132. [Google Scholar] [CrossRef] [PubMed]
- Masui, T.; Mann, M.A.; Borgeson, D.C.; Garland, M.E.; Okamura, T.; Fujii, H.; Pelling, C.J.; Cohen, M.S. Sequencing analysis of HA-RAS, KI-RAS, and N-RAS genes in rat urinary-bladder tumors induced by N-[4-(5-Nitro-2-furyl)-2-thiazolyl]formamide (FANFT) and sodium saccharin. Terat. Carcin. Mutagen. 1993, 13, 225–233. [Google Scholar] [CrossRef] [PubMed]
- Garland, M.E.; Sakata, T.; Fisher, M.J.; Masui, T.; Cohen, M.S. Influences of Diet and Strain on the Proliferative Effect on the Rat Urinary Bladder Induced by Sodium Saccharin. Cancer Res. 1989, 49, 3789–3794. [Google Scholar] [PubMed]
- Groutas, W.C.; Houser-Archield, N.; Chong, L.S.; Venkataraman, R.; Epp, J.B.; Huang, H.; McClenahan, J.J. Efficient inhibition of human-leukocyte elastase and cathepsin-G by saccharin derivatives. J. Med. Chem. 1993, 36, 3178–3181. [Google Scholar] [CrossRef] [PubMed]
- Groutas, W.C.; Chong, L.S.; Venkataraman, R.; Kuang, R.; Epp, J.B.; Houser-Archield, N.; Huang, H.; Hoydal, R.J. Amino acid-derivative phthalimide and saccharin derivatives as inhibitors of human leukocyte elastase, cathepsin G, and proteinase 3. Arch. Biochem. Biophys. 1996, 332, 335–340. [Google Scholar] [CrossRef] [PubMed]
- Groutas, W.C.; Epp, J.B.; Venkataraman, R.; Kuang, R.; Truong, T.M.; McClenahan, J.J.; Prakash, O. Design, synthesis, and in vitro inhibitory activity toward human leukocyte elastase, cathepsin G, and proteinase 3 of saccharin-derived sulfones and congeners. Bioorg. Med. Chem. 1996, 4, 1393–1400. [Google Scholar] [CrossRef]
- Elghamry, I.; Youssef, M.M.; Al-Omair, M.A.; Elsawy, H. Synthesis, antimicrobial, DNA cleavage and antioxidant activities of tricyclic sultams derived from saccharin. Eur. J. Med. Chem. 2017, 139, 107–113. [Google Scholar] [CrossRef] [PubMed]
- Apella, M.C.; Totaro, R.; Baran, E.J. Determination of superoxide dismutase-like activity in some divalent metal saccharinates. Biol. Trace Elem. Res. 1993, 37, 293–299. [Google Scholar] [CrossRef] [PubMed]
- Guenther, U.; Wrigge, H.; Theuerkauf, N.; Boettcher, M.F.; Wensing, G.; Zinserling, J.; Putensen, C.; Hoeft, A. Repinotan, a selective 5-HT1A-R-agonist, antagonizes morphine-induced ventilator depression in anesthetized rats. Anesth. Analg. 2010, 111, 901–907. [Google Scholar] [PubMed]
- Malinka, W.; Ryng, S.; Sieklucka-Dziuba, M.; Rajtar, G.; Gownial, A.; Kleinrok, Z. 2-Substituted-3-oxoisothiazolo[5,4-b]pyridines as potential central nervous system and antimycobacterial agents. Farmaco 1998, 53, 504–512. [Google Scholar] [CrossRef]
- Malinka, W.; Ryng, S.; Sieklucka-Dziuba, M.; Rajtar, G.; Gownial, A.; Kleinrok, Z. Synthesis and preliminary screening of derivatives of 2-(4-arylpiperazine-1-ylalkyl)-3-oxoisothiazolo[5,4,b]pyridines as CNS and antimycobacterial agents. Pharmazie 2000, 55, 416–425. [Google Scholar] [PubMed]
- Csakai, A.; Smith, C.; Davis, E.; Martinko, A.; Coulp, S.; Yin, H. Saccharin derivatives as inhibitors of interferon-mediated inflammation. J. Med. Chem. 2014, 57, 5348–5355. [Google Scholar] [CrossRef] [PubMed]
