Secretory Phospholipase A2-IIA Protein and mRNA Pools in Extracellular Vesicles of Bronchoalveolar Lavage Fluid from Patients with Early Acute Respiratory Distress Syndrome: A New Perception in the Dissemination of Inflammation?
Abstract
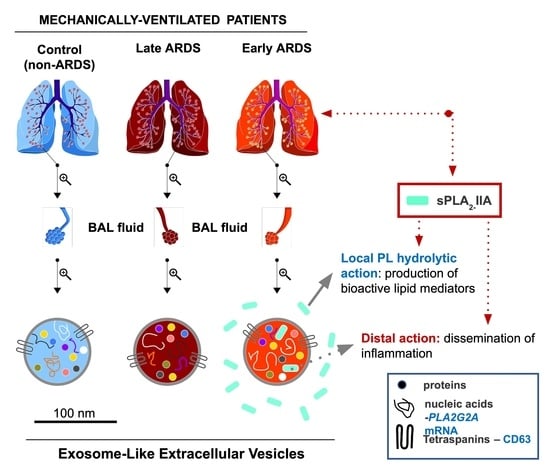
:1. Introduction
2. Results
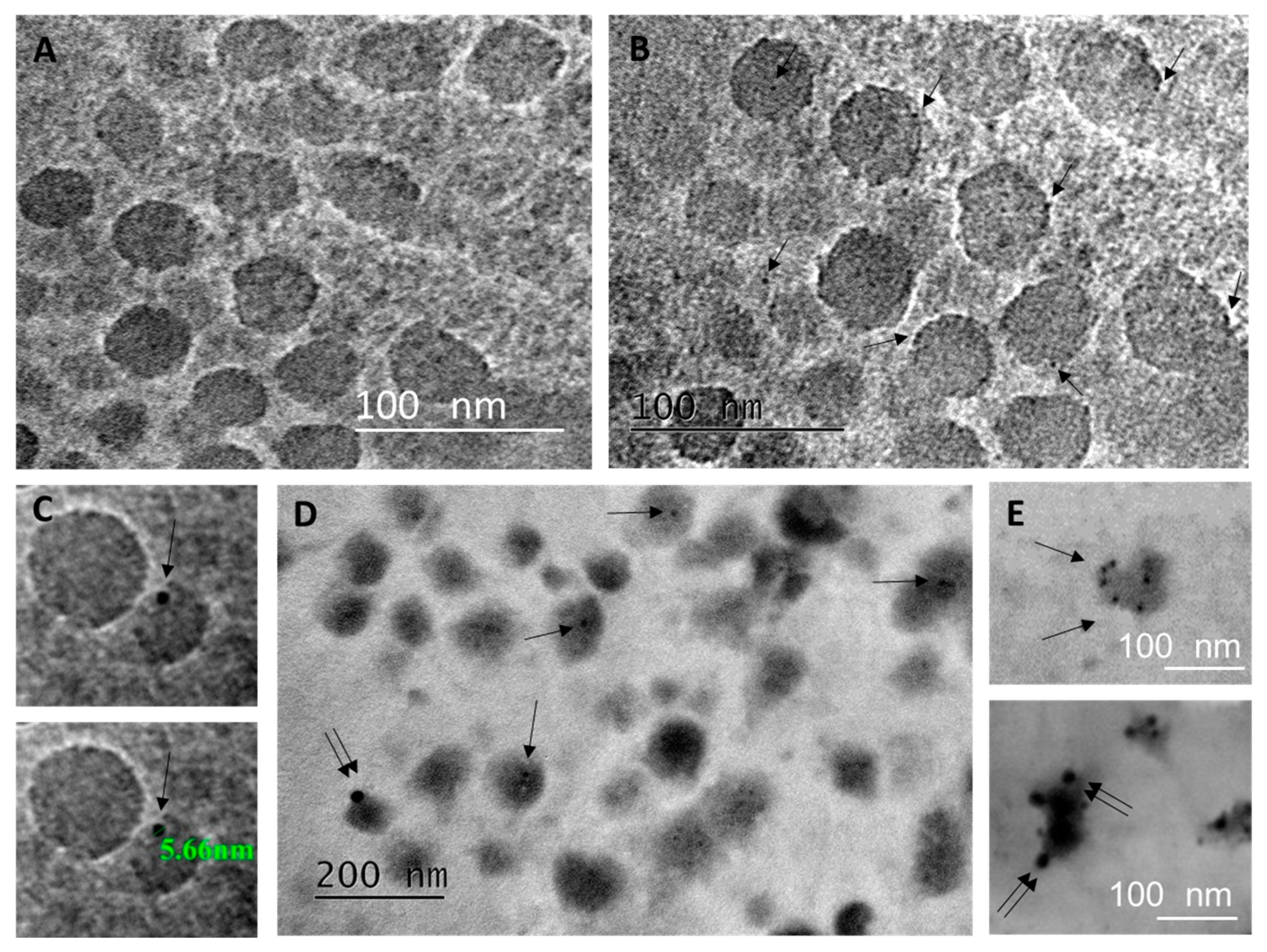
2.1. Characterization of BAL Fluid Derived EVs
2.2. sPLA2-IIA in BAL Fluid-Derived EVs
2.3. sPLA2-IIA mRNA Content in BAL Fluid-Derived EVs Is Increased in Early ARDS Patients
3. Discussion
4. Materials and Methods
4.1. Patients
4.2. Bronchoalveolar Lavage
4.3. Isolation and Purification of EVs
4.4. Dynamic Light Scattering
4.5. Total RNA Isolation and qRT-PCR
4.6. Western Blotting
4.7. Confocal Microscopy
4.8. Transmission Electron Microscopy
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Balsinde, J.; Balboa, M.A.; Insel, P.A.; Dennis, E.A. Regulation and Inhibition of Phospholipase A2. Annu. Rev. Pharmacol. Toxicol. 1999, 39, 175–189. [Google Scholar] [PubMed]
- Gimenez, A.P.; Wu, Y.Z.; Paya, M.; Delclaux, C.; Touqui, L.; Goossens, P.L. High Bactericidal Efficiency of Type IIA Phospholipase A2 against Bacillus anthracis and Inhibition of its Secretion by the Lethal Toxin. J. Immunol. 2004, 173, 521–530. [Google Scholar] [PubMed]
- Prescott, S.M.; Zimmerman, G.A.; Stafforini, D.M.; McIntyre, T.M. Platelet-Activating Factor and Related Lipid Mediators. Annu. Rev. Biochem. 2000, 69, 419–445. [Google Scholar] [PubMed]
- Murakami, M.; Shimbara, S.; Kambe, T.; Kuwata, H.; Winstead, M.V.; Tischfield, J.A.; Kudo, I. The functions of five distinct mammalian phospholipase A2s in regulating arachidonic acid release. Type IIa and type V secretory phospholipase A2 are functionally redundant and act in concert with cytosolic phospholipase A2. J. Biol. Chem. 1998, 273, 4411–14423. [Google Scholar]
- Dabral, D.; Coorssen, J.R. Phospholipase A(2:) Potential roles in native membrane fusion. Int. J. Biochem. Cell Biol. 2017, 85, 1–5. [Google Scholar]
- Brown, W.J.; Chambers, K.; Doody, A. Phospholipase A2 (PLA2) enzymes in membrane trafficking: Mediators of membrane shape and function. Traffic 2003, 4, 214–221. [Google Scholar]
- Moses, G.S.; Jensen, M.D.; Lue, L.F.; Walker, D.G.; Sun, A.Y.; Simonyi, A.; Sun, G.Y. Secretory PLA2-IIA: A new inflammatory factor for Alzheimer’s disease. J. Neuroinflamm. 2006, 3, 28. [Google Scholar]
- Crowl, R.M.; Stoller, T.J.; Conroy, R.R.; Stoner, C.R. Induction of phospholipase A2 gene expression in human hepatoma cells by mediators of the acute phase response. J. Biol. Chem. 1991, 266, 2647–2651. [Google Scholar]
