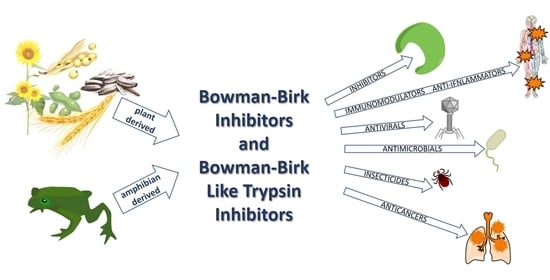
Bowman-Birk Inhibitors: Insights into Family of Multifunctional Proteins and Peptides with Potential Therapeutical Applications
Abstract
:1. Introduction
2. Bowman-Birk Inhibitors (BBIs)
3. Canonical Inhibitors (Standard Mechanism Inhibitors)
4. BBIs Hallmarks
4.1. Mono- and Double-Headed Structure
4.2. Self-Association of BBIs
4.3. Presence of Isoforms
4.4. Extreme Stability
5. Biological Properties
5.1. Anticarcinogenic Activity
Putative Mechanisms of Anticarcinogenic Activity
5.2. Anti-Inflammatory and Immunomodulatory Properties
5.2.1. Inflammatory Disorders of Gastrointestinal (GI) Tract
5.2.2. Experimental Autoimmune Encephalomyelitis
5.2.3. Experimental Autoimmune Neuritis
5.2.4. Alzheimer’s Disease
5.2.5. Anti-Inflammatory Properties of Animal-Derived Bowman-Birk Like Inhibitors
5.2.6. Putative Mechanisms of Anti-Inflammatory Activity
5.3. Antimicrobial Activity
5.3.1. Antiviral Activity
5.3.2. Antifungal Activity
5.3.3. Antibacterial Activity
5.4. Insecticidal Activity
5.5. Other Putative Functions
5.6. Antinutritional Activity
5.7. SFTI—An Exceptional Member of BBIs
5.8. Amphibian-Derived Bowman-Birk-Like Trypsin Inhibitors (BBLTIs)
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Puente, X.S.; Sánchez, L.M.; Gutiérrez-Fernández, A.; Velasco, G.; López-Otín, C. A genomic view of the complexity of mammalian proteolytic systems. Biochem. Soc. Trans. 2005, 33, 331–334. [Google Scholar] [CrossRef] [PubMed]
- Rawlings, N.D.; Barrett, A.J.; Thomas, P.D.; Huang, X.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef] [PubMed]
- Dudani, J.S.; Warren, A.D.; Bhatia, S.N. Harnessing Protease Activity to Improve Cancer Care. Annu. Rev. Cancer Biol. 2018, 2, 353–376. [Google Scholar] [CrossRef]
- Soualmia, F.; El Amri, C. Serine protease inhibitors to treat inflammation: A patent review (2011–2016). Expert Opin. Ther. Pat. 2018, 28, 93–110. [Google Scholar] [CrossRef]
- Sharony, R.; Yu, P.J.; Park, J.; Galloway, A.C.; Mignatti, P.; Pintucci, G. Protein targets of inflammatory serine proteases and cardiovascular disease. J. Inflamm. 2010, 7, 45. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Silva, J.G.; Español, Y.; Velasco, G.; Quesada, V. The Degradome database: Expanding roles of mammalian proteases in life and disease. Nucleic Acids Res. 2016, 44, D351–D355. [Google Scholar] [CrossRef]
- Craik, C.S.; Page, M.J.; Madison, E.L. Proteases as therapeutics. Biochem. J. 2011, 435, 1–16. [Google Scholar] [CrossRef] [Green Version]
- Martin, C.E.; List, K. Cell surface–anchored serine proteases in cancer progression and metastasis. Cancer Metastasis Rev. 2019, 38, 357–387. [Google Scholar] [CrossRef]
- Protease Inhibitors—DrugBank. Available online: https://www.drugbank.ca/categories/DBCAT000002 (accessed on 2 September 2020).
- Laskowski, M.; Qasim, M.A. What can the structures of enzyme-inhibitor complexes tell us about the structures of enzyme substrate complexes? Biochim. Biophys. Acta 2000, 1477, 324–337. [Google Scholar] [CrossRef]
- Hellinger, R.; Gruber, C.W. Peptide-based protease inhibitors from plants. Drug Discov. Today 2019, 24, 1877–1889. [Google Scholar] [CrossRef]
- Touil, T.; Ciric, B.; Ventura, E.; Shindler, K.S.; Gran, B.; Rostami, A. Bowman-Birk inhibitor suppresses autoimmune inflammation and neuronal loss in a mouse model of multiple sclerosis. J. Neurol. Sci. 2008, 271, 191–202. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Losso, J.N. The biochemical and functional food properties of the Bowman-Birk inhibitor. Crit. Rev. Food Sci. Nutr. 2008, 48, 94–118. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A.; Del Carmen Arques, M. Bowman-Birk inhibitors from legumes as colorectal chemopreventive agents. World J. Gastroenterol. 2014, 20, 10305–10315. [Google Scholar] [CrossRef] [PubMed]
- Srikanth, S.; Chen, Z. Plant protease inhibitors in therapeutics-focus on cancer therapy. Front. Pharmacol. 2016, 7, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bendre, A.D.; Ramasamy, S.; Suresh, C.G. Analysis of Kunitz inhibitors from plants for comprehensive structural and functional insights. Int. J. Biol. Macromol. 2018, 113, 933–943. [Google Scholar] [CrossRef] [PubMed]
- Bowman, D.E. Differentiation of Soy Bean Antitryptic Factors. Exp. Biol. Med. 1946, 63, 547–550. [Google Scholar] [CrossRef] [PubMed]
- Birk, Y.; Gertler, A.; Khalef, S. A pure trypsin inhibitor from soya beans. Biochem. J. 1963, 87, 281–284. [Google Scholar] [CrossRef] [Green Version]
- Sessa, D.J.; Wolf, W.J. Bowman-Birk inhibitors in soybean seed coats. Ind. Crops Prod. 2001, 14, 73–83. [Google Scholar] [CrossRef]
- Fields, C.; Mallee, P.; Muzard, J.; Lee, G.U. Isolation of Bowman-Birk-Inhibitor from soybean extracts using novel peptide probes and high gradient magnetic separation. Food Chem. 2012, 134, 1831–1838. [Google Scholar] [CrossRef] [Green Version]
- Palavalli, M.H.; Natarajan, S.S.; Wang, T.T.Y.; Krishnan, H.B. Imbibition of soybean seeds in warm water results in the release of copious amounts of Bowman-Birk protease inhibitor, a putative anticarcinogenic agent. J. Agric. Food Chem. 2012, 60, 3135–3143. [Google Scholar] [CrossRef]
- Kennedy, A.R. Chemopreventive agents: Protease inhibitors. Pharmacol. Ther. 1998, 78, 167–209. [Google Scholar] [CrossRef]
- Clemente, A.; Sonnante, G.; Domoney, C. Bowman-Birk inhibitors from legumes and human gastrointestinal health: Current status and perspectives. Curr. Protein Pept. Sci. 2011, 12, 358–373. [Google Scholar] [CrossRef] [PubMed]
- Shan, L.; Li, C.; Chen, F.; Zhao, S.; Xia, G. A Bowman-Birk type protease inhibitor is involved in the tolerance to salt stress in wheat. Plant Cell Environ. 2008, 31, 1128–1137. [Google Scholar] [CrossRef] [PubMed]
- Dramé, K.N.; Passaquet, C.; Repellin, A.; Zuily-Fodil, Y. Cloning, characterization and differential expression of a Bowman-Birk inhibitor during progressive water deficit and subsequent recovery in peanut (Arachis hypogaea) leaves. J. Plant Physiol. 2013, 170, 225–229. [Google Scholar] [CrossRef] [PubMed]
- Malefo, M.B.; Mathibela, E.O.; Crampton, B.G.; Makgopa, M.E. Investigating the role of Bowman-Birk serine protease inhibitor in Arabidopsis plants under drought stress. Plant Physiol. Biochem. 2020, 149, 286–293. [Google Scholar] [CrossRef]
- Zhang, L.; Nakanishi Itai, R.; Yamakawa, T.; Nakanishi, H.; Nishizawa, N.K.; Kobayashi, T. The Bowman-Birk Trypsin Inhibitor IBP1 Interacts with and Prevents Degradation of IDEF1 in Rice. Plant Mol. Biol. Report. 2014, 32, 841–851. [Google Scholar] [CrossRef]
- Clemente, M.; Corigliano, M.G.; Pariani, S.A.; Sánchez-López, E.F.; Sander, V.A.; Ramos-Duarte, V.A. Plant serine protease inhibitors: Biotechnology application in agriculture and molecular farming. Int. J. Mol. Sci. 2019, 20, 1345. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Park, S.C.; Hwang, I.; Cheong, H.; Nah, J.W.; Hahm, K.S.; Park, Y. Protease inhibitors from plants with antimicrobial activity. Int. J. Mol. Sci. 2009, 10, 2860–2872. [Google Scholar] [CrossRef] [Green Version]
- Wang, T.; Jiang, Y.; Chen, X.; Wang, L.; Ma, C.; Xi, X.; Zhang, Y.; Chen, T.; Shaw, C.; Zhou, M. Ranacyclin-NF, a Novel Bowman–Birk Type Protease Inhibitor from the Skin Secretion of the East Asian Frog, Pelophylax nigromaculatus. Biology 2020, 9, 149. [Google Scholar] [CrossRef]
- Li, J.; Zhang, C.; Xu, X.; Wang, J.; Yu, H.; Lai, R.; Gong, W. Trypsin inhibitory loop is an excellent lead structure to design serine protease inhibitors and antimicrobial peptides. FASEB J. 2007, 21, 2466–2473. [Google Scholar] [CrossRef] [Green Version]
- Miao, Y.; Chen, G.; Xi, X.; Ma, C.; Wang, L.; Burrows, J.F.; Duan, J.; Zhou, M.; Chen, T. Discovery and rational design of a novel bowman-birk related protease inhibitor. Biomolecules 2019, 9, 280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Otlewski, J.; Jelen, F.; Zakrzewska, M.; Oleksy, A. The many faces of protease-protein inhibitor interaction. EMBO J. 2005, 24, 1303–1310. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laskowski, M.; Kato, I. Protein Inhibitors of Proteinases. Annu. Rev. Biochem. 1980, 49, 593–626. [Google Scholar] [CrossRef] [PubMed]
- Apostoluk, W.; Otlewski, J. Variability of the canonical loop conformations in serine proteinases inhibitors and other proteins. Proteins Struct. Funct. Genet. 1998, 32, 459–474. [Google Scholar] [CrossRef]
- Krowarsch, D.; Cierpicki, T.; Jelen, F.; Otlewski, J. Canonical protein inhibitors of serine proteases. Cell. Mol. Life Sci. 2003, 60, 2427–2444. [Google Scholar] [CrossRef]
- Brauer, A.B.E.; Kelly, G.; Matthews, S.J.; Leatherbarrow, R.J. The 1H-NMR solution structure of the antitryptic core peptide of Bowman-Birk inhibitor proteins: A minimal “canonical loop”. J. Biomol. Struct. Dyn. 2002, 20, 59–70. [Google Scholar] [CrossRef]
- Brauer, A.B.E.; Kelly, G.; McBride, J.D.; Cooke, R.M.; Matthews, S.J.; Leatherbarrow, R.J. The Bowman-Birk inhibitor reactive site loop sequence represents an independent structural β-hairpin motif. J. Mol. Biol. 2001, 306, 799–807. [Google Scholar] [CrossRef] [Green Version]
- Schechter, I.; Berger, A. On the size of the active site in proteases. Biochem. Biophys. Res. Commun. 1967, 27, 157–162. [Google Scholar] [CrossRef]
- Piergiovanni, A.R.; Galasso, I. Polymorphism of trypsin and chymotrypsin binding loops in Bowman-Birk inhibitors from common bean (Phaseolus vulgaris L.). Plant Sci. 2004, 166, 1525–1531. [Google Scholar] [CrossRef]
- Radisky, E.S.; Koshland, D.E. A clogged gutter mechanism for protease inhibitors. Proc. Natl. Acad. Sci. USA 2002, 99, 10316–10321. [Google Scholar] [CrossRef] [Green Version]
- Marx, U.C.; Korsinczky, M.L.J.; Schirra, H.J.; Jones, A.; Condie, B.; Otvos, L.; Craik, D.J. Enzymatic cyclization of a potent bowman-birk protease inhibitor, sunflower trypsin inhibitor-1, and solution structure of an acyclic precursor peptide. J. Biol. Chem. 2003, 278, 21782–21789. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gáspári, Z.; Várnai, P.; Szappanos, B.; Perczel, A. Reconciling the lock-and-key and dynamic views of canonical serine protease inhibitor action. FEBS Lett. 2010, 584, 203–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, H.K.; Kim, Y.S.; Yang, J.K.; Moon, J.; Lee, J.Y.; Suh, S.W. Crystal structure of a 16 kDa double-headed Bowman-Birk trypsin inhibitor from barley seeds at 1.9 Å resolution. J. Mol. Biol. 1999, 293, 1133–1144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meenu Krishnan, V.G.; Murugan, K. Purification, characterization and kinetics of protease inhibitor from fruits of Solanum aculeatissimum Jacq. Food Sci. Hum. Wellness 2015, 4, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Kyte, J.; Doolittle, R.F. A simple method for displaying the hydropathic character of a protein. J. Mol. Biol. 1982, 157, 105–132. [Google Scholar] [CrossRef] [Green Version]
- Craik, D.J.; Čemažar, M.; Wang, C.K.L.; Daly, N.L. The cyclotide family of circular miniproteins: Nature’s combinatorial peptide template. Biopolym. Pept. Sci. Sect. 2006, 84, 250–266. [Google Scholar] [CrossRef]