- Fischer, R.; Kretschik, O.; Schenke, T.; Schenkel, R.; Wiedemann, J.; Erdelen, C.; Loesel, P.; Drewes, M.W.; Feucht, D.; Andersch, W.W. Ger. Offen. DE 1999244668. 1999. Chem. Abstr. 2001, 134, 4932. [Google Scholar]
- Singh, H.; Chawla, A.S.; Kapoor, V.K.; Paul, D.; Malhotra, R.K. Medicinal chemistry of tetrazoles. Prog. Med. Chem. 1980, 17, 151–183. [Google Scholar] [PubMed]
- Noda, K.; Saad, Y.; Kinoshita, A.; Boyle, T.P.; Graham, R.M.; Husain, A.; Karnik, S.S. Tetrazole and carboxylate receptor antagonists bind to the same subsite by different mechanisms. J. Biol. Chem. 1995, 270, 2284–2289. [Google Scholar] [CrossRef] [PubMed]
- Mavromoustakos, T.; Kolocouris, A.; Zervou, M.; Roumelioti, P.; Matsoukas, J.; Weisemann, R. An effort to understand the molecular basis of hypertension through the study of conformational analysis of Losartan and Sarmesin using a combination of nuclear magnetic resonance spectroscopy and theoretical calculations. J. Med. Chem. 1999, 42, 1714–1722. [Google Scholar] [CrossRef] [PubMed]
- Toney, J.H.; Fitzgerald, P.M.D.; Grover-Sharma, N.; Olson, S.H.; May, W.J.; Sundelof, J.G.; Vanderwall, D.E.; Cleary, K.A.; Grant, S.K.; Wu, J.K.; et al. Antibiotic sensitization using biphenyl tetrazoles as potent inhibitors of Bacteroides fragilis metallo-β-lactamase. Chem. Biol. 1998, 5, 185–196. [Google Scholar] [CrossRef]
- Gao, C.; Chang, L.; Xu, Z.; Yan, X.-F.; Ding, C.; Zhao, F.; Wu, X.; Feng, L.-S. Recent advances of tetrazole derivatives as potential anti-tubercular and anti-malarial agents. Eur. J. Med. Chem. 2019, 163, 404. [Google Scholar] [CrossRef] [PubMed]
- Hashimoto, Y.; Ohashi, R.; Kurosawa, Y.; Minami, K.; Kaji, H.; Hayashida, K.; Narita, H.; Murata, S. Pharmacologic Profile of TA-606, a Novel Angiotensin II-Receptor Antagonist in the Rat. J. Cardiovasc. Pharmacol. 1998, 31, 568–575. [Google Scholar] [CrossRef] [PubMed]
- Desarro, A.; Ammendola, D.; Zappala, M.; Grasso, S.; Desarro, G.B. Relationship between Structure and Convulsant Properties of Some b-Lactam Antibiotics following Intracerebroventricular Microinjection in Rats. Antimicrob. Agents Chemother. 1995, 39, 232–237. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tamura, Y.; Watanabe, F.; Nakatani, T.; Yasui, K.; Fuji, M.; Komurasaki, T.; Tsuzuki, H.; Maekawa, R.; Yoshioka, T.; Kawada, K.; et al. Highly Selective and Orally Active Inhibitors of Type IV Collagenase (MMP-9 and MMP-2): N-Sulfonylamino Acid Derivatives. J. Med. Chem. 1998, 41, 640–649. [Google Scholar] [CrossRef] [PubMed]
- Abell, A.D.; Foulds, G.J. Synthesis of a cis-conformationally restricted peptide bond isostere and its application to the inhibition of the HIV-1 protease. J. Chem. Soc. Perkin Trans. 1997, 1, 2475–2482. [Google Scholar] [CrossRef]
- Sandmann, G.; Schneider, C.; Boger, P. A New Non-Radioactive Assay of Phytoene Desaturase to Evaluate Bleaching Herbicides. Z. Naturforsch. C 1996, 51, 534–538. [Google Scholar] [CrossRef] [PubMed]
- Koldobskii, G.I.; Ostrovskii, V.A.; Poplavskii, V.S. Advances in tetrazole chemistry. Khim. Geterotsikl. Soedin. 1981, 10, 1299. [Google Scholar]
