Higher S100B Levels Predict Persistently Elevated Anhedonia with Escitalopram Monotherapy Versus Antidepressant Combinations: Findings from CO-MED Trial
Abstract
:1. Introduction
2. Methods
2.1. Study Overview
2.2. Medications
2.3. Assessments
2.3.1. Quick Inventory of Depressive Symptomatology Self-Report (QIDS-SR)
2.3.2. Inventory of Depressive Symptomatology Clinician Rated (IDS-C)
2.4. Measurement of S100B Levels in Plasma
2.5. Statistical Analyses
3. Results
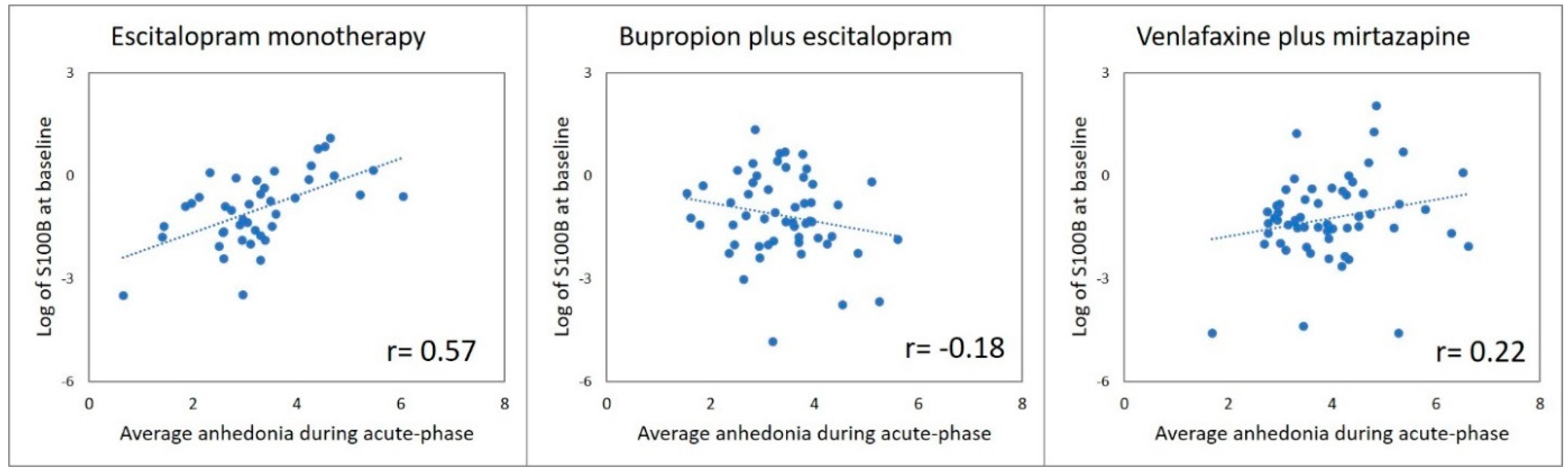
3.1. Does Baseline S100B Differentially Predict Changes in Anhedonia with Escitalopram Monotherapy versus Antidepressant Combinations?
3.2. Does Baseline S100B Differentially Predict Changes in Overall Depression Severity with Escitalopram Monotherapy versus Antidepressant Combinations?