- Moore, S.J.; Leung, C.L.; Cochran, J.R. Knottins: Disulfide-bonded therapeutic and diagnostic peptides. Drug Discov. Today Technol. 2012, 9, e3–e11. [Google Scholar] [CrossRef]
- Luckett, S.; Garcia, R.S.; Barker, J.J.; Konarev, A.V.; Shewry, P.R.; Clarke, A.R.; Brady, R.L. High-resolution structure of a potent, cyclic proteinase inhibitor from sunflower seeds. J. Mol. Biol. 1999, 290, 525–533. [Google Scholar] [CrossRef] [Green Version]
- Mylne, J.S.; Colgrave, M.L.; Daly, N.L.; Chanson, A.H.; Elliott, A.G.; McCallum, E.J.; Jones, A.; Craik, D.J. Albumins and their processing machinery are hijacked for cyclic peptides in sunflower. Nat. Chem. Biol. 2011, 7, 257–259. [Google Scholar] [CrossRef]
- Jayasena, A.S.; Fisher, M.F.; Panero, J.L.; Secco, D.; Bernath-Levin, K.; Berkowitz, O.; Taylor, N.L.; Schilling, E.E.; Whelan, J.; Mylne, J.S. Stepwise evolution of a buried inhibitor peptide over 45 My. Mol. Biol. Evol. 2017, 34, 1505–1516. [Google Scholar] [CrossRef]
- Kumar, V.; Murugeson, S.; Vithani, N.; Prakash, B.; Gowda, L.R. A salt-bridge stabilized C-terminal hook is critical for the dimerization of a Bowman Birk inhibitor. Arch. Biochem. Biophys. 2015, 566, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Rao, K.N.; Suresh, C.G. Bowman-Birk protease inhibitor from the seeds of Vigna unguiculata forms a highly stable dimeric structure. Biochim. Biophys. Acta Proteins Proteom. 2007, 1774, 1264–1273. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Rao, A.G.A.; Hariharaputran, S.; Chandra, N.; Gowda, L.R. Molecular mechanism of dimerization of Bowman-Birk inhibitors: Pivotal role of Asp76 in the dimerzation. J. Biol. Chem. 2004, 279, 30425–30432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mohanraj, S.S.; Gujjarlapudi, M.; Lokya, V.; Mallikarjuna, N.; Dutta-Gupta, A.; Padmasree, K. Purification and characterization of Bowman-Birk and Kunitz isoinhibitors from the seeds of Rhynchosia sublobata (Schumach.) Meikle, a wild relative of pigeonpea. Phytochemistry 2019, 159, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Lokya, V.; Swathi, M.; Mallikarjuna, N.; Padmasree, K. Response of Midgut Trypsin- and Chymotrypsin-Like Proteases of Helicoverpa armigera Larvae Upon Feeding With Peanut BBI: Biochemical and Biophysical Characterization of PnBBI. Front. Plant Sci. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Honda, D.E.; Martins, J.B.L.; Ventura, M.M.; Eyrilmez, S.M.; Lepšík, M.; Hobza, P.; Pecina, A.; de Freitas, S.M. Interface Interactions of the Bowman-Birk Inhibitor BTCI in a Ternary Complex with Trypsin and Chymotrypsin Evaluated by Semiempirical Quantum Mechanical Calculations. European J. Org. Chem. 2018, 2018, 5203–5211. [Google Scholar] [CrossRef]
- Brand, G.D.; Pires, D.A.T.; Furtado, J.R.; Cooper, A.; Freitas, S.M.; Bloch, C. Oligomerization affects the kinetics and thermodynamics of the interaction of a Bowman-Birk inhibitor with proteases. Arch. Biochem. Biophys. 2017, 618, 9–14. [Google Scholar] [CrossRef]
- Li De La Sierra, I.; Quillien, L.; Flecker, P.; Gueguen, J.; Brunie, S. Dimeric crystal structure of a Bowman-Birk protease inhibitor from pea seeds. J. Mol. Biol. 1999, 285, 1195–1207. [Google Scholar] [CrossRef]
- Souza, L.D.C.; Camargo, R.; Demasi, M.; Santana, J.M.; De Sá, C.M.; De Freitas, S.M. Effects of an anticarcinogenic bowman-birk protease inhibitor on purified 20S proteasome and MCF-7 breast cancer cells. PLoS ONE 2014, 9, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Silva, L.P.; Azevedo, R.B.; Morais, P.C.; Ventura, M.M.; Freitas, S.M. Oligomerization states of Bowman-Birk inhibitor by atomic force microscopy and computational approaches. Proteins Struct. Funct. Genet. 2005, 61, 642–648. [Google Scholar] [CrossRef]
- Koepke, J.; Ermler, U.; Warkentin, E.; Wenzl, G.; Flecker, P. Crystal structure of cancer chemopreventive Bowman-Birk inhibitor in ternary complex with bovine trypsin at 2.3 Å resolution. Structural basis of Janus-faced serine protease inhibitor specificity. J. Mol. Biol. 2000, 298, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, J.A.R.G.; Silva, L.P.; Teles, R.C.L.; Esteves, G.F.; Azevedo, R.B.; Ventura, M.M.; De Freitas, S.M. Crystal structure of the Bowman-Birk inhibitor from Vigna unguiculata seeds in complex with β-trypsin at 1.55 Å resolution and its structural properties in association with proteinases. Biophys. J. 2007, 92, 1638–1650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Catalano, M.; Ragona, L.; Molinari, H.; Tava, A.; Zetta, L. Anticarcinogenic Bowman Birk inhibitor isolated from snail medic seeds (Medicago scutellata): Solution structure and analysis of self-association behavior. Biochemistry 2003, 42, 2836–2846. [Google Scholar] [CrossRef] [PubMed]
- Deshimaru, M.; Hanamoto, R.; Kusano, C.; Yoshimi, S.; Terada, S. Purification and Characterization of Proteinase Inhibitors from Wild Soja (Glycine soja) Seeds. Biosci. Biotechnol. Biochem. 2002, 66, 1897–1903. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A.; Moreno, F.J.; Marín-Manzano, M.D.C.; Jiménez, E.; Domoney, C. The cytotoxic effect of Bowman-Birk isoinhibitors, IBB1 and IBBD2, from soybean (Glycine max) on HT29 human colorectal cancer cells is related to their intrinsic ability to inhibit serine proteases. Mol. Nutr. Food Res. 2010, 54, 396–405. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.W.; Huang, S.C.; Lin-Shiau, S.Y.; Lin, J.K. Bowman-Birk inhibitor abates proteasome function and suppresses the proliferation of MCF7 breast cancer cells through accumulation of MAP kinase phosphatase-1. Carcinogenesis 2005, 26, 1296–1306. [Google Scholar] [CrossRef] [Green Version]
- Voss, R.H.; Ermler, U.; Essen, L.O.; Wenzl, G.; Kim, Y.M.; Flecker, P. Crystal structure of the bifunctional soybean Bowman-Birk inhibitor at 0.28-nm resolution. Structural peculiarities in a folded protein conformation. Eur. J. Biochem. 1996, 242, 122–131. [Google Scholar] [CrossRef]
- Werner, M.H.; Wemmer, D.E. Three-Dimensional Structure of Soybean Trypsin/Chymotrypsin Bowman-Birk Inhibitor in Solution. Biochemistry 1992, 31, 999–1010. [Google Scholar] [CrossRef]
- Esteves, G.F.; Teles, R.C.L.; Cavalcante, N.S.; Neves, D.; Ventura, M.M.; Barbosa, J.A.R.G.; Freitas, S.M. RCSB PDB—3RU4: Crystal structure of the Bowman-Birk serine protease inhibitor BTCI in complex with trypsin and chymotrypsin. 2012. Available online: https://www.rcsb.org/structure/3RU4 (accessed on 25 November 2020). [CrossRef]
- Cotabarren, J.; Broitman, D.J.; Quiroga, E.; Obregón, W.D. GdTI, the first thermostable trypsin inhibitor from Geoffroea decorticans seeds. A novel natural drug with potential application in biomedicine. Int. J. Biol. Macromol. 2020, 148, 869–879. [Google Scholar] [CrossRef]
- Zhang, Y.; Kouzuma, Y.; Miyaji, T.; Yonekura, M. Purification, characterization, and cDNA cloning of a Bowman-Birk type trypsin inhibitor from Apios americana medikus tubers. Biosci. Biotechnol. Biochem. 2008, 72, 171–178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scarafoni, A.; Consonni, A.; Galbusera, V.; Negri, A.; Tedeschi, G.; Rasmussen, P.; Magni, C.; Duranti, M. Identification and characterization of a Bowman-Birk inhibitor active towards trypsin but not chymotrypsin in Lupinus albus seeds. Phytochemistry 2008, 69, 1820–1825. [Google Scholar] [CrossRef] [PubMed]
- Martins, T.F.; Vasconcelos, I.M.; Silva, R.G.G.; Silva, F.D.A.; Souza, P.F.N.; Varela, A.L.N.; Albuquerque, L.M.; Oliveira, J.T.A. A Bowman-Birk Inhibitor from the Seeds of Luetzelburgia auriculata Inhibits Staphylococcus aureus Growth by Promoting Severe Cell Membrane Damage. J. Nat. Prod. 2018, 81, 1497–1507. [Google Scholar] [CrossRef] [PubMed]
- Prasad, E.R.; Merzendorfer, H.; Madhurarekha, C.; Dutta-Gupta, A.; Padmasree, K. Bowman-birk proteinase inhibitor from Cajanus cajan seeds: Purification, characterization, and insecticidal properties. J. Agric. Food Chem. 2010, 58, 2838–2847. [Google Scholar] [CrossRef]
- Swathi, M.; Lokya, V.; Swaroop, V.; Mallikarjuna, N.; Kannan, M.; Dutta-Gupta, A.; Padmasree, K. Structural and functional characterization of proteinase inhibitors from seeds of Cajanus cajan (cv. ICP 7118). Plant Physiol. Biochem. 2014, 83, 77–87. [Google Scholar] [CrossRef]
- Kuhar, K.; Kansal, R.; Subrahmanyam, B.; Koundal, K.R.; Miglani, K.; Gupta, V.K. A Bowman-Birk protease inhibitor with antifeedant and antifungal activity from Dolichos biflorus. Springer 2013, 35, 1887–1903. [Google Scholar] [CrossRef]
- Prasad, E.R.; Dutta-Gupta, A.; Padmasree, K. Purification and characterization of a Bowman-Birk proteinase inhibitor from the seeds of black gram (Vigna mungo). Phytochemistry 2010, 71, 363–372. [Google Scholar] [CrossRef]
- De Paola, D.; Blanco, E.; Pierri, C.L.; Sonnante, G. Isolation and characterization of novel variants of BBI coding genes from the legume Lathyrus sativus. Plant Physiol. Biochem. 2012, 57, 45–53. [Google Scholar] [CrossRef]
- Al-Maiman, S.A.; Gassem, M.A.; Osman, M.A.; Mohamed Ahmed, I.A.; Al-Juhaimi, F.Y.; Abdel Rahman, I.E.; Weber, C. Bowman-Birk proteinase inhibitor from tepary bean (Phaseolus acutifolius) seeds: Purification and biochemical properties. Int. Food Res. J. 2019, 26, 1123–1131. [Google Scholar]
- Dantzger, M.; Vasconcelos, I.M.; Scorsato, V.; Aparicio, R.; Marangoni, S.; Macedo, M.L.R. Bowman-Birk proteinase inhibitor from Clitoria fairchildiana seeds: Isolation, biochemical properties and insecticidal potential. Phytochemistry 2015, 118, 224–235. [Google Scholar] [CrossRef]
- Bueno, N.R.; Fritz, H.; Auerswald, E.A.; Mentele, R.; Sampaio, M.; Sampaio, C.A.M.; Oliva, M.L.V. Primary structure of Dioclea glabra trypsin inhibitor, DgTI, a Bowman-Birk inhibitor. Biochem. Biophys. Res. Commun. 1999, 261, 838–843. [Google Scholar] [CrossRef] [PubMed]
- Fei Fang, E.; Abd Elazeem Hassanien, A.; Ho Wong, J.; Shui Fern Bah, C.; Saad Soliman, S.; Bun Ng, T. Isolation of a New Trypsin Inhibitor from the Faba Bean (Vicia faba cv. Giza 843) with Potential Medicinal Applications. Protein Pept. Lett. 2011, 18, 64–72. [Google Scholar] [CrossRef] [PubMed]
- Rahbé, Y.; Ferrasson, E.; Rabesona, H.; Quillien, L. Toxicity to the pea aphid Acyrthosiphon pisum of anti-chymotrypsin isoforms and fragments of Bowman-Birk protease inhibitors from pea seeds. Insect Biochem. Mol. Biol. 2003, 33, 299–306. [Google Scholar] [CrossRef]
- Ragg, E.M.; Galbusera, V.; Scarafoni, A.; Negri, A.; Tedeschi, G.; Consonni, A.; Sessa, F.; Duranti, M. Inhibitory properties and solution structure of a potent Bowman-Birk protease inhibitor from lentil (Lens culinaris, L) seeds. FEBS J. 2006, 273, 4024–4039. [Google Scholar] [CrossRef]
- Paiva, P.M.G.; Oliva, M.L.V.; Fritz, H.; Coelho, L.C.B.B.; Sampaio, C.A.M. Purification and primary structure determination of two Bowman-Birk type trypsin isoinhibitors from Cratylia mollis seeds. Phytochemistry 2006, 67, 545–552. [Google Scholar] [CrossRef]
- Ceciliani, F.; Tava, A.; Iori, R.; Mortarino, M.; Odoardi, M.; Ronchi, S. A trypsin inhibitor from snail medic seeds active against pest proteases. Phytochemistry 1997, 44, 393–398. [Google Scholar] [CrossRef]