- Ostrovskii, V.A.; Pevzner, M.S.; Kofman, T.P.; Shcherbinin, M.B.; Tselinskii, I.V. Targets in Heterocyclic Systems. Chemistry and Properties; Attanasi, O.A., Spinelli, D., Eds.; Societa Chimica Italiana: Rome, Italy, 1999; Volume 3, p. 467. [Google Scholar]
- Moore, D.S.; Robinson, S.D. Catenated Nitrogen Ligands Part II. Transition Metal Derivatives of Triazoles, Tetrazoles, Pentazoles, and Hexazine. Adv. Inorg. Chem. 1988, 32, 171–239. [Google Scholar]
- Balaban, A.T.; Oniciu, D.C.; Katritzky, A.R. Aromaticity as a cornerstone of heterocyclic chemistry. Chem. Rev. 2004, 104, 2777–2812. [Google Scholar] [CrossRef] [PubMed]
- Hu, Y.; Li, C.-Y.; Wang, X.-M.; Yang, Y.-H.; Zhu, H.-L. 1,3,4-Thiadiazole: Synthesis, reactions, and applications in medicinal, agricultural, and materials chemistry. Chem. Rev. 2014, 114, 5572–5610. [Google Scholar] [CrossRef] [PubMed]
- Almajan, G.L.; Barbuceanu, S.F.; Bancescu, G.; Saramet, I.; Saramet, G.; Draghici, C. Synthesis and antimicrobial evaluation of some fused heterocyclic [1,2,4]triazolo[3,4-b][1,3,4]thiadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 6139–6146. [Google Scholar] [CrossRef] [PubMed]
- Kolavi, G.; Hegde, V.; Khazi, I.; Gadad, P. Synthesis and evaluation of antitubercular activity of imidazo[2,1-b][1,3,4]thiadiazole derivatives. Bioorg. Med. Chem. 2006, 14, 3069–3080. [Google Scholar] [CrossRef] [PubMed]
- Khan, I.; Ali, S.; Hameed, S.; Rama, N.H.; Hussain, M.T.; Wadood, A.; Uddin, R.; Ul-Haq, Z.; Khan, A.; Ali, S.; et al. Synthesis, antioxidant activities and urease inhibition of some new 1,2,4-triazole and 1,3,4-thiadiazole derivatives. Eur. J. Med. Chem. 2010, 45, 5200–5207. [Google Scholar] [CrossRef] [PubMed]
- Hafez, H.N.; Hegab, M.I.; Ahmed-Farag, I.S.; El-Gazzar, A.B.A. A facile regioselective synthesis of novel spiro-thioxanthene and spiro-xanthene-9′,2-[1,3,4]thiadiazole derivatives as potential analgesic and anti-inflammatory agents. Bioorg. Med. Chem. Lett. 2008, 18, 4538–4543. [Google Scholar] [CrossRef] [PubMed]
- Jatav, V.; Mishra, P.; Kashaw, S.; Stables, J.P. CNS depressant and anticonvulsant activities of some novel 3-[5-substituted 1,3,4-thiadiazole-2-yl]-2-styryl quinazoline-4(3H)-ones. Eur. J. Med. Chem. 2008, 43, 1945–1954. [Google Scholar] [CrossRef] [PubMed]
- Clerici, F.; Pocar, D.; Guido, M.; Loche, A.; Perlini, V.; Brufani, M. Synthesis of 2-amino-5-sulfanyl-1,3,4-thiadiazole derivatives and evaluation of their antidepressant and anxiolytic activity. J. Med. Chem. 2001, 44, 931–936. [Google Scholar] [CrossRef] [PubMed]
- Hasui, T.; Matsunaga, N.; Ora, T.; Ohyabu, N.; Nishigaki, N.; Imura, Y.; Igata, Y.; Matsui, H.; Motoyaji, T.; Tanaka, T.; et al. Identification of benzoxazin-3-one derivatives as novel, potent, and selective nonsteroidal mineralocorticoid receptor antagonists. J. Med. Chem. 2011, 54, 8616–8631. [Google Scholar] [CrossRef] [PubMed]