- Rosenthal, M.D.; Gordon, M.N.; Buescher, E.S.; Slusser, J.H.; Harris, L.K.; Franson, R.C. Human neutrophils store type II 14-kDa phospholipase A2 in granules and secrete active enzyme in response to soluble stimuli. Biochem. Biophys. Res. Commun. 1995, 208, 650–656. [Google Scholar]
- Hatzidaki, E.; Nakos, G.; Galiatsou, E.; Lekka, M.E. Impaired phospholipases A2 production by stimulated macrophages from patients with acute respiratory distress syndrome. Biochim. Biophys. Acta 2010, 1802, 986–994. [Google Scholar]
- Birts, C.N.; Barton, C.H.; Wilton, D.C. Catalytic and non-catalytic functions of human IIA phospholipase A2. Trends Biochem. Sci. 2010, 35, 28–35. [Google Scholar] [PubMed]
- Takada, Y.; Fujita, M. Secreted Phospholipase A2 Type IIA (sPLA2-IIA) activates integrins in an allosteric manner. Adv. Exp. Med. Biol. 2017, 925, 103–115. [Google Scholar] [PubMed]
- Kitsiouli, E.; Nakos, G.; Lekka, M.E. Phospholipase A2 subclasses in Acute Respiratory Distress Syndrome. Biochim. Biophys. Acta 2009, 1792, 941–953. [Google Scholar] [PubMed] [Green Version]
- Kitsiouli, E.I.; Nakos, G.; Lekka, M.E. Differential determination of phospholipase A(2) and PAF-acetylhydrolase in biological fluids using fluorescent substrates. J. Lipid Res. 1999, 40, 2346–2356. [Google Scholar] [PubMed]
- Nakos, G.; Kitsiouli, E.; Hatzidaki, E.; Koulouras, V.; Touqui, L.; Lekka, M.E. Phospholipases A2 and platelet-activating-factor acetylhydrolase in patients with acute respiratory distress syndrome. Crit. Care Med. 2005, 33, 772–779. [Google Scholar]
- Tsangaris, I.; Lekka, M.E.; Kitsiouli, E.I.; Constantopoulos, S.; Nakos, G. Bronchoalveolar lavage alterations during prolonged ventilation of patients without acute lung injury. Eur. Respir. J. 2003, 21, 495–501. [Google Scholar]
- Blondonnet, R.; Constantin, J.M.; Sapin, V.; Jabaudon, M. A Pathophysiologic approach to biomarkers in Acute Respiratory Distress Syndrome. Dis. Markers 2016, 2016, 3501373. [Google Scholar]
- Kollef, M.H.; Schuster, D.P. The acute respiratory distress syndrome. N. Engl. J. Med. 1995, 332, 27–37. [Google Scholar]
- Nakos, G.; Kitsiouli, E.; Tsangaris, I.; Lekka, M.E. Bronchoalveolar lavage fluid characteristics of early intermediate and late phases of ARDS. Alterations in leukocytes, proteins, PAF and surfactant components. Intensive Care Med. 1998, 24, 296–303. [Google Scholar]
- Vincent, J.L.; Sakr, Y.; Groeneveld, J.; Zandstra, D.F.; Hoste, E.; Malledant, Y.; Lei, K.; Sprung, C.L. ARDS of early or late onset: Does it make a difference? Chest 2010, 137, 81–87. [Google Scholar]