- Capaldi, S.; Perduca, M.; Faggion, B.; Carrizo, M.E.; Tava, A.; Ragona, L.; Monaco, H.L. Crystal structure of the anticarcinogenic Bowman-Birk inhibitor from snail medic (Medicago scutellata) seeds complexed with bovine trypsin. J. Struct. Biol. 2007, 158, 71–79. [Google Scholar] [CrossRef]
- Tanaka, A.S.; Sampaio, M.U.; Oliva, M.L.V.; Sampaio, C.A.M.; Marangoni, S.; de Oliveira, B.; Novelle, J.C.; Fink, E. Purification and Primary Structure Determination of a Bowman-BirkTrypsin Inhibitor from Torresea cearensis Seeds. Biol. Chem. 1997, 378, 273–282. [Google Scholar] [CrossRef]
- Spengler, J.; Jiménez, J.C.; Burger, K.; Giralt, E.; Albericio, F. Abbreviated nomenclature for cyclic and branched homo- and hetero-detic peptides. J. Pept. Res. 2005, 65, 550–555. [Google Scholar] [CrossRef]
- Korsinczky, M.L.J.; Schirra, H.J.; Rosengren, K.J.; West, J.; Condie, B.A.; Otvos, L.; Anderson, M.A.; Craik, D.J. Solution structures by1H NMR of the novel cyclic trypsin inhibitor SFTI-1 from sunflower seeds and an acyclic permutant. J. Mol. Biol. 2001, 311, 579–591. [Google Scholar] [CrossRef]
- Yuan, C.; Chen, L.; Meehan, E.J.; Daly, N.; Craik, D.J.; Huang, M.; Ngo, J.C. Structure of catalytic domain of Matriptase in complex with Sunflower trypsin inhibitor-1. BMC Struct. Biol. 2011, 11, 30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gitlin, A.; Dębowski, D.; Karna, N.; Łęgowska, A.; Stirnberg, M.; Gütschow, M.; Rolka, K. Inhibitors of Matriptase-2 Based on the Trypsin Inhibitor SFTI-1. ChemBioChem 2015, 16, 1601–1607. [Google Scholar] [CrossRef] [PubMed]
- De Veer, S.J.; Swedberg, J.E.; Akcan, M.; Rosengren, K.J.; Brattsand, M.; Craik, D.J.; Harris, J.M. Engineered protease inhibitors based on sunflower trypsin inhibitor-1 (SFTI-1) provide insights into the role of sequence and conformation in Laskowski mechanism inhibition. Biochem. J. 2015, 469, 243–253. [Google Scholar] [CrossRef]
- de Veer, S.J.; Li, C.Y.; Swedberg, J.E.; Schroeder, C.I.; Craik, D.J. Engineering potent mesotrypsin inhibitors based on the plant-derived cyclic peptide, sunflower trypsin inhibitor-1. Eur. J. Med. Chem. 2018, 155, 695–704. [Google Scholar] [CrossRef] [PubMed]
- Dȩbowski, D.; Pikuła, M.; Lubos, M.; Langa, P.; Trzonkowski, P.; Lesner, A.; Łȩgowska, A.; Rolka, K. Inhibition of human and yeast 20S proteasome by analogs of trypsin inhibitor SFTI-1. PLoS ONE 2014, 9. [Google Scholar] [CrossRef] [PubMed]
- Fittler, H.; Avrutina, O.; Empting, M.; Kolmar, H. Potent inhibitors of human matriptase-1 based on the scaffold of sunflower trypsin inhibitor. J. Pept. Sci. 2014, 20, 415–420. [Google Scholar] [CrossRef]
- Dębowski, D.; Cichorek, M.; Lubos, M.; Wójcik, S.; Łęgowska, A.; Rolka, K. Noncovalent inhibitors of human 20S and 26S proteasome based on trypsin inhibitor SFTI-1. Biopolymers 2016, 106, 685–696. [Google Scholar] [CrossRef]
- Quimbar, P.; Malik, U.; Sommerhoff, C.P.; Kaas, Q.; Chan, L.Y.; Huang, Y.H.; Grundhuber, M.; Dunse, K.; Craik, D.J.; Anderson, M.A.; et al. High-affinity cyclic peptide matriptase inhibitors. J. Biol. Chem. 2013, 288, 13885–13896. [Google Scholar] [CrossRef] [Green Version]
- Zabłotna, E.; Jaśkiewicz, A.; Łęgowska, A.; Miecznikowska, H.; Lesner, A.; Rolka, K. Design of serine proteinase inhibitors by combinatorial chemistry using trypsin inhibitor SFTI-1 as a starting structure. J. Pept. Sci. Off. Publ. Eur. Pept. Soc. 2007, 749–755. [Google Scholar] [CrossRef]
- Łęgowska, A.; Dębowski, D.; Lesner, A.; Wysocka, M.; Rolka, K. Introduction of non-natural amino acid residues into the substrate-specific P1 position of trypsin inhibitor SFTI-1 yields potent chymotrypsin and cathepsin G inhibitors. Bioorganic Med. Chem. 2009, 17, 3302–3307. [Google Scholar] [CrossRef]
- Zabłotna, E.; Kret, A.; Jaśkiewicz, A.; Olma, A.; Leplawy, M.T.; Rolka, K. Introduction of α-hydroxymethyamino acid residues in substrate specificity P1 position of trypsin inhibitor SFTI-1 from sunflower seeds retains its activity. Biochem. Biophys. Res. Commun. 2006, 340, 823–828. [Google Scholar] [CrossRef] [PubMed]
- Gitlin-Domagalska, A.; Dębowski, D.; Łęgowska, A.; Stirnberg, M.; Okońska, J.; Gütschow, M.; Rolka, K. Design and chemical syntheses of potent matriptase-2 inhibitors based on trypsin inhibitor SFTI-1 isolated from sunflower seeds. Biopolymers 2017, 108, e23031. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.; Kinsler, V.A.; Macmillan, D.; Di, W.-L. Tissue Kallikrein Inhibitors Based on the Sunflower Trypsin Inhibitor Scaffold—A Potential Therapeutic Intervention for Skin Diseases. PLoS ONE 2016, 11, e0166268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Veer, S.J.; Wang, C.K.; Harris, J.M.; Craik, D.J.; Swedberg, J.E. Improving the Selectivity of Engineered Protease Inhibitors: Optimizing the P2 Prime Residue Using a Versatile Cyclic Peptide Library. J. Med. Chem. 2015, 58, 8257–8268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hilpert, K.; Hansen, G.; Wessner, H.; Volkmer-Engert, R.; Höhne, W. Complete substitutional analysis of a sunflower trypsin inhibitor with different serine proteases. J. Biochem. 2005, 138, 383–390. [Google Scholar] [CrossRef] [PubMed]
- Swedberg, J.E.; Wu, G.; Mahatmanto, T.; Durek, T.; Caradoc-Davies, T.T.; Whisstock, J.C.; Law, R.H.P.; Craik, D.J. Highly Potent and Selective Plasmin Inhibitors Based on the Sunflower Trypsin Inhibitor-1 Scaffold Attenuate Fibrinolysis in Plasma. J. Med. Chem. 2019, 62, 552–560. [Google Scholar] [CrossRef]
- Li, C.Y.; Yap, K.; Swedberg, J.E.; Craik, D.J.; De Veer, S.J. Binding Loop Substitutions in the Cyclic Peptide SFTI-1 Generate Potent and Selective Chymase Inhibitors. J. Med. Chem. 2020, 63, 816–826. [Google Scholar] [CrossRef]
- Swedberg, J.E.; Li, C.Y.; De Veer, S.J.; Wang, C.K.; Craik, D.J. Design of potent and selective cathepsin G inhibitors based on the sunflower trypsin inhibitor-1 scaffold. J. Med. Chem. 2017, 60, 658–667. [Google Scholar] [CrossRef]
- Fittler, H.; Depp, A.; Avrutina, O.; Dahms, S.O.; Than, M.E.; Empting, M.; Kolmar, H. Engineering a Constrained Peptidic Scaffold towards Potent and Selective Furin Inhibitors. ChemBioChem 2015, 16, 2441–2444. [Google Scholar] [CrossRef]
- Swedberg, J.E.; Nigon, L.V.; Reid, J.C.; de Veer, S.J.; Walpole, C.M.; Stephens, C.R.; Walsh, T.P.; Takayama, T.K.; Hooper, J.D.; Clements, J.A.; et al. Substrate-Guided Design of a Potent and Selective Kallikrein-Related Peptidase Inhibitor for Kallikrein 4. Chem. Biol. 2009, 16, 633–643. [Google Scholar] [CrossRef]
- Riley, B.T.; Ilyichova, O.; Costa, M.G.S.; Porebski, B.T.; De Veer, S.J.; Swedberg, J.E.; Kass, I.; Harris, J.M.; Hoke, D.E.; Buckle, A.M. Direct and indirect mechanisms of KLK4 inhibition revealed by structure and dynamics. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Riley, B.T.; Ilyichova, O.; De Veer, S.J.; Swedberg, J.E.; Wilson, E.; Hoke, D.E.; Harris, J.M.; Buckle, A.M. KLK4 Inhibition by Cyclic and Acyclic Peptides: Structural and Dynamical Insights into Standard-Mechanism Protease Inhibitors. Biochemistry 2019, 58, 2524–2533. [Google Scholar] [CrossRef] [PubMed]
- Li, C.Y.; De Veer, S.J.; White, A.M.; Chen, X.; Harris, J.M.; Swedberg, J.E.; Craik, D.J. Amino Acid Scanning at P5′ within the Bowman-Birk Inhibitory Loop Reveals Specificity Trends for Diverse Serine Proteases. J. Med. Chem. 2019, 62, 3696–3706. [Google Scholar] [CrossRef] [PubMed]
- de Veer, S.J.; Furio, L.; Swedberg, J.E.; Munro, C.A.; Brattsand, M.; Clements, J.A.; Hovnanian, A.; Harris, J.M. Selective Substrates and Inhibitors for Kallikrein-Related Peptidase 7 (KLK7) Shed Light on KLK Proteolytic Activity in the Stratum Corneum. J. Investig. Dermatol. 2017, 137, 430–439. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kocsis, A.; Kékesi, K.A.; Szász, R.; Végh, B.M.; Balczer, J.; Dobó, J.; Závodszky, P.; Gál, P.; Pál, G. Selective Inhibition of the Lectin Pathway of Complement with Phage Display Selected Peptides against Mannose-Binding Lectin-Associated Serine Protease (MASP)-1 and -2: Significant Contribution of MASP-1 to Lectin Pathway Activation. J. Immunol. 2010, 185, 4169–4178. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Veer, S.J.; White, A.M.; Craik, D. Sunflower Trypsin Inhibitor-1 (SFTI-1): Sowing Seeds in the Fields of Chemistry and Biology. Angew. Chem. Int. Ed. Engl. 2020. [Google Scholar] [CrossRef]
- Tian, S.; Swedberg, J.E.; Li, C.Y.; Craik, D.J.; De Veer, S.J. Iterative optimization of the cyclic peptide sfti-1 yields potent inhibitors of neutrophil proteinase 3. ACS Med. Chem. Lett. 2019, 10, 1234–1239. [Google Scholar] [CrossRef]
- Chen, X.; Riley, B.T.; de Veer, S.J.; Hoke, D.E.; Van Haeften, J.; Leahy, D.; Swedberg, J.E.; Brattsand, M.; Hartfield, P.J.; Buckle, A.M.; et al. Potent, multi-target serine protease inhibition achieved by a simplified β-sheet motif. PLoS ONE 2019, 14, e0210842. [Google Scholar] [CrossRef]
- Oliás, R.; Becerra-Rodríguez, C.; Soliz-Rueda, J.R.; Moreno, F.J.; Delgado-Andrade, C.; Clemente, A. Glycation affects differently the main soybean Bowman-Birk isoinhibitors, IBB1 and IBBD2, altering their antiproliferative properties against HT29 colon cancer cells. Food Funct. 2019, 10, 6193–6202. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Huerta, E.; Fernández-Tomé, S.; Arques, M.C.; Amigo, L.; Recio, I.; Clemente, A.; Hernández-Ledesma, B. The protective role of the Bowman-Birk protease inhibitor in soybean lunasin digestion: The effect of released peptides on colon cancer growth. Food Funct. 2015, 6, 2626–2635. [Google Scholar] [CrossRef] [Green Version]
- Arques, M.C.; Marín-Manzano, M.C.; Da Rocha, L.C.B.; Hernandez-Ledesma, B.; Recio, I.; Clemente, A. Quantitative determination of active Bowman-Birk isoinhibitors, IBB1 and IBBD2, in commercial soymilks. Food Chem. 2014, 155, 24–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Harsulkar, A.M.; Giri, A.P.; Patankar, A.G.; Gupta, V.S.; Sainani, M.N.; Ranjekar, P.K.; Deshpande, V.V. Successive use of non-host plant proteinase inhibitors required for effective inhibition of Helicoverpa armigera gut proteinases and larval growth. Plant Physiol. 1999, 121, 497–506. [Google Scholar] [CrossRef] [Green Version]
- Jongsma, M.A.; Bakker, P.L.; Peters, J.; Bosch, D.; Stiekema, W.J. Adaptation of Spodoptera exigua larvae to plant proteinase inhibitors by induction of gut proteinase activity insensitive to inhibition. Proc. Natl. Acad. Sci. USA 1995, 92, 8041–8045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brito, L.O.; Lopes, A.R.; Parra, J.R.P.; Terra, W.R.; Silva-Filho, M.C. Adaptation of tobacco budworm Heliothis virescens to proteinase inhibitors may be mediated by the synthesis of new proteinases. Comp. Biochem. Physiol. B 2001, 128, 365–375. [Google Scholar] [CrossRef]
- Domoney, C.; Welham, T.; Sidebottom, C.; Firmin, J.L. Multiple isoforms of Pisum trypsin inhibitors result from modification of two primary gene products. FEBS Lett. 1995, 360, 15–20. [Google Scholar] [CrossRef] [Green Version]