- Noolvi, M.N.; Patel, H.M.; Singh, N.; Gadad, A.K.; Cameotra, S.S.; Badiger, A. Synthesis and anticancer evaluation of novel 2-cyclopropylimidazo[2,1-b][1,3,4]-thiadiazole derivatives. Eur. J. Med. Chem. 2011, 46, 4411–4418. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.H.; Shi, Y.X.; Ma, Y.; Zhang, C.Y.; Dong, W.L.; Pan, L.; Wang, B.L.; Li, B.J.; Li, Z.M. Synthesis, antifungal activities and 3D-QSAR study of N-(5-substituted-1,3,4-thiadiazol-2-yl)cyclopropanecarboxamides. Eur. J. Med. Chem. 2009, 44, 2782–2786. [Google Scholar] [CrossRef] [PubMed]
- Kaur, I.P.; Smitha, R.; Aggarwal, D.; Kapil, M. Acetazolamide: Future perspective in topical glaucoma therapeutics. Int. J. Pharm. 2002, 248, 1–14. [Google Scholar] [CrossRef]
- Luks, A.M.; McIntosh, S.E.; Grissom, C.K.; Auerbach, P.S.; Rodway, G.W.; Schoene, R.B.; Zafren, K.; Hackett, P.H. Wilderness Medical Society consensus guidelines for the prevention and treatment of acute altitude illness. Wilderness Environ. Med. 2010, 21, 146–155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wolf, P. Acute drug administration in epilepsy: A review. CNS Neurosci. Ther. 2011, 17, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Rangwala, L.M.; Liu, G.T. Pediatric idiopathic intracranial hypertension. Surv. Ophthalmol. 2007, 52, 597–617. [Google Scholar] [CrossRef] [PubMed]
- Russell, M.B.; Ducros, A. Sporadic and familial hemiplegic migraine: Pathophysiological mechanisms, clinical characteristics, diagnosis, and management. Lancet Neurol. 2011, 10, 457–470. [Google Scholar] [CrossRef]
- Tiselius, H.G. New horizons in the management of patients with cystinuria. Curr. Opin. Urol. 2010, 20, 169–173. [Google Scholar] [CrossRef] [PubMed]
- Jalandhara, N.B.; Patel, A.; Arora, R.R.; Jalandhara, P. Obstructive sleep apnea: A cardiopulmonary perspective and medical therapeutics. Am. J. Ther. 2009, 16, 257–263. [Google Scholar] [CrossRef] [PubMed]
- Schara, U.; Lochmuller, H. Therapeutic strategies in congenital myasthenic syndromes. Neurotherapeutics 2008, 5, 542–547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Superti-Furga, G.; Cochran, J.; Crews, C.M.; Frye, S.; Neubauer, G.; Prinjha, R.; Shokat, K. Where is the Future of Drug Discovery for Cancer? Cell 2017, 168, 564–565. [Google Scholar]
- Gulçin, I. Antioxidant activity of food constituents: An overview. Arch. Toxicol. 2012, 86, 345–391. [Google Scholar] [CrossRef] [PubMed]
- Walker, B.; Barrett, S.; Polasky, S.; Galaz, V.; Folke, C.; Engstrom, G.; Ackerman, F.; Arrow, K.; Carpenter, S.; Chopra, K.; et al. Environment. Looming global-scale failures and missing institutions. Science 2009, 325, 1345–1346. [Google Scholar] [CrossRef] [PubMed]
- D’Costa, V.M.; King, C.E.; Kalan, L.; Morar, M.; Sung, W.W.; Schwarz, C.; Froese, D.; Zazula, G.; Calmels, F.; Debruyne, R.; et al. Antibiotic resistance is ancient. Nature 2011, 477, 457. [Google Scholar] [CrossRef] [PubMed]
- Andersson, D.I.; Hughes, D. Antibiotic resistance and its cost: Is it possible to reverse resistance? Nat. Rev. Microbiol. 2010, 8, 260. [Google Scholar] [CrossRef] [PubMed]