- Ware, L.B.; Matthay, M.A. The acute respiratory distress syndrome. N. Engl. J. Med. 2000, 342, 1334–1349. [Google Scholar] [PubMed]
- Spadaro, S.; Park, M.; Turrini, C.; Park, M.; Turrini, C.; Tunstall, T.; Thwaites, R.; Mauri, T.; Ragazzi, R.; Ruggeri, P.; et al. Biomarkers for Acute Respiratory Distress syndrome and prospects for personalised medicine. J. Inflamm. (Lond.) 2019, 16, 1. [Google Scholar]
- Théry, C.; Clayton, A.; Amigorena, S.; Raposo, G. Isolation and characterization of exosomes from cell culture supernatants and biological fluids. Curr. Protoc. Cell Biol. 2006, 30, 3–22. [Google Scholar]
- Tan, L.; Wu, H.; Liu, Y.; Zhao, M.; Li, D.; Lu, Q. Recent advances of exosomes in immune modulation and autoimmune diseases. Autoimmunity 2016, 3, 1–9. [Google Scholar]
- Caby, M.P.; Lankar, D.; Vincendeau-Scherrer, C.; Raposo, G.; Bonnerot, C. Exosomal-like vesicles are present in human blood plasma. Int. Immunol. 2005, 17, 879–887. [Google Scholar] [PubMed] [Green Version]
- Kostopanagiotou, G.; Routsi, C.; Smyrniotis, V.; Lekka, M.E.; Kitsiouli, E.; Arkadopoulos, N.; Nakos, G. Alterations in bronchoalveolar lavage fluid during fulminant hepatic failure. Hepatology 2003, 37, 1130–1138. [Google Scholar] [PubMed]
- Kostopanagiotou, G.G.; Kalimeris, K.A.; Arkadopoulos, N.P.; Pafiti, A.; Panagopoulos, D.; Smyrniotis, V.; Vlahakos, D.; Routsi, C.; Lekka, M.E.; Nakos, G. Desferrioxamine attenuates minor lung injury following surgical acute liver failure. Eur. Respir. J. 2009, 33, 1429–1436. [Google Scholar]
- Nakos, G.; Tsangaris, H.; Liokatis, S.; Kitsiouli, E.; Lekka, M.E. Ventilator-associated pneumonia and atelectasis: Evaluation through Bronchoalveolar Lavage Fluid analysis. Intensive Care Med. 2003, 29, 555–563. [Google Scholar]
- Bone, R.C. Immunologic dissonance: A continuing evolution in our understanding of the systemic inflammatory response syndrome (SIRS) and the multiple organ dysfunction syndrome (MODS). Ann. Intern. Med. 1996, 125, 680–687. [Google Scholar]
- Kostopanagiotou, G.; Avgerinos, E.D.; Costopanagiotou, C.; Arkadopoulos, N.; Andreadou, I.; Diamantopoulou, K.; Lekka, M.E.; Smyrniotis, V.; Nakos, G. Acute lung injury in a rat model of intestinal ischemia-reperfusion: The potential time depended role of phospholipases A(2). J. Surg. Res. 2008, 147, 108–116. [Google Scholar]
- Buzas, E.I.; György, B.; Nagy, G.; Falus, A.; Gay, S. Emerging role of extracellular vesicles in inflammatory diseases. Nat. Rev. Rheumatol. 2014, 10, 356–364. [Google Scholar] [PubMed]