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Slavich, G.M.; Irwin, M.R. From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychol. Bull. 2014, 140, 774–815. [Google Scholar] [CrossRef]
- Cheng, Y.; Desse, S.; Martinez, A.; Worthen, R.J.; Jope, R.S.; Beurel, E. TNFalpha disrupts blood brain barrier integrity to maintain prolonged depressive-like behavior in mice. Brain Behav. Immun. 2018, 69, 556–567. [Google Scholar] [CrossRef] [PubMed]
- Banks, W.A. The blood-brain barrier in neuroimmunology: Tales of separation and assimilation. Brain Behav. Immun. 2015, 44, 1–8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Felger, J.C.; Haroon, E.; Patel, T.A.; Goldsmith, D.R.; Wommack, E.C.; Woolwine, B.J.; Le, N.-A.; Feinberg, R.; Tansey, M.G.; Miller, A.H. What does plasma CRP tell us about peripheral and central inflammation in depression? Mol. Psychiatry 2018, 1. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Miller, A.H.; Minhajuddin, A.; Trivedi, M.H. Association of T and non-T cell cytokines with anhedonia: Role of gender differences. Psychoneuroendocrinology 2018, 95, 1–7. [Google Scholar] [CrossRef]
- Treadway, M.T.; Admon, R.; Arulpragasam, A.R.; Mehta, M.; Douglas, S.; Vitaliano, G.; Olson, D.P.; Cooper, J.A.; Pizzagalli, D.A. Association Between Interleukin-6 and Striatal Prediction-Error Signals Following Acute Stress in Healthy Female Participants. Boil. Psychiatry 2017, 82, 570–577. [Google Scholar] [CrossRef]
- Gelenberg, A.J. Practice Guideline for the Treatment of Patients with Major Depressive Disorder Third Edition. Am. J. Psychiatry 2010, 167, 1. [Google Scholar]
- Jha, M.K. Anti-Inflammatory Treatments for Major Depressive Disorder: What’s on the Horizon? J. Clin. Psychiatry 2019, 80, 1–3. [Google Scholar] [CrossRef]
- Jha, M.K.; Trivedi, M.H. Pharmacogenomics and Biomarkers of Depression. Handb. Exp. Pharmacol. 2019, 250, 101–113. [Google Scholar]
- Jha, M.K.; Trivedi, M.H. Personalized Antidepressant Selection and Pathway to Novel Treatments: Clinical Utility of Targeting Inflammation. Int. J. Mol. Sci. 2018, 19, 233. [Google Scholar] [CrossRef] [Green Version]
- Jha, M.K.; Minhajuddin, A.; Gadad, B.S.; Greer, T.; Grannemann, B.; Soyombo, A.; Mayes, T.L.; Rush, A.J.; Trivedi, M.H. Can C-reactive protein inform antidepressant medication selection in depressed outpatients? Findings from the CO-MED trial. Psychoneuroendocrinology 2017, 78, 105–113. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Minhajuddin, A.; Gadad, B.S.; Greer, T.L.; Mayes, T.L.; Trivedi, M.H. Interleukin 17 selectively predicts better outcomes with bupropion-SSRI combination: Novel T cell biomarker for antidepressant medication selection. Brain Behav. Immun. 2017, 66, 103–110. [Google Scholar] [CrossRef] [PubMed]