- Quillien, L.; Ferrasson, E.; Molle, D.; Gueguen, J. Trypsin inhibitor polymorphism: Multigene family expression and posttranslational modification. J. Protein Chem. 1997, 16, 195–203. [Google Scholar] [CrossRef] [PubMed]
- Kalume, D.E.; Sousa, M.V.; Morhy, L. Purification, characterization, sequence determination, and mass spectrometric analysis of a trypsin inhibitor from seeds of the brazilian tree Dipteryx alata (leguminosae). J. Protein Chem. 1995, 14, 685–693. [Google Scholar] [CrossRef]
- Mohanraj, S.S.; Tetali, S.D.; Mallikarjuna, N.; Dutta-Gupta, A.; Padmasree, K. Biochemical properties of a bacterially-expressed Bowman-Birk inhibitor from Rhynchosia sublobata (Schumach.) Meikle seeds and its activity against gut proteases of Achaea janata. Phytochemistry 2018, 151, 78–90. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Gowda, L.R. The contribution of two disulfide bonds in the trypsin binding domain of horsegram (Dolichos biflorus) Bowman-Birk inhibitor to thermal stability and functionality. Arch. Biochem. Biophys. 2013, 537, 49–61. [Google Scholar] [CrossRef]
- Chen, Y.; Xu, Z.; Zhang, C.; Kong, X.; Hua, Y. Heat-induced inactivation mechanisms of Kunitz trypsin inhibitor and Bowman-Birk inhibitor in soymilk processing. Food Chem. 2014, 154, 108–116. [Google Scholar] [CrossRef]
- Dipietrc, C.M.; Liener, I.E. Heat Inactivation of the Kunitz and Bowman-Birk Soybean Protease Inhibitors. J. Agric. Food Chem. 1989, 37, 39–44. [Google Scholar] [CrossRef]
- He, H.; Li, X.; Kong, X.; Hua, Y.; Chen, Y. Heat-induced inactivation mechanism of soybean Bowman-Birk inhibitors. Food Chem. 2017, 232, 712–720. [Google Scholar] [CrossRef] [PubMed]
- Ramasarma, P.R.; Appu Rao, A.G.; Rajagopal Rao, D. Role of disulfide linkages in structure and activity of proteinase inhibitor from horsegram (Dolichos biflorus). Biochim. Biophys. Acta 1995, 1248, 35–42. [Google Scholar] [CrossRef]
- Clemente, A.; Jimenez, E.; Marin-Manzano, M.C.; Rubio, L.A. Active Bowman–Birk inhibitors survive gastrointestinal digestion at the terminal ileum of pigs fed chickpea-based diets. J. Sci. Food Agric. 2008, 88, 513–521. [Google Scholar] [CrossRef]
- Persiani, S.; Yeung, A.; Shen, W.C.; Kennedy, A.R. Polylysine conjugates of bowman-birk protease inhibitor as targeted anti-carcinogenic agents. Carcinogenesis 1991, 12, 1149–1152. [Google Scholar] [CrossRef]
- Clemente, A.; Domoney, C. Biological significance of polymorphism in legume protease inhibitors from the Bowman-Birk family. Curr. Protein Pept. Sci. 2006, 7, 201–216. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Billings, P.C.; Wan, X.S.; Newberne, P.M. Effects of Bowman-Birk inhibitor on rat colon carcinogenesis. Nutr. Cancer 2002, 43, 174–186. [Google Scholar] [CrossRef]
- Ware, J.H.; Wan, X.S.; Newberne, P.; Kennedy, A.R. Bowman-Birk inhibitor concentrate reduces colon inflammation in mice with dextran sulfate sodium-induced ulcerative colitis. Dig. Dis. Sci. 1999, 44, 986–990. [Google Scholar] [CrossRef]
- de Paula Carli, A.; de Abreu Vieira, P.M.; Silva, K.T.S.; de Sá Cota, R.G.; Carneiro, C.M.; Castro-Borges, W.; de Andrade, M.H.G. Bowman-Birk inhibitors, proteasome peptidase activities and colorectal pre neoplasias induced by 1,2-dimethylhydrazine in Swiss mice. Food Chem. Toxicol. 2012, 50, 1405–1412. [Google Scholar] [CrossRef]
- Billings, P.C.; Newberne, P.M.; Kennedy, A.R. Protease inhibitor suppression of colon and anal gland carcinogenesis induced by dimethylhydrazine. Carcinogenesis 1990, 11, 1083–1086. [Google Scholar] [CrossRef]
- Wan, X.S.; Ware, J.H.; Zhang, L.; Newberne, P.M.; Evans, S.M.; Clark, L.C.; Kennedy, A.R. Treatment with soybean-derived Bowman Birk inhibitor increases serum prostate-specific antigen concentration while suppressing growth of human prostate cancer xenografts in nude mice. Prostate 1999, 41, 243–252. [Google Scholar] [CrossRef]
- Kennedy, A.R.; Wan, X.S. Effects of the Bowman-Birk inhibitor on growth, invasion, and clonogenic survival of human prostate epithelial cells and prostate cancer cells. Prostate 2002, 50, 125–133. [Google Scholar] [CrossRef] [PubMed]
- Bruce Malkowicz, S.; McKenna, W.G.; Vaughn, D.J.; Wan, X.S.; Propert, K.J.; Rockwell, K.; Marks, S.H.F.; Wein, A.J.; Kennedy, A.R. Effects of Bowman-Birk inhibitor concentrate (BBIC) in patients with benign prostatic hyperplasia. Prostate 2001, 48, 16–28. [Google Scholar] [CrossRef] [PubMed]
- McCormick, D.L.; Johnson, W.D.; Bosland, M.C.; Lubet, R.A.; Steele, V.E. Chemoprevention of rat prostate carcinogenesis by soy isoflavones and by Bowman-Birk inhibitor. Nutr. Cancer 2007, 57, 184–193. [Google Scholar] [CrossRef]
- Kaneko, S.; Yamazaki, T.; Kohno, K.; Sato, A.; Kato, K.; Yano, T. Combination effect of bowman-birk inhibitor and α-tocopheryl succinate on prostate cancer stem-like cells. J. Nutr. Sci. Vitaminol. (Tokyo) 2019, 65, 272–277. [Google Scholar] [CrossRef] [Green Version]
- Tang, M.; Asamoto, M.; Ogawa, K.; Naiki-Ito, A.; Sato, S.; Takahashi, S.; Shirai, T. Induction of apoptosis in the LNCaP human prostate carcinoma cell line and prostate adenocarcinomas of SV40T antigen transgenic rats by the Bowman-Birk inhibitor. Pathol. Int. 2009, 59, 790–796. [Google Scholar] [CrossRef]
- Wan, X.S.; Hamilton, T.C.; Ware, J.H.; Donahue, J.J.; Kennedy, A.R. Growth inhibition and cytotoxicity induced by Bowman-Birk inhibitor concentrate in cisplatin-resistant human ovarian cancer cells. Nutr. Cancer 1998, 31, 8–17. [Google Scholar] [CrossRef]
- Sakurai Connexin 43-dependent tumor-suppressing effect of the Bowman-Birk protease inhibitor on M5076 ovarian sarcoma-bearing mice. Mol. Med. Rep. 2008, 1, 689–693. [CrossRef] [Green Version]
- Suzuki, K.; Yano, T.; Sadzuka, Y.; Sugiyama, T.; Seki, T.; Asano, R. Restoration of connexin 43 by Bowman-Birk protease inhibitor in M5076 bearing mice. Oncol. Rep. 2005, 13, 1247–1250. [Google Scholar] [CrossRef]
- Zhang, L.; Wan, X.S.; Donahue, J.J.; Ware, J.H.; Kennedy, A.R. Effects of the Bowman-Birk inhibitor on clonogenic survival and cisplatin- or radiation-induced cytotoxicity in human breast, cervical, and head and neck cancer cells. Nutr. Cancer 1999, 33, 165–173. [Google Scholar] [CrossRef]
- Ho, V.S.M.; NG, T.B. A Bowman-Birk trypsin inhibitor with antiproliferative activity from Hokkaido large black soybeans. J. Pept. Sci. 2008, 14, 278–282. [Google Scholar] [CrossRef] [PubMed]
- Hsieh, C.C.; Hernández-Ledesma, B.; Jeong, H.J.; Park, J.H.; De Lumen, B.O. Complementary roles in cancer prevention: Protease inhibitor makes the cancer preventive peptide Lunasin bioavailable. PLoS ONE 2010, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, W.B.; Kennedy, A.R.; Wan, X.S.; Atiba, J.; McLaren, C.E.; Meyskens, F.L. Single-Dose Administration of Bowman-Birk Inhibitor Concentrate in Patients with Oral Leukoplakia. Cancer Epidemiol. Prev. Biomarkers 2000, 9, 43–47. [Google Scholar]
- Armstrong, W.B.; Kennedy, A.R.; Steven Wan, X.; Taylor, T.H.; Nguyen, Q.A.; Jensen, J.; Thompson, W.; Lagerberg, W.; Meyskens, F.L. Clinical modulation of oral leukoplakia and protease activity by Bowman-Birk inhibitor concentrate in a phase IIa chemoprevention trial. Clin. Cancer Res. 2000, 6, 4684–4691. [Google Scholar] [PubMed]
- Kennedy, A.R.; Billings, P.C.; Maki, P.A. Effects of Various Preparations of Dietary Protease Inhibitors on Oral Carcinogenesis in Hamsters Induced by DMBA. Nutr. Cancer 1993, 19, 191–200. [Google Scholar] [CrossRef]
- Clair, W.H.S.; Billings, P.C.; Carew, J.A.; Keller-McGandy, C.; Newberne, P.; Kennedy, A.R. Suppression of Dimethylhydrazine-induced Carcinogenesis in Mice by Dietary Addition of the Bowman-Birk Protease Inhibitor. Cancer Res. 1990, 50, 580–586. [Google Scholar] [CrossRef]
- Saito, T.; Sato, H.; Virgona, N.; Hagiwara, H.; Kashiwagi, K.; Suzuki, K.; Asano, R.; Yano, T. Negative growth control of osteosarcoma cell by Bowman-Birk protease inhibitor from soybean; involvement of connexin 43. Cancer Lett. 2007, 253, 249–257. [Google Scholar] [CrossRef]
- Wang, W.; Bringe, N.A.; Berhow, M.A.; De Mejia, E.G. β-conglycinins among sources of bioactives in hydrolysates of different soybean varieties that inhibit leukemia cells in vitro. J. Agric. Food Chem. 2008, 56, 4012–4020. [Google Scholar] [CrossRef]
- Mehdad, A.; Brumana, G.; Souza, A.A.; Barbosa, J.A.R.G.; Ventura, M.M.; de Freitas, S.M. A bowman–birk inhibitor induces apoptosis in human breast adenocarcinoma through mitochondrial impairment and oxidative damage following proteasome 20S inhibition. Cell Death Discov. 2016, 2, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Joanitti, G.A.; Azevedo, R.B.; Freitas, S.M. Apoptosis and lysosome membrane permeabilization induction on breast cancer cells by an anticarcinogenic Bowman-Birk protease inhibitor from Vigna unguiculata seeds. Cancer Lett. 2010, 293, 73–81. [Google Scholar] [CrossRef]
- Chan, Y.S.; Zhang, Y.; Ng, T.B. Brown kidney bean bowman-birk trypsin inhibitor is heat and pH stable and exhibits anti-proliferative activity. Appl. Biochem. Biotechnol. 2013, 169, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Magee, P.J.; Owusu-Apenten, R.; McCann, M.J.; Gill, C.I.; Rowland, I.R. Chickpea (Cicer arietinum) and other plant-derived protease inhibitor concentrates inhibit breast and prostate cancer cell proliferation in vitro. Nutr. Cancer 2012, 64, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A.; Gee, J.M.; Johnson, I.T.; MacKenzie, D.A.; Domoney, C. Pea (Pisum sativum L.) protease inhibitors from the Bowman—Birk class influence the growth of human colorectal adenocarcinoma HT29 cells in vitro. J. Agric. Food Chem. 2005, 53, 8979–8986. [Google Scholar] [CrossRef] [PubMed]
- Clemente, A.; Carmen Marín-Manzano, M.; Jiménez, E.; Carmen Arques, M.; Domoney, C. The anti-proliferative effect of TI1B, a major Bowman-Birk isoinhibitor from pea (Pisum sativum L.), on HT29 colon cancer cells is mediated through protease inhibition. Br. J. Nutr. 2012, 108. [Google Scholar] [CrossRef] [Green Version]
- Caccialupi, P.; Ceci, L.R.; Siciliano, R.A.; Pignone, D.; Clemente, A.; Sonnante, G. Bowman-Birk inhibitors in lentil: Heterologous expression, functional characterisation and anti-proliferative properties in human colon cancer cells. Food Chem. 2010, 120, 1058–1066. [Google Scholar] [CrossRef]
- Zhang, L.; Chen, X.; Wu, Y.; Zhou, M.; Ma, C.; Xi, X.; Chen, T.; Walker, B.; Shaw, C.; Wang, L. A Bowman-Birk type chymotrypsin inhibitor peptide from the amphibian, Hylarana erythraea. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef] [Green Version]
- Lyu, P.; Ge, L.; Ma, R.; Wei, R.; McCrudden, C.M.; Chen, T.; Shaw, C.; Kwok, H.F. Identification and pharmaceutical evaluation of novel frog skin-derived serine proteinase inhibitor peptide–PE-BBI (Pelophylax esculentus Bowman-Birk inhibitor) for the potential treatment of cancer. Sci. Rep. 2018, 8. [Google Scholar] [CrossRef]
- Chen, X.; Chen, D.; Huang, L.; Chen, X.; Zhou, M.; Xi, X.; Ma, C.; Chen, T.; Wang, L. Identification and Target-Modification of SL-BBI: A Novel Bowman-Birk Type Trypsin Inhibitor from Sylvirana latouchii. Biomolecules 2020, 10, 1254. [Google Scholar] [CrossRef]