- Wahlund, C.J.E.; Eklund, A.; Grunewald, J.; Gabrielsson, S. Pulmonary extracellular vesicles as mediators of local and systemic inflammation. Front. Cell Dev. Biol. 2017, 5, 39. [Google Scholar] [PubMed] [Green Version]
- Gregson, A.; Hoji, A.; Injean, P.; Poynter, S.T.; Briones, C.; Palchevskiy, V.; Weigt, S.S.; Shino, M.Y.; Derhovanessian, A.; Sayah, D.M.; et al. Altered exosomal RNA profiles in bronchoalveolar lavage from lung transplants with acute rejection. Am. J. Respir. Crit. Care Med. 2015, 192, 1490–1503. [Google Scholar] [PubMed]
- Gunasekaran, M.; Xu, Z.; Nayak, D.K.; Sharma, M.; Hachem, R.; Walia, R.; Bremner, R.M.; Smith, M.A.; Mohanakumar, T. Donor-Derived exosomes with lung self-antigens in human lung allograft rejection. Am. J. Transplant. 2017, 17, 474–484. [Google Scholar] [PubMed] [Green Version]
- Van Golde, L.M.; Batenburg, J.J.; Robertson, B. The pulmonary surfactant system: Biochemical aspects and functional significance. Physiol. Rev. 1988, 68, 374–455. [Google Scholar]
- Díaz-Flores, L.; Gutiérrez, R.; Alvarez-Argüelles, H.; Díaz-Flores, L., Jr.; González, R.; Martín-Vasallo, P.; Carrasco, J.L. Extracellular multivesicular bodies in tissues affected by inflammation/repair and tumors. Ultrastruct. Pathol. 2018, 42, 448–457. [Google Scholar]
- McVey, M.; Tabuchi, A.; Kuebler, W.M. Microparticles and acute lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 2018, 303, L364–L381. [Google Scholar]
- Sun, X.; Singleton, P.A.; Letsiou, E.; Zhao, J.; Belvitch, P.; Sammani, S.; Chiang, E.T.; Moreno-Vinasco, L.; Wade, M.S.; Zhou, T.; et al. Sphingosine-1-phosphate receptor-3 is a novel biomarker in acute lung injury. Am. J. Respir. Cell. Mol. Biol. 2012, 47, 628–636. [Google Scholar]
- Letsiou, E.; Sammani, S.; Zhang, W.; Zhou, T.; Quijada, H.; Moreno-Vinasco, L.; Dudek, S.M.; Garcia, J.G. Pathologic mechanical stress and endotoxin exposure increases lung endothelial microparticle shedding. Am. J. Respir. Cell. Mol. Biol. 2015, 52, 193–204. [Google Scholar]
- Bhatnagar, S.; Shinagawa, K.; Castellino, F.J.; Schorey, J.S. Exosomes released from macrophages infected with intracellular pathogens stimulate a proinflammatory response in vitro and in vivo. Blood 2007, 110, 3234–3244. [Google Scholar]
- Zhu, M.; Li, Y.; Shi, J.; Feng, W.; Nie, G.; Zhao, Y. Exosomes as extrapulmonary signaling conveyors for nanoparticle-induced systemic immune activation. Small 2012, 8, 404–412. [Google Scholar] [PubMed]