- Uher, R.; Tansey, K.E.; Dew, T.; Maier, W.; Mors, O.; Hauser, J.; Dernovsek, M.Z.; Henigsberg, N.; Souery, D.; Farmer, A.; et al. An Inflammatory Biomarker as a Differential Predictor of Outcome of Depression Treatment with Escitalopram and Nortriptyline. Am. J. Psychiatry 2014, 171, 1278–1286. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Trivedi, M.H.; Jha, M.K. Is C-reactive protein ready for prime time in the selection of antidepressant medications? Psychoneuroendocrinology 2017, 84, 206. [Google Scholar] [CrossRef] [PubMed]
- Jha, M.K.; Minhajuddin, A.; Gadad, B.S.; Trivedi, M.H. Platelet-Derived Growth Factor as an Antidepressant Treatment Selection Biomarker: Higher Levels Selectively Predict Better Outcomes with Bupropion-SSRI Combination. Int. J. Neuropsychopharmacol. 2017, 20, 919–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jha, M.K.; Minhajuddin, A.; Chin-Fatt, C.; Greer, T.L.; Carmody, T.J.; Trivedi, M.H. Sex differences in the association of baseline c-reactive protein (CRP) and acute-phase treatment outcomes in major depressive disorder: Findings from the EMBARC study. J. Psychiatr. Res. 2019, 113, 165–171. [Google Scholar] [CrossRef]
- Green, E.; Goldstein-Piekarski, A.N.; Schatzberg, A.F.; Rush, A.J.; Ma, J.; Williams, L. Personalizing antidepressant choice by sex, body mass index, and symptom profile: An iSPOT-D report. Pers. Med. Psychiatry 2017, 1, 65–73. [Google Scholar] [CrossRef] [Green Version]
- Jha, M.K.; Wakhlu, S.; Dronamraju, N.; Minhajuddin, A.; Greer, T.L.; Trivedi, M.H. Validating pre-treatment body mass index as moderator of antidepressant treatment outcomes: Findings from CO-MED trial. J. Affect. Disord. 2018, 234, 34–37. [Google Scholar] [CrossRef]
- Franklin, T.C.; Xu, C.; Duman, R.S. Depression and sterile inflammation: Essential role of danger associated molecular patterns. Brain Behav. Immun. 2018, 72, 2–13. [Google Scholar] [CrossRef]
- Thelin, E.P.; Johannesson, L.; Nelson, D.; Bellander, B.-M. S100B Is an Important Outcome Predictor in Traumatic Brain Injury. J. Neurotrauma 2013, 30, 519–528. [Google Scholar] [CrossRef]
- Koh, S.X.; Lee, J.K. S100B as a marker for brain damage and blood-brain barrier disruption following exercise. Sports Med. 2014, 44, 369–385. [Google Scholar] [CrossRef] [PubMed]
- Huppert, J.; Closhen, D.; Croxford, A.; White, R.; Kulig, P.; Pietrowski, E.; Bechmann, I.; Becher, B.; Luhmann, H.J.; Waisman, A.; et al. Cellular mechanisms of IL-17-induced blood-brain barrier disruption. FASEB J. 2010, 24, 1023–1034. [Google Scholar] [CrossRef] [PubMed]
- Kebir, H.; Kreymborg, K.; Ifergan, I.; Dodelet-Devillers, A.; Cayrol, R.; Bernard, M.; Giuliani, F.; Arbour, N.; Becher, B.; Prat, A. Human TH17 lymphocytes promote blood-brain barrier disruption and central nervous system inflammation. Nat. Med. 2007, 13, 1173–1175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wohleb, E.S.; McKim, D.B.; Sheridan, J.F.; Godbout, J.P. Monocyte trafficking to the brain with stress and inflammation: A novel axis of immune-to-brain communication that influences mood and behavior. Front Neurosci. 2014, 8, 447. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.H.; Haroon, E.; Felger, J.C. Therapeutic Implications of Brain-Immune Interactions: Treatment in Translation. Neuropsychopharmacology 2017, 42, 334–359. [Google Scholar] [CrossRef] [PubMed]