- Yavelow, J.; Collins, M.; Birk, Y.; Troll, W.; Kennedy, A.R. Nanomolar concentrations of Bowman-Birk soybean protease inhibitor suppress x-ray-induced transformation in vitro. Proc. Natl. Acad. Sci. USA 1985, 82, 5395–5399. [Google Scholar] [CrossRef] [Green Version]
- Ware, J.H.; Wan, X.S.; Rubin, H.; Schechter, N.M.; Kennedy, A.R. Soybean Bowman-Birk protease inhibitor is a highly effective inhibitor of human mast cell chymase. Arch. Biochem. Biophys. 1997, 344, 133–138. [Google Scholar] [CrossRef]
- Newberne, P.M.; Billings, P.C. Preparation and production of a cancer chemopreventive agent, bowman-birk inhibitor concentrate. Nutr. Cancer 1993, 19, 281–302. [Google Scholar] [CrossRef]
- Lin, L.L.; Mick, R.; Ware, J.; Metz, J.; Lustig, R.; Vapiwala, N.; Rengan, R.; Kennedy, A.R. Phase I randomized double-blind placebo-controlled single-dose safety studies of Bowman-Birk inhibitor concentrate. Oncol. Lett. 2014, 7, 1151–1158. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Armstrong, W.B.; Taylor, T.H.; Kennedy, A.R.; Melrose, R.J.; Messadi, D.V.; Gu, M.; Le, A.D.; Perloff, M.; Civantos, F.; Goodwin, W.J.; et al. Bowman birk inhibitor concentrate and oral leukoplakia: A randomized phase IIb trial. Cancer Prev. Res. 2013, 6, 410–418. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, X.; Lu, L.; Anderson, K.; Ware, J.; Kennedy, A. Urinary excretion of Bowman-Birk inhibitor in humans after soy consumption as determined by a monoclonal antibody-based immunoassay. Cancer Epidemiol. Biomark. Prev. 2000, 7, 741–747. [Google Scholar]
- Billings, P.C.; Brandon, D.L.; Habres, J.M. Internalisation of the Bowman-Birk protease inhibitor by intestinal epithelial cells. Eur. J. Cancer Clin. Oncol. 1991, 27, 903–908. [Google Scholar] [CrossRef]
- Clair, W.H.S.; Clair, D.K.S. Effect of the Bowman-Birk Protease Inhibitor on the Expression of Oncogenes in the Irradiated Rat Colon. Cancer Res. 1991, 51, 4539–4543. [Google Scholar]
- Caggana, M.; Kennedy, A.R. C-fos mrna levels are reduced in the presence of antipain and bowman-birk inhibitor. Carcinogenesis 1989, 10, 2145–2148. [Google Scholar] [CrossRef]
- Giltrap, A.M.; Cergol, K.M.; Pang, A.; Britton, W.J.; Payne, R.J. Total synthesis of fellutamide b and deoxy-fellutamides B, C, and D. Mar. Drugs 2013, 11, 2382–2397. [Google Scholar] [CrossRef] [Green Version]
- Dittmann, K.; Mayer, C.; Kehlbach, R.; Rodemann, H.P. The radioprotector Bowman-Birk proteinase inhibitor stimulates DNA repair via epidermal growth factor receptor phosphorylation and nuclear transport. Radiother. Oncol. 2008, 86, 375–382. [Google Scholar] [CrossRef]
- Fereidunian, A.; Sadeghalvad, M.; Oscoie, M.O.; Mostafaie, A. Soybean bowman-birk protease inhibitor (BBI): Identification of the mechanisms of BBI suppressive effect on growth of two adenocarcinoma cell lines: AGS and HT29. Arch. Med. Res. 2014, 45, 455–461. [Google Scholar] [CrossRef]
- Rasouli, H.; Parvaneh, S.; Mahnam, A.; Rastegari-Pouyani, M.; Hoseinkhani, Z.; Mansouri, K. Anti-angiogenic potential of trypsin inhibitor purified from Cucumis melo seeds: Homology modeling and molecular docking perspective. Int. J. Biol. Macromol. 2017, 96, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Tomé, S.; Hernández-Ledesma, B. Current state of art after twenty years of the discovery of bioactive peptide lunasin. Food Res. Int. 2019, 116, 71–78. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.; Wong, J.; Ng, T. Trypsin-Chymotrypsin Inhibitors from Vigna mungo Seeds. Protein Pept. Lett. 2009, 16, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Safavi, F.; Rostami, A. Role of serine proteases in inflammation: Bowman-Birk protease inhibitor (BBI) as a potential therapy for autoimmune diseases. Exp. Mol. Pathol. 2012, 93, 428–433. [Google Scholar] [CrossRef]
- Sadeghalvad, M.; Mohammadi-Motlagh, H.R.; Karaji, A.G.; Mostafaie, A. In vivo anti-inflammatory efficacy of the combined Bowman-Birk trypsin inhibitor and genistein isoflavone, two biological compounds from soybean. J. Biochem. Mol. Toxicol. 2019, 33, 1–9. [Google Scholar] [CrossRef]
- Yanagita, M.; Kobayashi, R.; Kashiwagi, Y.; Shimabukuro, Y.; Murakami, S. Thrombin regulates the function of human blood dendritic cells. Biochem. Biophys. Res. Commun. 2007, 364, 318–324. [Google Scholar] [CrossRef]
- Lefrançais, E.; Roga, S.; Gautier, V.; Gonzalez-de-Peredo, A.; Monsarrat, B.; Girard, J.P.; Cayrol, C. IL-33 is processed into mature bioactive forms by neutrophil elastase and cathepsin G. Proc. Natl. Acad. Sci. USA 2012, 109, 1673–1678. [Google Scholar] [CrossRef] [Green Version]
- Novick, D.; Rubinstein, M.; Azam, T.; Rabinkov, A.; Dinarello, C.A.; Kim, S.H. Proteinase 3 is an IL-32 binding protein. Proc. Natl. Acad. Sci. USA 2006, 103, 3316–3321. [Google Scholar] [CrossRef] [Green Version]
- Müllbacher, A.; Waring, P.; Hla, R.T.; Tran, T.; Chin, S.; Stehle, T.; Museteanu, C.; Simon, M.M. Granzymes are the essential downstream effector molecules for the control of primary virus infections by cytolytic leukocytes. Proc. Natl. Acad. Sci. USA 1999, 96, 13950–13955. [Google Scholar] [CrossRef] [Green Version]
- Clemente, A.; Marin-Manzano, M.C.; Arques, M.C.; Domoney, C. Bowman-Birk Inhibitors from Legumes: Utilisation in Disease Prevention and Therapy. In Bioactive Food Peptides in Health and Disease; InTechOpen Limited: London, UK, 2013. [Google Scholar] [CrossRef] [Green Version]
- Frenkel, K.; Chrzan, K.; Ryan, C.A.; Wiesner, R.; Troll, W. Chymotrypsin-specific protease inhibitors decrease H2O2 formation by activated human polymorphonuclear leukocytes. Carcinogenesis 1987, 8, 1207–1212. [Google Scholar] [CrossRef]
- Ware, J.H.; Wan, X.S.; Kennedy, A.R. Bowman-Birk inhibitor suppresses production of superoxide anion radicals in differentiated HL-60 cells. Nutr. Cancer 1999, 33, 174–177. [Google Scholar] [CrossRef] [PubMed]
- Juritsch, A.F.; Moreau, R. Role of soybean-derived bioactive compounds in inflammatory bowel disease. Nutr. Rev. 2018, 76, 618–638. [Google Scholar] [CrossRef] [PubMed]
- Utrilla, M.P.; Peinado, M.J.; Ruiz, R.; Rodriguez-Nogales, A.; Algieri, F.; Rodriguez-Cabezas, M.E.; Clemente, A.; Galvez, J.; Rubio, L.A. Pea (Pisum sativum L.) seed albumin extracts show anti-inflammatory effect in the DSS model of mouse colitis. Mol. Nutr. Food Res. 2015, 59, 807–819. [Google Scholar] [CrossRef] [PubMed]
- Lichtenstein, G.R.; Deren, J.J.; Katz, S.; Lewis, J.D.; Kennedy, A.R.; Ware, J.H. Bowman-Birk inhibitor concentrate: A novel therapeutic agent for patients with active ulcerative colitis. Dig. Dis. Sci. 2008, 53, 175–180. [Google Scholar] [CrossRef]
- Moussa, L.; Bézirard, V.; Salvador-Cartier, C.; Bacquié, V.; Lencina, C.; Lévêque, M.; Braniste, V.; Ménard, S.; Théodorou, V.; Houdeau, E. A low dose of fermented soy germ alleviates gut barrier injury, hyperalgesia and faecal protease activity in a rat model of inflammatory bowel disease. PLoS ONE 2012, 7, 49547. [Google Scholar] [CrossRef] [Green Version]
- A’t Hart, B.; Amor, S. The use of animal models to investigate the pathogenesis of neuroinflammatory disorders of the central nervous system. Curr. Opin. Neurol. 2003, 16, 375–383. [Google Scholar] [CrossRef]
- Gran, B.; Tabibzadeh, N.; Martin, A.; Ventura, E.S.; Ware, J.H.; Zhang, G.X.; Parr, J.L.; Kennedy, A.R.; Rostami, A.M. The protease inhibitor, Bowman-Birk Inhibitor, suppresses experimental autoimmune encephalomyelitis: A potential oral therapy for multiple sclerosis. Mult. Scler. 2006, 12, 688–697. [Google Scholar] [CrossRef]
- Dai, H.; Ciric, B.; Zhang, G.X.; Rostami, A. Interleukin-10 plays a crucial role in suppression of experimental autoimmune encephalomyelitis by Bowman-Birk inhibitor. J. Neuroimmunol. 2012, 245, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Safavi, F.; Thome, R.; Li, Z.; Wang, L.; Rasouli, J.; Ciric, B.; Zhang, G.X.; Rostami, A. A serine protease inhibitor induces type 1 regulatory T cells through IFN-γ/STAT1 signaling. Cell. Mol. Immunol. 2020, 2–4. [Google Scholar] [CrossRef]
- Jin, T.; Yu, H.; Wang, D.; Zhang, H.; Zhang, B.; Quezada, H.C.; Zhu, J.; Zhu, W. Bowman-Birk inhibitor concentrate suppresses experimental autoimmune neuritis via shifting macrophages from M1 to M2 subtype. Immunol. Lett. 2016, 171, 15–25. [Google Scholar] [CrossRef]
- Dimachkie, M.M.; Barohn, R.J. Guillain-Barré syndrome and variants. Neurol. Clin. 2013, 31, 491–510. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, J.; Mix, E.; Link, H. Cytokine production and the pathogenesis of experimental autoimmune neuritis and Gulllain-Barre syndrome. J. Neuroimmunol. 1998, 84, 40–52. [Google Scholar] [CrossRef]
- Akbari, S.; Akrami, H.; Mostafaei, A.; Kiani, S. Bowman-Birk inhibitor modifies transcription of autophagy and apoptosis genes in an in vitro model of Alzheimer’s disorder. J. Cell. Biochem. 2019, 120, 11150–11157. [Google Scholar] [CrossRef] [PubMed]
- Salmon, A.L.; Cross, L.J.M.; Irvine, A.E.; Lappin, T.R.J.; Dathell, M.; Krausell, G.; Canning, P.; Thim, L.; Beyermann, M.; Rothemund, S.; et al. Peptide Leucine Arginine, a Potent Immunomodulatory Peptide Isolated and Structurally Characterized from the Skin of the Northern Leopard Frog, Rana pipiens. J. Biol. Chem. 2001, 276, 10145–10152. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cairns, J.A. Inhibitors of mast cell tryptase beta as therapeutics for the treatment of asthma and inflammatory disorders. Pulm. Pharmacol. Ther. 2005, 18, 55–66. [Google Scholar] [CrossRef] [PubMed]
- Rothemund, S.; Sönnichsen, F.D.; Polte, T. Therapeutic potential of the peptide leucine arginine as a new nonplant Bowman-Birk-like serine protease inhibitor. J. Med. Chem. 2013, 56, 6732–6744. [Google Scholar] [CrossRef] [PubMed]
- Graham, C.; Irvine, A.E.; McClean, S.; Richter, S.C.; Flatt, P.R.; Shaw, C. Peptide Tyrosine Arginine, a potent immunomodulatory peptide isolated and structurally characterized from the skin secretions of the dusky gopher frog, Rana sevosa. Peptides 2005, 26, 737–743. [Google Scholar] [CrossRef]
- Arbogast, S.; Smith, J.; Matuszczak, Y.; Hardin, B.J.; Moylan, J.S.; Smith, J.D.; Ware, J.; Kennedy, A.R.; Reid, M.B. Bowman-Birk inhibitor concentrate prevents atrophy, weakness, and oxidative stress in soleus muscle of hindlimb-unloaded mice. J. Appl. Physiol. 2007, 102, 956–964. [Google Scholar] [CrossRef] [Green Version]
- Larionova, N.V.; Malykh, E.V.; Villemson, A.L.; Krasota, A.J.; Duchene, D.; Ollivon, M.; Gernet, M.V.; Belousova, R.V.; Shen, W.C.; Larionova, N.I. Effect of membranotropic and mucoadhesive formulations of protein proteinase inhibitors on bovine herpes virus-1 reproduction. Int. J. Pharm. 2003, 256, 191–198. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, X.Q.; Zhang, B.; Gu, J.; Meng, F.Z.; Liu, H.; Zhou, L.; Wang, X.; Hou, W.; Ho, W.Z. Bowman-birk inhibitor suppresses herpes simplex virus type 2 infection of human cervical epithelial cells. Viruses 2018, 10, 557. [Google Scholar] [CrossRef] [Green Version]