- Cassir, N.; Rolain, J.M.; Brouqui, P. A new strategy to fight antimicrobial resistance: The revival of old antibiotics. Front. Microbiol. 2014, 5, 551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brigas, A.F.; Fonseca, C.S.C.; Johnstone, R.A.W. Preparation of 3-Chloro-1,2-Benzisothiazole 1,1-Dioxide (Pseudo-Saccharyl Chloride). J. Chem. Res. 2002, 6, 299–300. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Alegria, E.C.B.A.; Sutradhar, M.; Cristiano, M.L.S.; Ismael, A.; Kopylovich, M.N.; Pombeiro, A.J.L. Copper(II) and cobalt(II) tetrazole-saccharinate complexes as effective catalysts for oxidation of secondary alcohols. J. Mol. Catal. A Chem. 2016, 425, 283–290. [Google Scholar] [CrossRef]
- Frija, L.M.T.; Fausto, R.; Loureiro, R.M.S.S.; Cristiano, M.L.S. Synthesis and structure of novel benzisothiazole-tetrazolyl derivatives for potential application as nitrogen ligands. J. Mol. Catal. A Chem. 2009, 305, 142–146. [Google Scholar] [CrossRef]
- Ismael, A.; Paixão, J.A.; Fausto, R.; Cristiano, M.L.S. Molecular structure of nitrogen-linked methyltetrazole-saccharinates. J. Mol. Struct. 2012, 1023, 128–142. [Google Scholar] [CrossRef]
- Cabral, L.; Brás, E.; Henriques, M.; Marques, C.; Frija, L.M.T.; Barreira, L.; Paixão, J.A.; Fausto, R.; Cristiano, M.L.S. Synthesis, Structure, and Cytotoxicity of a New Sulphanyl-Bridged Thiadiazolyl-Saccharinate Conjugate: The Relevance of S⋅⋅⋅N Interaction. Chem. Eur. J. 2018, 24, 3251–3262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijo, P.; Duarte, A.; Francisco, A.P.; Semedo-Lemsaddek, T.; Simões, M.F. In vitro antimicrobial activity of royleanone derivatives against Gram-positive bacterial pathogens. Phytother. Res. 2014, 8, 76–81. [Google Scholar] [CrossRef] [PubMed]
- Malik, E.; Dennison, S.R.; Harris, F.; Phoenix, D.A. pH Dependent Antimicrobial Peptides and Proteins, Their Mechanisms of Action and Potential as Therapeutic Agents. Pharmaceuticals 2016, 9, 67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rijo, P.; Falé, P.L.; Serralheiro, M.L.; Simões, M.F.; Gomes, A.; Reis, C. Optimization of medicinal plant extraction methods and their encapsulation through extrusion technology. Measurement 2014, 58, 249–255. [Google Scholar] [CrossRef]
- Alanís-Garza, B.A.; González-González, G.M.; Salazar-Aranda, R.; Waksman de Torres, N.; Rivas-Galindo, V.M. Screening of antifungal activity of plants from the northeast of Mexico. J. Ethnopharmacol. 2007, 114, 468–471. [Google Scholar] [CrossRef] [PubMed]





| E. faecalis | S. aureus | P. aeruginosa | E. coli | S. cerevisiae | C. albicans | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sample | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| TSMT | 236.5 | 1892.1 | 118.3 | 1892.1 | 118.3 | 946.0 | 236.5 | 473.0 | 473.0 | 946.0 | 236.5 | 1892.1 |
| MTSB | 219.0 | 1752.1 | 109.5 | 1752.1 | 109.5 | 876.1 | 219.0 | 438.0 | 438.0 | 876.1 | 219.0 | 1752.1 |
| TS | 249.8 | 1998.1 | 124.9 | 1998.1 | 124.9 | 999.0 | 249.8 | 499.5 | 499.5 | 999.0 | 249.8 | 1998.1 |
| 2MTS | 236.5 | 1892.1 | 118.3 | 1892.1 | 118.3 | 946.0 | 236.5 | 473.0 | 473.0 | 946.0 | 236.5 | 1892.1 |
| Positivecontrol | 1.95 | nt | 1.95 | nt | 0.977 | nt | 0.488 | nt | 15.6 | nt | 7.81 | nt |
| VAN | VAN | NOR | NOR | NYS | NYS | |||||||
| S. aureus CIP6538 | E. faecalis ATCC51299 (VRE) | S. aureus CIP106760 (MRSA) | E. faecalis 29212 | |||||