- Felger, J.C.; Li, Z.; Haroon, E.; Woolwine, B.J.; Jung, M.Y.; Hu, X.; Miller, A.H. Inflammation is associated with decreased functional connectivity within corticostriatal reward circuitry in depression. Mol. Psychiatry 2015, 21, 1358–1365. [Google Scholar] [CrossRef] [Green Version]
- Swardfager, W.; Rosenblat, J.D.; Benlamri, M.; McIntyre, R.S. Mapping inflammation onto mood: Inflammatory mediators of anhedonia. Neurosci. Biobehav. Rev. 2016, 64, 148–166. [Google Scholar] [CrossRef]
- Schroeter, M.L.; Abdul-Khaliq, H.; Krebs, M.; Diefenbacher, A.; Blasig, I.E. Serum markers support disease-specific glial pathology in major depression. J. Affect. Disord. 2008, 111, 271–280. [Google Scholar] [CrossRef]
- Polyakova, M.; Sander, C.; Arelin, K.; Lampe, L.; Luck, T.; Luppa, M.; Kratzsch, J.; Hoffmann, K.-T.; Riedel-Heller, S.; Villringer, A.; et al. First evidence for glial pathology in late life minor depression: S100B is increased in males with minor depression. Front. Cell. Neurosci. 2015, 9, 523. [Google Scholar] [CrossRef]
- Pearlman, D.M.; Brown, J.R.; MacKenzie, T.A.; Hernandez, F.; Najjar, S. Blood Levels of S-100 Calcium-Binding Protein B, High-Sensitivity C-Reactive Protein, and Interleukin-6 for Changes in Depressive Symptom Severity after Coronary Artery Bypass Grafting: Prospective Cohort Nested within a Randomized, Controlled Trial. PLoS ONE 2014, 9, e111110. [Google Scholar] [CrossRef]
- Ambrée, O.; Bergink, V.; Grosse, L.; Alferink, J.; Drexhage, H.A.; Rothermundt, M.; Arolt, V.; Birkenhäger, T.K. S100B Serum Levels Predict Treatment Response in Patients with Melancholic Depression. Int. J. Neuropsychopharmacol. 2015, 19, 103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jang, B.-S.; Kim, H.; Lim, S.-W.; Jang, K.-W.; Kim, D.-K. Serum S100B Levels and Major Depressive Disorder: Its Characteristics and Role in Antidepressant Response. Psychiatry Investig. 2008, 5, 193–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rush, A.J.; Trivedi, M.H.; Stewart, J.W.; Nierenberg, A.A.; Fava, M.; Kurian, B.T.; Warden, D.; Morris, D.W.; Luther, J.F.; Husain, M.M.; et al. Combining medications to enhance depression outcomes (CO-MED): Acute and long-term outcomes of a single-blind randomized study. Am. J. Psychiatry 2011, 168, 689–701. [Google Scholar] [CrossRef] [PubMed]
- Trivedi, M.H.; Rush, A.; Wisniewski, S.R.; Nierenberg, A.A.; Warden, D.; Ritz, L.; Norquist, G.; Howland, R.H.; Lebowitz, B.; McGrath, P.J.; et al. Evaluation of Outcomes With Citalopram for Depression Using Measurement-Based Care in STAR*D: Implications for Clinical Practice. Am. J. Psychiatry 2006, 163, 28–40. [Google Scholar] [CrossRef] [PubMed]
- Rush, A.; Trivedi, M.H.; Ibrahim, H.M.; Carmody, T.J.; Arnow, B.; Klein, D.N.; Markowitz, J.C.; Ninan, P.T.; Kornstein, S.; Manber, R.; et al. The 16-Item quick inventory of depressive symptomatology (QIDS), clinician rating (QIDS-C), and self-report (QIDS-SR): A psychometric evaluation in patients with chronic major depression. Boil. Psychiatry 2003, 54, 573–583. [Google Scholar] [CrossRef]