- Admyre, C.; Grunewald, J.; Thyberg, J.; Gripenbäck, S.; Tornling, G.; Eklund, A.; Scheynius, A.; Gabrielsson, S. Exosomes with major histocompatibility complex class II and co-stimulatory molecules are present in human BAL fluid. Eur. Respir. J. 2003, 22, 578–583. [Google Scholar] [PubMed]
- Qazi, K.R.; Paredes, P.T.; Dahlberg, B.; Grunewald, J.; Eklund, A.; Gabrielsson, S. Proinflammatory exosomes in bronchoalveolar lavage fluid of patients with sarcoidosis. Thorax 2010, 65, 1016–1024. [Google Scholar] [PubMed] [Green Version]
- Levänen, B.; Bhakta, N.R.; Torregrosa Paredes, P.; Barbeau, R.; Hiltbrunner, S.; Pollack, J.L.; Sköld, C.M.; Svartengren, M.; Grunewald, J.; Gabrielsson, S.; et al. Altered microRNA profiles in bronchoalveolar lavage fluid exosomes in asthmatic patients. J. Allergy Clin. Immunol. 2013, 131, 894–903. [Google Scholar] [PubMed] [Green Version]
- Torregrosa Paredes, P.; Esser, J.; Admyre, C.; Nord, M.; Rahman, Q.K.; Lukic, A.; Rådmark, O.; Grönneberg, R.; Grunewald, J.; Eklund, A.; et al. Bronchoalveolar lavage fluid exosomes contribute to cytokine and leukotriene production in allergic asthma. Allergy 2012, 67, 911–919. [Google Scholar] [PubMed]
- Moon, H.G.; Cao, Y.; Yang, J.; Lee, J.H.; Choi, H.S.; Jin, Y. Lung epithelial cell-derived extracellular vesicles activate macrophage-mediated inflammatory responses via ROCK1 pathway. Cell Death Dis. 2015, 6, e2016. [Google Scholar]
- Arbibe, L.; Vial, D.; Rosinski-Chupin, I.; Havet, N.; Huerre, M.; Vargaftig, B.B.; Touqui, L. Endotoxin induces expression of type II phospholipase A2 in macrophages during acute lung injury in guinea pigs: Involvement of TNF-alpha in lipopolysaccharide-induced type II phospholipase A2 synthesis. J. Immunol. 1997, 159, 391–400. [Google Scholar]
- Spyridakis, S.; Leondaritis, G.; Nakos, G.; Lekka, M.E.; Galanopoulou, D. A specific phospholipase C activity regulates phosphatidylinositol levels in lung surfactant of patients with acute respiratory distress syndrome. Am. J. Respir. Cell Mol. Biol. 2010, 42, 357–362. [Google Scholar]
- Anderson, B.O.; Moore, E.E.; Banerjee, A. Phospholipase A2 regulates critical inflammatory mediators of multiple organ failure. J. Surg. Res. 1994, 56, 199–205. [Google Scholar]
- Putman, E.; Creuwels, L.A.; van Golde, L.M.; Haagsman, H.P. Surface properties, morphology and protein composition of pulmonary surfactant subtypes. Biochem. J. 1996, 320 Pt 2, 599–605. [Google Scholar]