|---|---|---|---|---|---|---|---|---|
| Sample | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| TSMT | 473.0 | 1892.1 | 236.5 | 1892.1 | 236.5 | 473.0 | 236.5 | 1892.1 |
| MTSB | 13.7 | 109.5 | 13.7 | 109.5 | 3.42 | 1.71 | 3.42 | 27.4 |
| TS | 62.5 | 499.5 | 31.2 | 249.8 | 3.90 | 1.95 | 7.80 | 62.5 |
| 2MTS | 29.6 | 236.5 | 14.8 | 118.3 | 3.70 | 1.85 | 473.0 | 1892.1 |
| Positive control | 1.95 | nt | 1.95 | nt | 0.977 | nt | 0.488 | nt |
| VAN | VAN | NOR | NOR | |||||
| S. aureus CIP6538 | E. faecalis ATCC51299 (VRE) | S. aureus CIP106760 (MRSA) | E. faecalis 29212 | |||||
|---|---|---|---|---|---|---|---|---|
| Sample | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| TSMT | 473.0 | 1892.1 | 236.5 | 1892.1 | 473.0 | 1892.1 | 946.0 | 1892.1 |
| MTSB | 438.0 | 1752.1 | 219.0 | 1752.1 | 438.0 | 1752.1 | 876.1 | 1752.1 |
| TS | 499.5 | 1998.1 | 249.8 | 1998.1 | 499.5 | 1998.1 | 999.0 | 1998.1 |
| 2MTS | 473.0 | 1892.1 | 236.5 | 1892.1 | 473.0 | 1892.1 | 946.0 | 1892.1 |
| Positive control | 1.95 | nt | 1.95 | nt | 0.977 | nt | 0.488 | nt |
| VAN | VAN | NOR | NOR | |||||
| S. aureus CIP6538 | E. faecalis ATCC51299 (VRE) | S. aureus CIP106760 (MRSA) | E. faecalis 29212 | |||||
|---|---|---|---|---|---|---|---|---|
| Sample | MIC | MBC | MIC | MBC | MIC | MBC | MIC | MBC |
| TSMT | 946.0 | 1892.1 | 473.0 | 1892.1 | 473.0 | 1892.1 | 473.0 | 1892.1 |
| MTSB | 438.0 | 1752.1 | 219.0 | 1752.1 | 438.0 | 1752.1 | 438.0 | 1752.1 |
| TS | 499.5 | 1998.1 | 499.5 | 1998.1 | 999.0 | 1998.1 | 499.5 | 1998.1 |
| 2MTS | 473.0 | 1892.1 | 473.0 | 1892.1 | 473.0 | 1892.1 | 473.0 | 1892.1 |
| Positive control | 1.95 | nt | 1.95 | nt | 0.977 | nt | 0.488 | nt |
| VAN | VAN | NOR | NOR | |||||
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frija, L.M.T.; Ntungwe, E.; Sitarek, P.; Andrade, J.M.; Toma, M.; Śliwiński, T.; Cabral, L.; S. Cristiano, M.L.; Rijo, P.; Pombeiro, A.J.L. In Vitro Assessment of Antimicrobial, Antioxidant, and Cytotoxic Properties of Saccharin–Tetrazolyl and –Thiadiazolyl Derivatives: The Simple Dependence of the pH Value on Antimicrobial Activity. Pharmaceuticals 2019, 12, 167. https://0-doi-org.brum.beds.ac.uk/10.3390/ph12040167
Frija LMT, Ntungwe E, Sitarek P, Andrade JM, Toma M, Śliwiński T, Cabral L, S. Cristiano ML, Rijo P, Pombeiro AJL. In Vitro Assessment of Antimicrobial, Antioxidant, and Cytotoxic Properties of Saccharin–Tetrazolyl and –Thiadiazolyl Derivatives: The Simple Dependence of the pH Value on Antimicrobial Activity. Pharmaceuticals. 2019; 12(4):167. https://0-doi-org.brum.beds.ac.uk/10.3390/ph12040167
Chicago/Turabian StyleFrija, Luís M. T., Epole Ntungwe, Przemysław Sitarek, Joana M. Andrade, Monika Toma, Tomasz Śliwiński, Lília Cabral, M. Lurdes S. Cristiano, Patrícia Rijo, and Armando J. L. Pombeiro. 2019. "In Vitro Assessment of Antimicrobial, Antioxidant, and Cytotoxic Properties of Saccharin–Tetrazolyl and –Thiadiazolyl Derivatives: The Simple Dependence of the pH Value on Antimicrobial Activity" Pharmaceuticals 12, no. 4: 167. https://0-doi-org.brum.beds.ac.uk/10.3390/ph12040167