- Rush, A.J.; Bernstein, I.H.; Trivedi, M.H.; Carmody, T.J.; Wisniewski, S.; Mundt, J.C.; Shores-Wilson, K.; Biggs, M.M.; Woo, A.; Nierenberg, A.A.; et al. An Evaluation of the Quick Inventory of Depressive Symptomatology and the Hamilton Rating Scale for Depression: A Sequenced Treatment Alternatives to Relieve Depression Trial Report. Biol. Psychiatry 2006, 59, 493–501. [Google Scholar] [CrossRef] [Green Version]
- Trivedi, M.H.; Rush, A.J.; Ibrahim, H.M.; Carmody, T.J.; Biggs, M.M.; Suppes, T.; Crismon, M.L.; Shores-Wilson, K.; Toprac, M.G.; Dennehy, E.B.; et al. The Inventory of Depressive Symptomatology, Clinician Rating (IDS-C) and Self-Report (IDS-SR), and the Quick Inventory of Depressive Symptomatology, Clinician Rating (QIDS-C) and Self-Report (QIDS-SR) in public sector patients with mood disorders: A psychometric evaluation. Psychol. Med. 2004, 34, 73–82. [Google Scholar]
- Rush, A.J.; Gullion, C.M.; Basco, M.R.; Jarrett, R.B.; Trivedi, M.H. The Inventory of Depressive Symptomatology (IDS): Psychometric properties. Psychol. Med. 1996, 26, 477–486. [Google Scholar] [CrossRef]
- Snaith, R.P.; Hamilton, M.; Morley, S.; Humayan, A.; Hargreaves, D.; Trigwell, P. A Scale for the Assessment of Hedonic Tone the Snaith–Hamilton Pleasure Scale. Br. J. Psychiatry 1995, 167, 99–103. [Google Scholar] [CrossRef]
- Salvadore, G.; Nash, A.; Bleys, C.; Hsu, B.; Saad, Z.; Gause, A.; Moyer, J.; Xi, L.; Manji, H.; Van Nueten, L.; et al. A double-blind, placebo-controlled, multicenter study of sirukumab as adjunctive treatment to monoaminergic antidepressants in adults with major depressive disorder. In Proceedings of the Selected Abstracts from the American College of Neuropsychopharmacology (ACNP) 57th Annual Meeting, Hollywood, FL, USA, 9–13 December 2018. [Google Scholar]
- Trivedi, M.H.; Chin Fatt, C.; Jha, M.K.; Cooper, C.M.; Trombello, J.M.; Mason, B.L.; Hughes, J.; Gadad, B.; Czysz, A.H.; Toll, R.T.; et al. Comprehensive phenotyping of depression disease trajectory and risk: Rationale and design of Texas Resilience Against Depression (T-RAD). J. Psychiatr. Res. 2019, in press. [Google Scholar] [CrossRef]
- Trivedi, M.H.; South, C.; Jha, M.K.; Rush, A.J.; Cao, J.; Kurian, B.; Phillips, M.; Pizzagalli, D.A.; Trombello, J.M.; Oquendo, M.A.; et al. A Novel Strategy to Identify Placebo Responders: Prediction Index of Clinical and Biological Markers in the EMBARC Trial. Psychother. Psychosom. 2018, 87, 285–295. [Google Scholar] [CrossRef] [PubMed]
| Total | Escitalopram Monotherapy | Bupropion Plus Escitalopram | Venlafaxine Plus Mirtazapine | p-Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Number | 153 | 44 | 53 | 56 | ||||||
| Categorical variables | N | % | N | % | N | % | N | % | χ2 (df) | |
| Sex | 0.03 (2) | 0.98 | ||||||||
| Male | 45 | 29.4 | 13 | 29.6 | 16 | 30.2 | 16 | 28.6 | ||
| Female | 108 | 70.6 | 31 | 70.4 | 37 | 69.8 | 40 | 71.4 | ||
| Race | 4.00 (4) | 0.41 | ||||||||