- Ma, T.C.; Zhou, R.H.; Wang, X.; Li, J.L.; Sang, M.; Zhou, L.; Zhuang, K.; Hou, W.; Guo, D.Y.; Ho, W.Z. Soybean-derived Bowman-Birk inhibitor (BBI) inhibits HIV replication in macrophages. Sci. Rep. 2016, 6. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, T.C.; Guo, L.; Zhou, R.H.; Wang, X.; Liu, J.B.; Li, J.L.; Zhou, Y.; Hou, W.; Ho, W.Z. Soybean-derived Bowman-Birk inhibitor (BBI) blocks HIV entry into macrophages. Virology 2018, 513, 91–97. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.; Tzi, B.N. A trypsin-chymotrypsin inhibitor with antiproliferative activity from small glossy black soybeans. Planta Med. 2009, 75, 550–556. [Google Scholar] [CrossRef] [PubMed]
- Ye, X.Y.; Ng, T.B.; Rao, P.F. A bowman-birk-type trypsin-chymotrypsin inhibitor from broad beans. Biochem. Biophys. Res. Commun. 2001, 289, 91–96. [Google Scholar] [CrossRef] [PubMed]
- Qu, L.J.; Chen, J.; Liu, M.; Pan, N.; Okamoto, H.; Lin, Z.; Li, C.; Li, D.; Wang, J.; Zhu, G.; et al. Molecular Cloning and Functional Analysis of a Novel Type of Bowman-Birk Inhibitor Gene Family in Rice. Plant Physiol. 2003, 133, 560–570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chilosi, G.; Caruso, C.; Caporale, C.; Leonardi, L.; Bertini, L.; Buzi, A.; Nobile, M.; Magro, P.; Buonocore, V. Antifungal Activity of a Bowman-Birk-type Trypsin Inhibitor from Wheat Kernel. J. Phytopathol. 2000, 148, 477–481. [Google Scholar] [CrossRef]
- Hou, S.; Jamieson, P.; He, P. The cloak, dagger, and shield: Proteases in plant–pathogen interactions. Biochem. J. 2018, 475, 2491–2509. [Google Scholar] [CrossRef]
- Zhang, C.; Fang, H.; Shi, X.; He, F.; Wang, R.; Fan, J.; Bai, P.; Wang, J.; Park, C.; Bellizzi, M.; et al. A Fungal Effector and a Rice NLR Protein Have Antagonistic Effects on a Bowman-Birk Trypsin Inhibitor. Plant Biotechnol. J. 2020. [Google Scholar] [CrossRef]
- Rohrmeier, T.; Lehle, L. WIP1, a wound-inducible gene from maize with homology to Bowman-Birk proteinase inhibitors. Plant Mol. Biol. 1993, 22, 783–792. [Google Scholar] [CrossRef]
- Singh, S.; Singh, A.; Kumar, S.; Mittal, P.; Singh, I.K. Protease inhibitors: Recent advancement in its usage as a potential biocontrol agent for insect pest management. Insect Sci. 2020, 27, 186–201. [Google Scholar] [CrossRef] [Green Version]
- Prasad, E.R.; Dutta-Gupta, A.; Padmasree, K. Insecticidal potential of Bowman-Birk proteinase inhibitors from red gram (Cajanus cajan) and black gram (Vigna mungo) against lepidopteran insect pests. Pestic. Biochem. Physiol. 2010, 98, 80–88. [Google Scholar] [CrossRef]
- Azzouz, H.; Cherqui, A.; Campan, E.D.M.; Rahbé, Y.; Duport, G.; Jouanin, L.; Kaiser, L.; Giordanengo, P. Effects of plant protease inhibitors, oryzacystatin I and soybean Bowman-Birk inhibitor, on the aphid Macrosiphum euphorbiae (Homoptera, Aphididae) and its parasitoid Aphelinus abdominalis (Hymenoptera, Aphelinidae). J. Insect Physiol. 2005, 51, 75–86. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.D.A.; Valencia-Jiménez, A.; Magalhães, C.P.; Prates, M.V.; Melo, J.A.T.; De Lima, L.M.; De Sales, M.P.; Nakasu, E.Y.T.; Da Silva, M.C.M.; Grossi-De-Sá, M.F. Effect of a Bowman-Birk proteinase inhibitor from Phaseolus coccineus on Hypothenemus hampei gut proteinases in vitro. J. Agric. Food Chem. 2007, 55, 10714–10719. [Google Scholar] [CrossRef] [PubMed]
- Francoa, O.L.; Dos Santos, R.C.; Batista, J.A.N.; Mendes, A.C.M.; De Araújo, M.A.M.; Monnerat, R.G.; Fátima Grossi-de-Sá, M.; De Freitas, S.M. Effects of black-eyed pea trypsin/chymotrypsin inhibitor on proteolytic activity and on development of Anthonomus grandis. Phytochemistry 2003, 63, 343–349. [Google Scholar] [CrossRef]
- Aguirre, C.; Valdés-Rodríguez, S.; Mendoza-Hernández, G.; Rojo-Domínguez, A.; Blanco-Labra, A. A novel 8.7 kDa protease inhibitor from chan seeds (Hyptis suaveolens L.) inhibits proteases from the larger grain borer Prostephanus truncatus (Coleoptera: Bostrichidae). Comp. Biochem. Physiol. B Biochem. Mol. Biol. 2004, 138, 81–89. [Google Scholar] [CrossRef]
- Oppert, B.; Morgan, T.D.; Culbertson, C.; Kramer, K.J. Dietary mixtures of cysteine and serine proteinase inhibitors exhibit synergistic toxicity toward the red flour beetle, Tribolium castaneum. Comp. Biochem. Physiol. Part C Comp. 1993, 105, 379–385. [Google Scholar] [CrossRef]
- Carvalho, A.F.; Santos-Neto, M.S.; Monteiro, H.S.A.; Freitas, S.M.; Morhy, L.; Nascimento, N.R.F.; Fonteles, M.C. BTCI enhances guanylin-induced natriuresis and promotes renal glomerular and tubular effects. Braz. J. Biol. 2008, 68, 149–154. [Google Scholar] [CrossRef] [Green Version]
- Álvares, A.; Schwartz, E.; Amaral, N.; Trindade, N.; Pedrino, G.; Silva, L.; de Freitas, S. Bowman-Birk Protease Inhibitor from Vigna unguiculata Seeds Enhances the Action of Bradykinin-Related Peptides. Molecules 2014, 19, 17536–17558. [Google Scholar] [CrossRef] [Green Version]
- de Freitas, M.A.G.; Amaral, N.O.; Álvares, A.D.C.M.; de Oliveira, S.A.; Mehdad, A.; Honda, D.E.; Bessa, A.S.M.; Ramada, M.H.S.; Naves, L.M.; Pontes, C.N.R.; et al. Blood pressure-lowering effects of a Bowman-Birk inhibitor and its derived peptides in normotensive and hypertensive rats. Sci. Rep. 2020, 10, 1–15. [Google Scholar] [CrossRef]
- Fekadu Gemede, H. Antinutritional Factors in Plant Foods: Potential Health Benefits and Adverse Effects. Int. J. Nutr. Food Sci. 2014, 3, 284. [Google Scholar] [CrossRef] [Green Version]
- Avilés-Gaxiola, S.; Chuck-Hernández, C.; Serna Saldívar, S.O. Inactivation Methods of Trypsin Inhibitor in Legumes: A Review. J. Food Sci. 2018, 83, 17–29. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hernández-Ledesma, B.; Hsieh, C.C.; de Lumen, B.O. Lunasin and Bowman-Birk protease inhibitor (BBI) in US commercial soy foods. Food Chem. 2009, 115, 574–580. [Google Scholar] [CrossRef]
- Chen, Z.; Chen, Y.; Xue, Z.; Gao, X.; Jia, Y.; Wang, Y.; Lu, Y.; Zhang, J.; Zhang, M.; Chen, H. Insight into the inactivation mechanism of soybean Bowman-Birk trypsin inhibitor (BBTI) induced by epigallocatechin gallate and epigallocatechin: Fluorescence, thermodynamics and docking studies. Food Chem. 2020, 303. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Xiang, Q.; Liu, X.; Ding, T.; Zhang, X.; Zhai, Y.; Bai, Y. Inactivation of soybean trypsin inhibitor by dielectric-barrier discharge (DBD) plasma. Food Chem. 2017, 232, 515–522. [Google Scholar] [CrossRef]
- Chan, L.Y.; Craik, D.J.; Daly, N.L. Dual-targeting anti-angiogenic cyclic peptides as potential drug leads for cancer therapy. Sci. Rep. 2016, 6, 1–13. [Google Scholar] [CrossRef] [Green Version]
- Durek, T.; Cromm, P.M.; White, A.M.; Schroeder, C.I.; Kaas, Q.; Weidmann, J.; Ahmad Fuaad, A.; Cheneval, O.; Harvey, P.J.; Daly, N.L.; et al. Development of Novel Melanocortin Receptor Agonists Based on the Cyclic Peptide Framework of Sunflower Trypsin Inhibitor-1. J. Med. Chem. 2018, 61, 3674–3684. [Google Scholar] [CrossRef]
- Qiu, Y.; Taichi, M.; Wei, N.; Yang, H.; Luo, K.Q.; Tam, J.P. An Orally Active Bradykinin B1 Receptor Antagonist Engineered as a Bifunctional Chimera of Sunflower Trypsin Inhibitor. J. Med. Chem. 2017, 60, 504–510. [Google Scholar] [CrossRef]
- Gunasekera, S.; Fernandes-Cerqueira, C.; Wennmalm, S.; Wähämaa, H.; Sommarin, Y.; Catrina, A.I.; Jakobsson, P.-J.; Göransson, U. Stabilized Cyclic Peptides as Scavengers of Autoantibodies: Neutralization of Anticitrullinated Protein/Peptide Antibodies in Rheumatoid Arthritis. ACS Chem. Biol. 2018. [Google Scholar] [CrossRef]
- Chaudhuri, D.; Aboye, T.; Camarero, J.A. Using backbone-cyclized Cys-rich polypeptides as molecular scaffolds to target protein–protein interactions. Biochem. J. 2019, 476, 67–83. [Google Scholar] [CrossRef]
- Northfield, S.E.; Wang, C.K.; Schroeder, C.I.; Durek, T.; Kan, M.W.; Swedberg, J.E.; Craik, D.J. Disulfide-rich macrocyclic peptides as templates in drug design. Eur. J. Med. Chem. 2014, 77, 248–257. [Google Scholar] [CrossRef]
- Franke, B.; Mylne, J.S.; Rosengren, K.J. Buried treasure: Biosynthesis, structures and applications of cyclic peptides hidden in seed storage albumins. Nat. Prod. Rep. 2018, 35, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Mangoni, M.L.; Papo, N.; Mignogna, G.; Andreu, D.; Shai, Y.; Barra, D.; Simmaco, M. Ranacyclins, a New Family of Short Cyclic Antimicrobial Peptides: Biological Function, Mode of Action, and Parameters Involved in Target Specificity. Biochemistry 2003, 42, 14023–14035. [Google Scholar] [CrossRef] [PubMed]
- James, A.M.; Jayasena, A.S.; Zhang, J.; Berkowitz, O.; Secco, D.; Knott, G.J.; Whelan, J.; Bond, C.S.; Mylne, J.S. Evidence for ancient origins of bowman-birk inhibitors from Selaginella moellendorffii. Plant Cell 2017, 29, 461–473. [Google Scholar] [CrossRef] [PubMed]
- Elliott, A.G.; Delay, C.; Liu, H.; Phua, Z.; Johan Rosengren, K.; Benfield, A.H.; Panero, J.L.; Colgrave, M.L.; Jayasena, A.S.; Dunse, K.M.; et al. Evolutionary origins of a bioactive peptide buried within Preproalbumin. Plant Cell 2014, 26, 981–995. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, K.; Jiang, Y.; Xie, Y.; Liu, H.; Liu, R.; Zhao, Z.; Lai, R.; Yang, L. Small-Anion Selective Transmembrane “holes” Induced by an Antimicrobial Peptide Too Short to Span Membranes. J. Phys. Chem. B 2015, 119, 8553–8560. [Google Scholar] [CrossRef]
- Gitlin-Domagalska, A.; Dębowski, D.; Gucwa, K.; Starego, D.; Ptaszyńska, N.; Sieradzan, A.; Karczyńska, A.; Samsonov, S.A.; Mangold, M.; Gütschow, M.; et al. Truncation of Huia versabilis Bowman-Birk inhibitor increases its selectivity, matriptase-1 inhibitory activity and proteolytic stability. Biochimie 2020, 171–172, 178–186. [Google Scholar] [CrossRef]
- Song, G.; Zhou, M.; Chen, W.; Chen, T.; Walker, B.; Shaw, C. HV-BBI-A novel amphibian skin Bowman-Birk-like trypsin inhibitor. Biochem. Biophys. Res. Commun. 2008, 372, 191–196. [Google Scholar] [CrossRef]
- Grudnik, P.; Dębowski, D.; Łęgowska, A.; Malicki, S.; Golik, P.; Karna, N.; Rolka, K.; Dubin, G. Atomic resolution crystal structure of HV-BBI protease inhibitor from amphibian skin in complex with bovine trypsin. Proteins Struct. Funct. Bioinforma. 2015, 83, 582–589. [Google Scholar] [CrossRef]
- Dębowski, D.; Łukajtis, R.; Łęgowska, A.; Karna, N.; Pikuła, M.; Wysocka, M.; Maliszewska, I.; Sieńczyk, M.; Lesner, A.; Rolka, K. Inhibitory and antimicrobial activities of OGTI and HV-BBI peptides, fragments and analogs derived from amphibian skin. Peptides 2012, 35, 276–284. [Google Scholar] [CrossRef]
- Lin, Y.; Hang, H.; Chen, T.; Zhou, M.; Wang, L.; Shaw, C. PLR-HL: A Novel Amphibian Bowman-Birk-type Trypsin Inhibitor from the Skin Secretion of the Broad-folded Frog, Hylarana latouchii. Chem. Biol. Drug Des. 2016, 87, 91–100. [Google Scholar] [CrossRef]
- Wang, M.; Wang, L.; Chen, T.; Walker, B.; Zhou, M.; Sui, D.; Conlon, J.M.; Shaw, C. Identification and molecular cloning of a novel amphibian Bowman Birk-type trypsin inhibitor from the skin of the Hejiang Odorous Frog; Odorrana hejiangensis. Peptides 2012, 33, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Long, Q.; Xu, Y.; Guo, S.; Chen, T.; Wang, L.; Zhou, M.; Zhang, Y.; Shaw, C.; Walker, B. A structural and functional analogue of a Bowman-Birk-type protease inhibitor from Odorrana schmackeri. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Reihill, J.A.; Ouyang, X.; Yang, Z.; Douglas, L.E.J.; Zhou, M.; Chen, T.; Lorraine Martin, S. A novel serine protease inhibitor PE-BBI ameliorates cockroach extract-mediated airway epithelial barrier dysfunction. Biomolecules 2020, 10, 515. [Google Scholar] [CrossRef] [PubMed]