- Subra, C.; Grand, D.; Laulagnier, K.; Stella, A.; Lambeau, G.; Paillasse, M.; De Medina, P.; Monsarrat, B.; Perret, B.; Silvente-Poirot, S.; et al. Exosomes account for vesicle-mediated transcellular transport of activatable phospholipases and prostaglandins. J. Lipid Res. 2010, 51, 2105–2120. [Google Scholar] [PubMed] [Green Version]
- ARDS Definition Task Force; Ranieri, V.M.; Rubenfeld, G.D.; Thompson, B.T.; Ferguson, N.D.; Caldwell, E.; Fan, E.; Camporota, L.; Slutsky, A.S. Acute Respiratory Distress Syndrome: The Berlin Definition. JAMA 2012, 307, 2526–2533. [Google Scholar]




| Sample Fractions | Density (g/mL) | Mean Diameter (nm) * | Z-ave (nm) † | PdI ‡ |
|---|---|---|---|---|
| 1 | 1.09 | 339.50 ± 24.52 | 379.90 ± 17.24 | 0.253 ± 0.154 |
| 2 | 1.11 | 55.70 ± 7.14 | 361.60 ± 23.41 | 0.582 ± 0.147 |
| 3 | 1.13 | 26.65 ± 2.30 | 249.80 ± 3.81 | 0.573 ± 0.120 |
| 4 | 1.14 | 31.38 ± 3.95 | 313.20 ± 16.60 | 0.484 ± 0.070 |
| 5 | 1.15 | 46.37 ± 9.02 | 299.55 ± 3.46 | 0.663 ± 0.240 |
| 6 | 1.17 | 51.51 ± 1.25 | 269.83 ± 39.26 | 0.504 ± 0.263 |
| 7 | 1.20 | 162.30 ± 12.30 | 233.56 ± 28.24 | 0.513 ± 0.134 |
| 8 | 1.22 | 103.52 ± 5.64 | 195.65 ± 12.65 | 0.605 ± 0.036 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Papadopoulos, S.; Kazepidou, E.; Antonelou, M.H.; Leondaritis, G.; Tsapinou, A.; Koulouras, V.P.; Avgeropoulos, A.; Nakos, G.; Lekka, M.E. Secretory Phospholipase A2-IIA Protein and mRNA Pools in Extracellular Vesicles of Bronchoalveolar Lavage Fluid from Patients with Early Acute Respiratory Distress Syndrome: A New Perception in the Dissemination of Inflammation? Pharmaceuticals 2020, 13, 415. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13110415
Papadopoulos S, Kazepidou E, Antonelou MH, Leondaritis G, Tsapinou A, Koulouras VP, Avgeropoulos A, Nakos G, Lekka ME. Secretory Phospholipase A2-IIA Protein and mRNA Pools in Extracellular Vesicles of Bronchoalveolar Lavage Fluid from Patients with Early Acute Respiratory Distress Syndrome: A New Perception in the Dissemination of Inflammation? Pharmaceuticals. 2020; 13(11):415. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13110415
Chicago/Turabian StylePapadopoulos, Stylianos, Eleftheria Kazepidou, Marianna H. Antonelou, George Leondaritis, Alexia Tsapinou, Vasilios P. Koulouras, Apostolos Avgeropoulos, George Nakos, and Marilena E. Lekka. 2020. "Secretory Phospholipase A2-IIA Protein and mRNA Pools in Extracellular Vesicles of Bronchoalveolar Lavage Fluid from Patients with Early Acute Respiratory Distress Syndrome: A New Perception in the Dissemination of Inflammation?" Pharmaceuticals 13, no. 11: 415. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13110415