| White | 100 | 65.4 | 24 | 54.6 | 37 | 69.8 | 39 | 69.6 | ||
| Black | 40 | 26.1 | 14 | 31.8 | 12 | 22.6 | 14 | 25.0 | ||
| Other | 13 | 8.5 | 6 | 13.6 | 4 | 7.6 | 3 | 5.4 | ||
| Hispanic ethnicity | 1.53 (2) | 0.46 | ||||||||
| No | 128 | 83.7 | 36 | 81.8 | 47 | 88.7 | 45 | 80.4 | ||
| Yes | 25 | 16.3 | 8 | 18.2 | 6 | 11.3 | 11 | 19.6 | ||
| Education | 4.23 (4) | 0.38 | ||||||||
| <12 years | 24 | 15.7 | 4 | 9.1 | 11 | 20.8 | 9 | 16.1 | ||
| 12–15 years | 91 | 59.5 | 31 | 70.4 | 27 | 50.9 | 33 | 58.9 | ||
| >15 years | 38 | 24.8 | 9 | 20.5 | 15 | 28.3 | 14 | 25.0 | ||
| Anxious features | 114 | 74.5 | 30 | 68.2 | 42 | 79.3 | 42 | 75.0 | 1.56 (2) | 0.49 |
| Onset of depression before age 18 | 64 | 41.8 | 17 | 38.6 | 23 | 43.4 | 24 | 42.9 | 0.26 (2) | 0.88 |
| Continuous variables | Mean | SD | Mean | SD | Mean | SD | Mean | SD | F value (df) | p value |
| Age in years | 43.8 | 11.8 | 46.8 | 11.4 | 45.2 | 12.0 | 40.2 | 11.2 | 4.64 (2, 150) | 0.01 |
| QIDS-SR | 15.7 | 4.0 | 16.1 | 3.0 | 15.1 | 4.8 | 16.1 | 4.0 | 1.03 (2, 150) | 0.36 |
| IDS anhedonia | 5.4 | 2.0 | 5.3 | 2.0 | 5.3 | 2.1 | 5.7 | 1.9 | 0.76 (2, 150) | 0.47 |
| Body mass index | 32.0 | 9.3 | 33.5 | 11.5 | 31.5 | 7.9 | 31.2 | 8.5 | 0.88 (2, 150) | 0.42 |
| Log of S100B | −1.1 | 1.3 | −0.85 | 1.1 | −1.1 | 1.4 | −1.19 | 1.31 | 0.88 (2, 150) | 0.42 |
| Anhedonia Severity | Overall Depression Severity | |||||
|---|---|---|---|---|---|---|
| F value | df | p | F value | df | p | |
| Age | 0.32 | 1, 142 | 0.57 | 0.49 | 1, 142 | 0.49 |
| Gender | 9.71 | 1, 142 | 0.002 | 2.91 | 1, 142 | 0.09 |
| Body Mass Index | 0.75 | 1, 142 | 0.39 | 0.05 | 1, 142 | 0.82 |
| Baseline Log S100B | 2.06 | 1, 142 | 0.15 | 0.55 | 1, 142 | 0.46 |
| Time | 68.18 | 7, 790 | <0.0001 | 103.58 | 7, 787 | <0.0001 |
| Group | 3.08 | 2, 142 | 0.049 | 1.68 | 2, 142 | 0.19 |
| Time-by-treatment arm interaction | 0.69 | 14, 790 | 0.78 | 0.44 | 14, 787 | 0.96 |
| Log S100B-by-treatment arm interaction | 3.21 | 2, 142 | 0.043 | 1.99 | 2, 142 | 0.14 |
© 2019 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jha, M.K.; Minhajuddin, A.; Gadad, B.S.; Chin Fatt, C.; Trivedi, M.H. Higher S100B Levels Predict Persistently Elevated Anhedonia with Escitalopram Monotherapy Versus Antidepressant Combinations: Findings from CO-MED Trial. Pharmaceuticals 2019, 12, 184. https://0-doi-org.brum.beds.ac.uk/10.3390/ph12040184
Jha MK, Minhajuddin A, Gadad BS, Chin Fatt C, Trivedi MH. Higher S100B Levels Predict Persistently Elevated Anhedonia with Escitalopram Monotherapy Versus Antidepressant Combinations: Findings from CO-MED Trial. Pharmaceuticals. 2019; 12(4):184. https://0-doi-org.brum.beds.ac.uk/10.3390/ph12040184
Chicago/Turabian StyleJha, Manish K., Abu Minhajuddin, Bharathi S. Gadad, Cherise Chin Fatt, and Madhukar H. Trivedi. 2019. "Higher S100B Levels Predict Persistently Elevated Anhedonia with Escitalopram Monotherapy Versus Antidepressant Combinations: Findings from CO-MED Trial" Pharmaceuticals 12, no. 4: 184. https://0-doi-org.brum.beds.ac.uk/10.3390/ph12040184