| Source and Name | Inhibitory Activity Expressed as Ki (nM) (If not Stated Otherwise) (Enzyme); PDB Code |
|---|---|
| Glycine max (Soybean) Bowman-Birk inhibitor (various isoinhibitors) BBI | ranged from 3.2 to 29.8 (trypsin) [65,66]; 3.3 (chymotrypsin) [66]; IC50 20 μM (proteasome ChT-L) [67]. Crystal structure with trypsin 1K9B [68]; ternary complex with trypsin 1D6R [62]; crystal structure with chymotrypsin 5J4Q; solution structure [69]. |
| Vigna unguiculata (Black-eyed pea) trypsin and chymotrypsin inhibitor BTCI | 20 (trypsin) and 0.42 (trypsin, using surface plasmon resonance); 120 (chymotrypsin) and 0.41 (chymotrypsin, using surface plasmon resonance) [63]; 100 (proteasome T-L); 700 (proteasome ChT-L); 1400 (proteasome C-L) [60]. Crystal structure 2R33 [53], structure with trypsin 2G81 [63], structure with trypsin and chymotrypsin 3RU4 [70] |
| Geoffroea decorticans trypsin inhibitor GdTI | 2.1 (trypsin) [71]; 0.18 μM (IC50, α-glucosidase) [71] |
| Apios americana trypsin inhibitor AATI | 3 (trypsin); 1000 (chymotrypsin) [72] |
| Inhibitor from Lupinus albus (White lupin) | 4.2 (trypsin) [73] |
| Luetzelburgia Auriculata ((Allemao) Ducke) Bowman-Birk inhibitor LzaBBI | 0.86 (trypsin); 1.2 (chymotrypsin) [74] |
| Inhibitors from Cajanus cajan (Red gram) | 292 (trypsin); 2265 (chymotrypsin) [75] 272 (trypsin); 3725 (chymotrypsin) [76] |
| Dolichus biflorus Bowman-Birk inhibitor | 40 (trypsin); 480 (chymotrypsin) [77] |
| Vigna mungo (Black gram) protease inhibitor BgPI | 309.8 (trypsin); 10,770 (chymotrypsin) [78] |
| Twelve Lathyrus sativus Bowman-Birk isoinhibitors Ls_BBI | ranged from 6.9 to 30.8 (trypsin); ranged from 11.7 to 26.0 (chymotrypsin); Ls_BBI3c 54.6 (elastase) [79] |
| Phaseolus acutifolius (Tepary bean) protease inhibitor TBPI | 280 (trypsin); 68 (chymotrypsin) [80] |
| Clitoria fairchildiana (Sombreiro) protease inhibitor CFPI | 0.33 (trypsin); 0.15 (chymotrypsin) [81] |
| Dioclea glabra trypsin inhibitor DgTI | 0.5 (trypsin) [82] |
| Vicia faba (Faba bean) trypsin inhibitor VFTI-G1 | 20.4 (trypsin) [83] |
| Pisum sativum (Winter peas) trypsin isoinhibitors PsTI | ranged from 1.2 to 0.84 (trypsin); ranged from 21 to 15 (chymotrypsin) [84]; crystal structure 1PBI [59] |
| Rhynchosia sublobata Bowman-Birk inhibitors RsBBI | 128.5 (trypsin); 807.8 (chymotrypsin) [55] |
| Lens culinaris (Lentil) trypsin inhibitor LCTI | 0.54 (trypsin); 7.25 (chymotrypsin). Solution structure 2AIH [85] |
| Cratylia mollis (Camaratu bean) trypsin inhibitor CmTI2 | 1.4 (trypsin) [86] |
| Medicago scutellata (Snail medic) trypsin inhibitor MsTI | 1.8 (trypsin) [87]. Crystal structure with trypsin 2ILN [88]. |
| Torresea cearensis trypsin inhibitor TcTI | 1 (trypsin); 36 (plasmin); 50 (chymotrypsin); 1450 factor XIIa [89] |
| SFTI-1 from Helianthus annuus and Its Synthetic Analogs | |
|---|---|
| Name (If Given) and Sequence | Ki (nM) (Enzyme) (Structure in PDB, If Reported) |
| Bicyclic(native) SFTI-1 &1GRC(&2)TKSIPPIC(&2)FPD&1 | 0.1 (trypsin) [49] (crystal structure 1SFI [49], 1JBL solution structure [91]); 102 (matriptase) (crystal structure with matriptase catalytic domain 3P8F [92]); 218 (matriptase-2) [93]; 0.15 (cathepsin G); 105,000 (elastase); 7400 (chymotrypsin); 136,000 (thrombin) [49]; 143 (KLK5); 25.1 (KLK14) [94]; 4960 (mesotrypsin) [95] |
| Monocyclic SFTI-1 GRC(&)TKSIPPIC(&)FPD | 0.02698 (trypsin) [96] (solution structure 1JBN [91]);703 (matriptase) [97]; 61 (matriptase); 1365 (matriptase-2) [93]; 26,980 (20S proteasome, ChT-L); 29,090 (20S proteasome, C-L) [98] |
| GRC(&)TKSIAPIC(&)FPD | 27 (matriptase); 0.035 (trypsin) [99] |
| GRC(&)TKSIPAIC(&)FPD | 370 (matriptase); 0.0017 (trypsin) [99] |
| GRC(&)TKSIPPIC(&)FAD | 240 (matriptase); 0.0037 (trypsin) [99] |
| GRC(&)TKSIPPIC(&)FPA | 1500 (matriptase); 0.01 (trypsin) [99] |
| &1GRC(&2)TRSIPPIC(&2)FPD&1 | 19 (matriptase-2); 269 (matriptase); 13.4 (trypsin) [93] |
| GRC(&)TRSIPPIC(&)FPD | 91 (matriptase); 115 (matriptase-2); 15.2 (trypsin) [93] |
| GRC(&)TFSIPPIC(&)FPD | 0.5 (chymotrypsin) [100] |
| GRC(&)TXSIPPIC(&)FPD X = 4-fluoro-l-phenylalanine | 0.03 (chymotrypsin) [101] (Ki calculated as a reciprocal value of originally published Ka 3.0 × 1010 M−1) |
| GRC(&)TVSIPPIC(&)FPD | 71 (neutrophil elastase) [102] |
| GRC(&)TKSIPPRC(&)FPD | 6.4 (matriptase); 0.0038 (trypsin) [99] |
| GRC(&)TKSIPPKC(&)FPD | 40 (matriptase); 0.0057 (trypsin) [99] |
| GKC(&)TKSIPPIC(&)FPD | 1200 (matriptase); 0.002 (trypsin) [99] |
| GRC(&)TRSIPXIC(&)FPD X = Abu (aminobutyric acid) | 0.5 (trypsin) [100] |
| &1GrC(&2)TRSIPPIC(&2)FPD&1 r = d-Arg | 280 (matriptase-2); 63,360 (matriptase) [93] |
| GrC(&)TRSIPPIC(&)FPD r = d-Arg | 433 (matriptase-2); 76,310 (matriptase) [93] |
| &1KRC(&2)TRSIPPIC(&2)FPD&1 | 127 (matriptase-2); 532 (matriptase) [103] |
| &1GRC(&2)TKSIPPRC(&2)HPD&1 | 3.6 (matriptase) [97] |
| SDMI-1 GRC(&)TKSIPPRC(&)HPD | 11.2 (matriptase) [97] |
| SDMI-3 KRC(&)TKSIPPRC(&)HPD | 2.1 (matriptase) [97] |
| &1KRC(&2)TKSIPPRC(&2)HPD&1 | 4.1 (matriptase) [97] |
| K(&1)RC(&2)TKSIPPRC(&2)HPD&1 | 4.1 (matriptase) [97] |
| K(&1)RC(&2)TKSIPPRC(&2)HP&1 | 7.2 (matriptase) [97] |
| K(&1)RC(&2)TKSIPPRC(&2)H&1 | 2.6 (matriptase) [97] |
| &1GRC(&2)TRSIPPRC(&2)HPD&1 | 15 (matriptase-2); 4.9 (matriptase) [103] |
| GRC(&)TRSIPPRC(&)HPD | 127 (matriptase-2); 532 (matriptase) [103] |
| &1KRC(&2)TRSIPPRC(&2)HPD&1 | 102 (matriptase-2); 8.3 (matriptase); 9 (trypsin) [103] |
| K(&1)RC(&2)TRSIPPRC(&2)HPD&1 | 257 (matriptase-2); 2.6 (matriptase); 5.1 (trypsin) [103] |
| KRC(&)TRSIPPRC(&)HPD | 318 (matriptase-2); 4.3 (matriptase); 5.3 (trypsin) [103] |
| &1GRC(&2)TRSIPPHC(&2)WPD&1 | 51 (KLK5) [104] |
| &1GRC(&2)TRSYPPIC(&2)FPD&1 | 214 (thrombin) [105] |
| GVC(&)TLSIPPIC(&)FPD | 300 (pancreatic elastase) [106] |
| &1GRC(&2)YKSKPPIC(&2)FPD&1 | 0.05 (plasmin, crystal structure 6D3X); 160 (trypsin); 29,000 (cathepsin G) [107] |
| &1GRC(&2)QXSEPPEC(&2)FPD&1 X= 4-chloro-l-phenylalanine | 1.8 (chymase); 330 (chymotrypsin); 150 (cathepsin G) [108] |
| &1GTC(&2) X1 X2SDPPIC(&2)FPN&1 X1 = norleucine; X2 = 4-guanidine-l-phenylalanine | 1.6 (cathepsin G) [109] |
| GRC(&)TXSIPPIC(&)FPD X = 4-guanidine-l-phenylalanine | 5.55 (chymotrypsin) [101] (Ki calculated as a reciprocal value of originally published Ka 1.8 108 M−1) |
| KRC(&)KKSIPPRC(&)HPD | 3.8 (furin) [110] |
| KRC(&)KKSIPPRC(&)F-NH2 | 0.49 (furin) [110] |
| &1GFC(&2)QRSIPPIC(&2)FPD&1 | 3.59 (KLK4 [111], crystal structure 4K1E [112]) |
| &1GFC(&2)QRSIPPIC(&2)FPN&1 | 0.04 (KLK4 [90], crystal structure 4KEL [113]) |
| &1GYC(&2)NRSYPPEC(&2)FPN&1 | 0.34 (KLK5); 18 (KLK14) [114] |
| &1GFC(&2)HRSYPPEC(&2)WPN&1 | 2.4 (KLK5, solution structure 6NOX) [114] 150 (KLK14) [114] |
| &1GKC(&2)LFSNPPIC(&2)FPN&1 | 0.14 (KLK7); 170 (chymotrypsin) [115] |
| &1GWC(&2)IRSKPPIC(&2)NPN&1 | 7.0 (KLK14); 19.9 (KLK4); 3200 (trypsin) [105] |
| SFMI-1 GIC(&)SRSLPPIC(&)IPD | 65 (MASP-1); 1030 (MASP-2); 260 (trypsin) [116] |
| SFMI-2 GYC(&)SRSYPPVC(&)IPD | 180 (MASP-2); 1000 (trypsin) [116] |
| GRC(&)TRSXPPIC(&)FPD X = 4,4′-biphenyl-l-alanine | 28 (mesotrypsin) [117] |
| &1GX1C(&2)YX2SYPPIC(&2)NPN&1 X1 = 4,4′-biphenyl-l-alanine; X2 = norvaline | 6.1 (proteinase 3); 16 (neutrophil elastase) [118] |
| &1GTC(&2)YXSYPPIC(&2)NPN&1X = Abu | 7.0 (proteinase 3); 3.2 (neutrophil elastase) [118] |
| GRC(&)TRSKKPIC(&)FPD | 310 (20S proteasome, ChT-L); 20,140 (20S proteasome, T-L); 680 (20S proteasome, C-L) [96] |
| &1GTC(&2)TRSIPPIC(&2)NPN&1 | 0.71 (trypsin [94], crystal structure 6BVH [119]) |
| RXC(&)TRSKKPIC(&)FPD X = N-arginine (peptoid monomer) | 80 (20S proteasome, ChT-L); 14,000 (20S proteasome, T-L); 140 (20S proteasome, C-L) [98] |
| O2Oc-GRC(&)TRSKKPIC(&)FPD | 310 (20S proteasome, ChT-L); 1150 (20S proteasome, T-L); 350 (20S proteasome, C-L) [98] |
| RGC(&1)TRSKKPIC(&2)GPGGGC(&2)TR--SKKPIC(&1)FPD | 30 (20S proteasome, ChT-L); 20 (20S proteasome, C-L) [98] |
| Inhibitor Source | Type of Cancer | Cell Line and/or Animal Model/Reference | The Observed Effect |
|---|---|---|---|
| Plant-Derived BBIs | |||
| Glycine max (Soybean) Bowman-Birk inhibitor BBI (or BBIC) | colorectal | HT29 cell line [66] | Inhibited proliferation at concentrations ranged from 31 μM to 125 μM, cell cycle arrest in the G0–G1 phase. |
| DMH-induced colon cancer in rat [138] | Suppressive effect on colon carcinogenesis. Diet supplemented with 0.1% and 0.5% of inhibitor. | ||
| dextran sulfate sodium-induced ulcerative colitis in mice [139] | Reduction of inflammation, lower mortality rate, delayed onset of mortality. Diet supplemented with 0.5% of inhibitor. | ||
| DMH-induced colorectal neoplasia in Swiss mice [140] | Protection from inflammatory processes and from the appearance of pre-malignant lesions. Diet supplemented with 0.1% of inhibitor. | ||
| DMH-induced colon carcinogenesis in mice [141] | Reduction of the incidence of adenocarcinomas of the colon by ∼50%. Diet supplemented with 0.1% of inhibitor. | ||
| prostate | LNCaP prostate cancer xenograft mouse model [142] | Suppressive effect on the tumor growth in nude mice and an increase of the serum PSA concentration. Diet supplemented with 0.1% of inhibitor. | |
| various normal and cancer cell lines, including LNCaP and PC-3 [143] | Cell growth inhibition at the concentration of 100 μg/mL;BBIC inhibited clonogenic survival. | ||
| patients with benign prostatic hyperplasia and lower urinary tract symptoms [144] | Phase I clinical trial. | ||
| N-methyl-N-nitrosourea + testosterone-induced prostate carcinogenesis in rats [145] | Inhibition of induced prostate carcinogenesis in the Wistar-Unilever rats. BBIC administered at 200 or 2000 mg/kg diet dose. | ||
| LNCaP cell line and LNCaP stem-like cells [146] | Combination of BBI and α-tocopheryl succinate results in cell growth inhibition and induction of apoptosis. BBI at the concentration of 200 μg/mL. | ||
| LNCaP cell line and the transgenic rats developing adenocarcinoma of the prostate [147] | Induction of Cx43 expression and apoptosis at the concentration of 500 μg/mL. Reduced progression of adenocarcinomas in the lateral prostate lobes in rats. | ||
| ovarian | A2780 cell line and its cisplatin-resistant sublines C30, C200 [148] | Suppression of the clonogenic cells survival and a boost of cisplatin-induced growth inhibition and/or cytotoxicity at the concentrations of 50 and 100 μg/mL. | |
| M5076 sarcoma xenograft mouse model [149,150] | Reduction of relative tumor weight associated with induced expression of Cx43. Diet supplemented with 0.5% BBI. | ||
| breast | MCF7 cell line [67,151,152] | Decreased clonogenic survival of cells at the concentration 100 μg/ml [151], with IC50 of about 35 μM [152]; downregulation of cyclin D1 and E1, upregulation of mitogen-activated protein kinase phosphatase 1 (MKP-1), and suppression of phosphorylated extracellular signal-related kinases (ERK1/2) activity upon treatment with 20 μM [67]. | |
| xenograft model of nude mice transplanted with MDA-MB-231 cells [153] | BBI injection at 20 mg/kg body weight shows no effect on tumor incidence. BBI protects lunasin, an actual bioactive agent, from digestion. | ||
| oral leukoplakia | patients with oral leukoplakia [154,155] | Phase IIa clinical trial. | |
| oral cavity | DMBA-induced oral carcinogenesis in hamster [156] | Suppression of the carcinogenesis at the concentrations ranging from 1% to 0.01%. | |
| head and neck carcinoma | SCC61 cell line [151] | Suppression of the clonogenic survival of cell line and enhancement of radiation-induced cell killing at the concentration of 100 μg/mL. | |
| hepatic | HepG2 cell line [152] | Inhibited proliferation with IC50 of about 140 μM. | |
| liver | DMH-induced liver carcinogenesis in mice [157] | Suppression of the DMH-induced carcinogenesis in the mouse liver and gastrointestinal tract. Diet supplemented with 0.5% and 0.1% inhibitor. | |
| osteosarcoma | U2OS cell line [158] | Cell growth inhibition, induction of Cx43, induction of apoptosis at the concentration 200 μg/ml; BBI-dependent negative growth control was based on cytostatic and cytotoxic effects. | |
| leukemia | L1210 cell line [159] | Cell growth inhibition with IC50 of about 22.5 μM. | |
| Vigna unguiculata (Black-eyed pea) trypsin and chymotrypsin inhibitor BTCI | breast | MCF-7 and/or MDA-MB-231 cell lines [160,161] | Cell growth inhibition, cytostatic effect at the G2/M phase, induction of apoptosis at the concentrations 100 μM (MDA-MB-231). |
| Phaseolus vulgaris (Kidney bean) Bowman-Birk inhibitor | breast | MCF7 cell line [162] | Inhibited proliferation with IC50 of about 71.5 μM. |
| prostate | LNCaP cell line [163] | Inhibited proliferation at the concentrations 200, 400 μg/mL. | |
| Vigna radiata (Mungbean) Bowman-Birk inhibitor | prostate | LNCaP cell line [163] | Inhibited proliferation at the concentrations 100, 200 μg/mL. |
| Cicer arietinum (Chickpea) Bowman-Birk inhibitor | prostate | PC-3 and LNCaP cell lines [163] | Inhibited proliferation at the concentrations 25–400 μg/mL. |
| breast | MDA-MB-231 cell line [163] | Inhibited proliferation at the concentrations 25–400 μg/mL. | |
| Pisum sativum (Pea) trypsin inhibitor TI1B | colorectal | HT29 cell line [164,165] | Inhibited proliferation with IC50 ranged from 32 μM (rTI1B) to 73 μM (rTI2B). |
| Vicia faba (Faba bean) trypsin inhibitor VFTI-G1 | hepatoma | HepG2 cell line [83] | Inhibited proliferation with IC50 of about 30 μM; induced chromatin condensation and cell apoptosis. |
| Lens culinaris (Lentil) Bowman-Birk inhibitor | colorectal | HT29 cell line [166] | Inhibited proliferation with IC50 of about 32 μM. |
| Macrotyloma axillare (Horsegram) Bowman-Birk inhibitor | colorectal | DMH-induced colorectal neoplasia in Swiss mice [140] | Protection from inflammatory processes and the appearance of pre-malignant lesions. Diet supplemented with 0.1% of inhibitor. |
| Animal-Derived BBLTIs | |||
| The skin secretion of Asian green frog, Hylarana erythraea | prostate | PC-3 cell line [167] | Inhibited proliferation at the concentration 1 mM. |
| lung | H157 cell line [167] | Inhibited proliferation at the concentration 1 mM. | |
| breast | MCF-7 cell line [167] | Inhibited proliferation at the concentration 1 mM. | |
| The skin secretion of frog Pelophylax esculentus | colorectal | DLD-1, DKS8, HCT116, and HKE3 cell lines [168] | Inhibited proliferation with IC50 50.1 µM, 9.8 µM, 35.4 µM, 50.2 µM, respectively. |
| The skin secretions of Pelophlax plancyi fukienesis (chimeric peptide called Tat-loop) | lung cancer | H460, H157 [32] | Inhibited proliferation at the concentration 100 µM. |
| The skin secretions of Sylvirana latouchii (F-SL analog) | human non-small cell lung cancer (NSCLC) | H157, H460, H838, and H23 [169] | Induced caspase 3/7 activation, which confirms induced apoptosis in H157 (IC50 of about 101.4 µM) and H838 (IC50 of about 59.74 µM). |
| breast | MCF-7 [169] | Inhibited proliferation with IC50 of about 201.7 µM. | |
| prostate | PC-3 [169] | Inhibited proliferation with IC50 of about 158.6 µM. | |
| Origin | Name (If Given) and Sequence | Ki (nM) (Enzyme) | Antimicrobial Potency (If Determined) |
|---|---|---|---|
| Lithobates pipiens, (formerly Rana pipiens) (Northern Leopard frog) [208] | pLR LVRGC(&)WTKSYPPKPC(&)FVR | 110 (trypsin) [208] | E. coli, Y. pseudotuberculosis, Ps. syringae pv tabaci (LC >100 μM); B. magaterium (LC 20 μM); S. lentus (LC 50 μM); M.luteus (LC 10 μM); C. albicans (LC >100 μM); C. tropicalis (LC 11 μM); C. guiller-mondii (LC 6.6 μM); P. nicotianae spores (LC 75 μM) [244] |
| &1LVRGC(&2)WTKSYPPKPC(&2)FVR&1 | 70 (trypsin) [208] | Not studied | |
| LVRGC(&)WTKSYPPKPC(&) | 322 (trypsin) [208] | Not studied | |
| C(&)WTKSYPPKPC(&) | 335 (trypsin) [208] | Not studied | |
| Lithobates capito (formerly Rana sevosa) (Dusky Gopher frog) [209] | pYR YLKGC(&)WTKSYPPKPC(&)FSR | Not studied | |
| Rana esculenta (Common Water Frog) [244] | Ranacyclin E SAPRGC(&)WTKSYPPKPC(&)K-NH2 | 129 (trypsin) [208] | E. coli (LC >100 μM); Y. pseudotuberculosis YP III (LC 9 μM); Ps. syringae pv tabaci (LC 80 μM); B. magaterium (LC 3 μM); S. lentus (LC 7 μM); M.luteus (LC 5 μM); C. albicans (LC >100 μM); C. tropicalis (LC 7.4 μM); C. guiller-mondii (LC 3.4 μM); P. nicotianae spores (LC 32 μM) [244] |
| Rana temporaria(European common frog) [244] | Ranacyclin T GALRGC(&)WTKSYPPKPC(&)K-NH2 | 116 (trypsin) [208] | E. coli D21 (LC 30 μM); Y. pseudotuberculosis YP III (LC 5 μM); Ps. syringae pv tabaci (LC 16 μM); B. magaterium (LC 3 μM); S. lentus (LC 10 μM); M.luteus (LC 7 μM); C. albicans (LC 22 μM); C. tropicalis (LC 14 μM); C. guiller-mondii (LC 1.0 μM); P. nicotianae spores (LC 16 μM) [244] |
| Odorrana graham [31] (Garaham’s frog) | ORB AALKGC(&)WTKSIPPKPC(&)FGKR | 3.06 × 105 (trypsin) [31] | E. coli (MIC 3.20 µg/ml); S. aureus (MIC 5.83 µg/ml); B. subtilis (MIC 1.85 µg/ml); C. albicans (MIC 2.40 µg/ml) [31] |
| ORB2 LKGC(&)WTKSIPPKPC(&)FG | 685 (trypsin) [31] | No antimicrobial activity (E. coli, S. aureus, B. subtilis, C. albicans) [31] | |
| ORB1 LKGC(&)WTKSIPPKPC(&)F | 4 × 106 (trypsin) [31] | E. coli (MIC 2.34 µg/ml); S. aureus (MIC 1.76 µg/ml); B. subtilis (MIC 2.34 µg/ml); C. albicans (MIC 4.69 µg/ml) [31] No antimicrobial activity at concentration range up to 250 mg/ml (S. aureus, S. epidermidis, B. subtilis, B. cereus, E. coli, P. aeruginosa) [248] | |
| ORB-CF C(&)WTKSIPPKPC(&)F | 2.2 × 106 (trypsin) [31] | E. coli (MIC 8.90 µg/ml); S. aureus (MIC 5.96 µg/ml); B. subtilis (MIC 10.50 µg/ml); C. albicans (MIC 19.4 µg/ml) [31] | |
| ORB-C C(&)WTKSIPPKPC(&) | 710 (trypsin) [31] | No antimicrobial activity (E. coli, S. aureus, B. subtilis, C. albicans) [31] | |
| ORB2-K LKGC(&)WTKSIPPKPC(&)FGK | 3 (trypsin) [250]; 886 (trypsin) [31] | No antimicrobial activity (E. coli, S. aureus, B. subtilis, C. albicans) [31] | |
| Huia versabilis (Bamboo Leaf Odorous Frog) [249] | HV BBI SVIGC(&)WTKSIPPRPC(&)FVK-NH2 | 18.8 (trypsin) [249]; 120 (trypsin) [248]; 155 (matriptase-1) [248]; 82 (plasmin) [248] | S. aureus (MIC 60 µg/ml at Davis Minimal Broth, no antimicrobial activity at Mueller–Hinton Broth) [251] |
| [Arg8]HV-BBI SVIGC(&)WTRSIPPRPC(&)FVK-NH2 | 54.2 (trypsin) [249] | Not studied | |
| [Phe8]HV-BBI SVIGC(&)WTFSIPPRPC(&)FVK-NH2 | 389 (chymotrypsin) [249] | No antimicrobial activity (E. coli, S. aureus) [251] | |
| HV BBI (4-16) GC(&)WTKSIPPRPC(&)F-NH2 | 8 (matriptase-1) [248]; 151 (trypsin) [248] | Not studied | |
| HV-BBI (3-18) IGC(&)WTKSIPPRPC(&)FVK-NH2 | 4 (trypsin) [251] | E. coli (MIC 160 µg/ml); S. aureus (MIC 80 µg/ml) (at Davis Minimal Broth, no antimicrobial activity at Mueller–Hinton Broth) [251] | |
| Odorrana hejiangensis [253] (Hejiang Odorous Frog) | HJTI GAPKGC(&)WTKSYPPQPC(&)S | 388 (trypsin) [253] | No antimicrobial activity (at concentrations up to and including 180 M; E. coli, S. aureus, and C. albicans) [253] |
| [Lys14,17]HJTI GAPKGC(&)WTKSYPPKPC(&)K | 217 (trypsin) [253] | E. coli (MIC 160 μM) | |
| Hylarana latouchii(Broadfolded Frog) [252] | pLR-HL LIGGC(&)WTKSIPPKPC(&)LV | 143 (trypsin) [252] | No antimicrobial activity (E. coli, S. aureus and C. albicans) [252] |
| LIGGC(&)WTFSIPPKPC(&)LV | 2141 (chymotrypsin) [252] | Not studied | |
| Odorrana schmackeri (Piebald Odorous Frog) [254] | OSTI AALKGC(&)WTKSIPPKPC(&)F-NH2 | 0.3 (trypsin) [254]; 2500 (tryptase) [254] | Not studied |
| [Phe9]OSTI AALKGC(&)WTFSIPPKPC(&)F | 1000 (chymotrypsin) [254] | Not studied | |
| Hylarana erythraea (Asian Green Frog) [167] | HECI TVLRGC(&)WTFSFPPKPC(&)I-NH2 | 3920 (chymotrypsin) [167]; 8550 (proteasome 20S C-L) [167] | Not studied |
| Pelophylax esculentus [168] (Green Frog) | PE-BBI GALKGC(&)WTKSIPPKPC(&)K-NH2 | 310 (trypsin) [168] | No antimicrobial activity [168] |
| Pelophlax plancyi Fukienesis [32] (Fukien Gold-Striped Pond Frog) | PPF-BBI ALRGC(&)WTKSIPPKPC(&)P-NH2 | 170 (trypsin) [32]; 30,730 (tryptase) [32] | E. coli (MIC 128 μM); S. aureus (MIC 128 μM); MRSA (MIC 512 μM); P.aureginosa (MIC > 512 μM); C. albicans (MIC > 128 μM) [32] |
| [Phe8]PPF-BBI ALRGC(&)WTFSIPPKPC(&)P-NH2 | 850 (chymotrypsin) [32] | E. coli (MIC > 512 μM); S. aureus (MIC > 512 μM); MRSA (MIC > 512 μM); P.aureginosa (MIC > 512 μM); C. albicans (MIC > 512 μM) [32] | |
| [Pro16]PPF-BBI ALRGC(&)WTKSIPPKPC(&)K-NH2 | 112 (trypsin) [32] | E. coli (MIC 128 μM); S. aureus (MIC 64 μM); MRSA (MIC > 512 μM); P.aureginosa (MIC 512 μM); C. albicans (MIC 128 μM) [32] | |
| Tat-loop RKKRRQRRRC(&)WTKSIPPKPC(&) | 607 (trypsin) [32] | E. coli (MIC 128 µM); S. aureus (MIC 128 µM); MRSA (MIC 256 µM); P.aureginosa (MIC 256 µM); C. albicans (MIC 4 μM) [32] | |
| Pelophylax nigromaculatus (East AsianFrog) [30] | Ranacyclin NF (RNF) GAPRGC(&)WTKSYPPQPC(&)F-NH2 | 447 (trypsin) [30]; 6774 (tryptase) [30] | S. aureus (MIC 512 µM) [30] |
| RNF1 GAPRGC(&)WTKSYPPQPC(&)F | 1300 (trypsin) [30]; 9059 (tryptase) [30] | No antimicrobial activity [30] | |
| RNF3L GALRGC(&)WTKSYPPQPC(&)F-NH2 | 201 (trypsin) [30]; 12,500 (tryptase) [30]; | No antimicrobial activity [30] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gitlin-Domagalska, A.; Maciejewska, A.; Dębowski, D. Bowman-Birk Inhibitors: Insights into Family of Multifunctional Proteins and Peptides with Potential Therapeutical Applications. Pharmaceuticals 2020, 13, 421. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13120421
Gitlin-Domagalska A, Maciejewska A, Dębowski D. Bowman-Birk Inhibitors: Insights into Family of Multifunctional Proteins and Peptides with Potential Therapeutical Applications. Pharmaceuticals. 2020; 13(12):421. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13120421
Chicago/Turabian StyleGitlin-Domagalska, Agata, Aleksandra Maciejewska, and Dawid Dębowski. 2020. "Bowman-Birk Inhibitors: Insights into Family of Multifunctional Proteins and Peptides with Potential Therapeutical Applications" Pharmaceuticals 13, no. 12: 421. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13120421