FDA-Approved Drugs with Potent In Vitro Antiviral Activity against Severe Acute Respiratory Syndrome Coronavirus 2
Abstract
:1. Introduction
2. Results
2.1. Antiviral Activity Screening for Commonly Prescribed FDA-Approved Analgesics, Antipyretics, Anti-Inflammatory Drugs, and Antibiotics
2.2. Cytotoxicity and Antiviral Activity of Selected FDA-Approved Drugs
2.3. Mechanism of Anti-SARS-CoV-2 Activity for Promising FDA-Approved Drugs
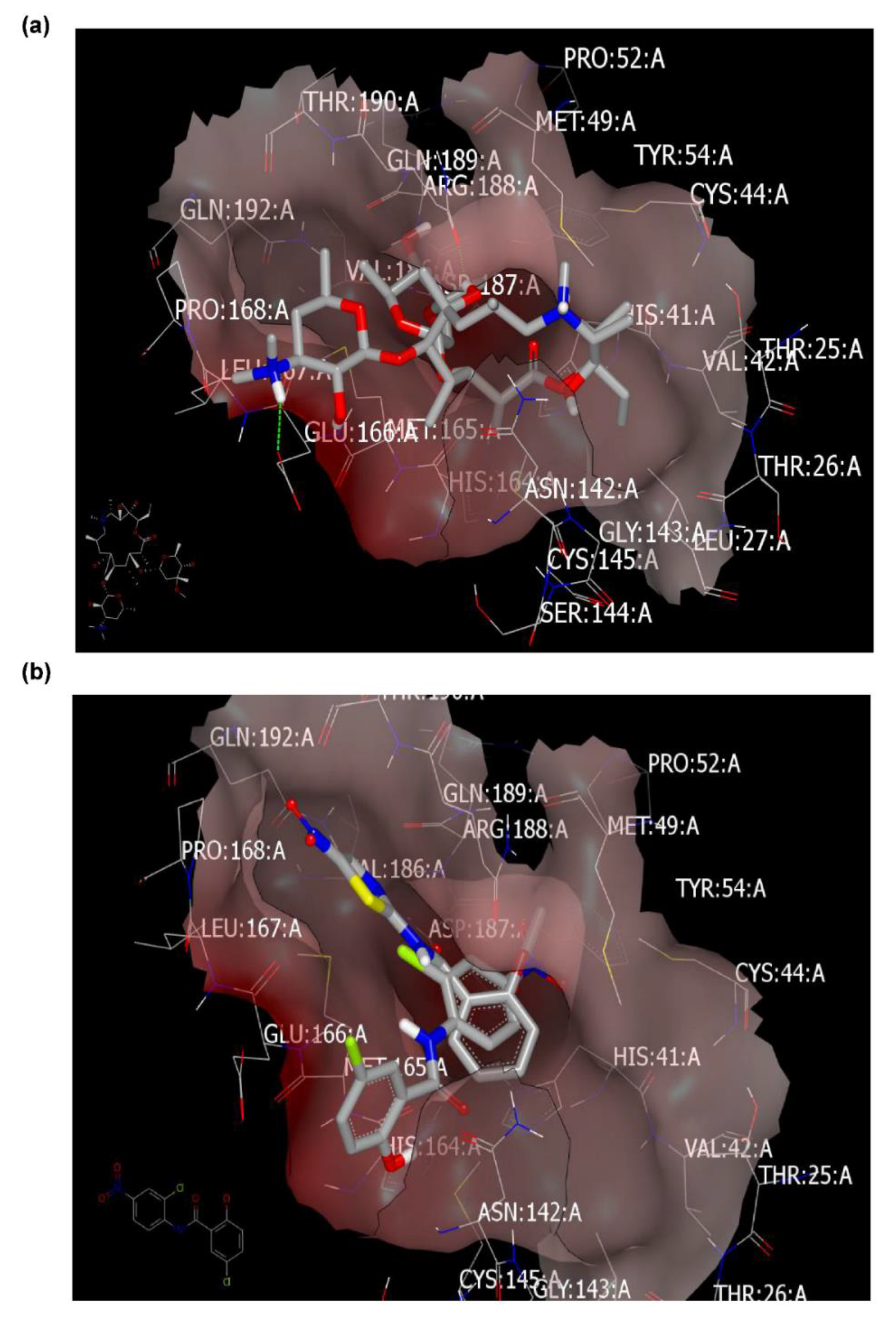
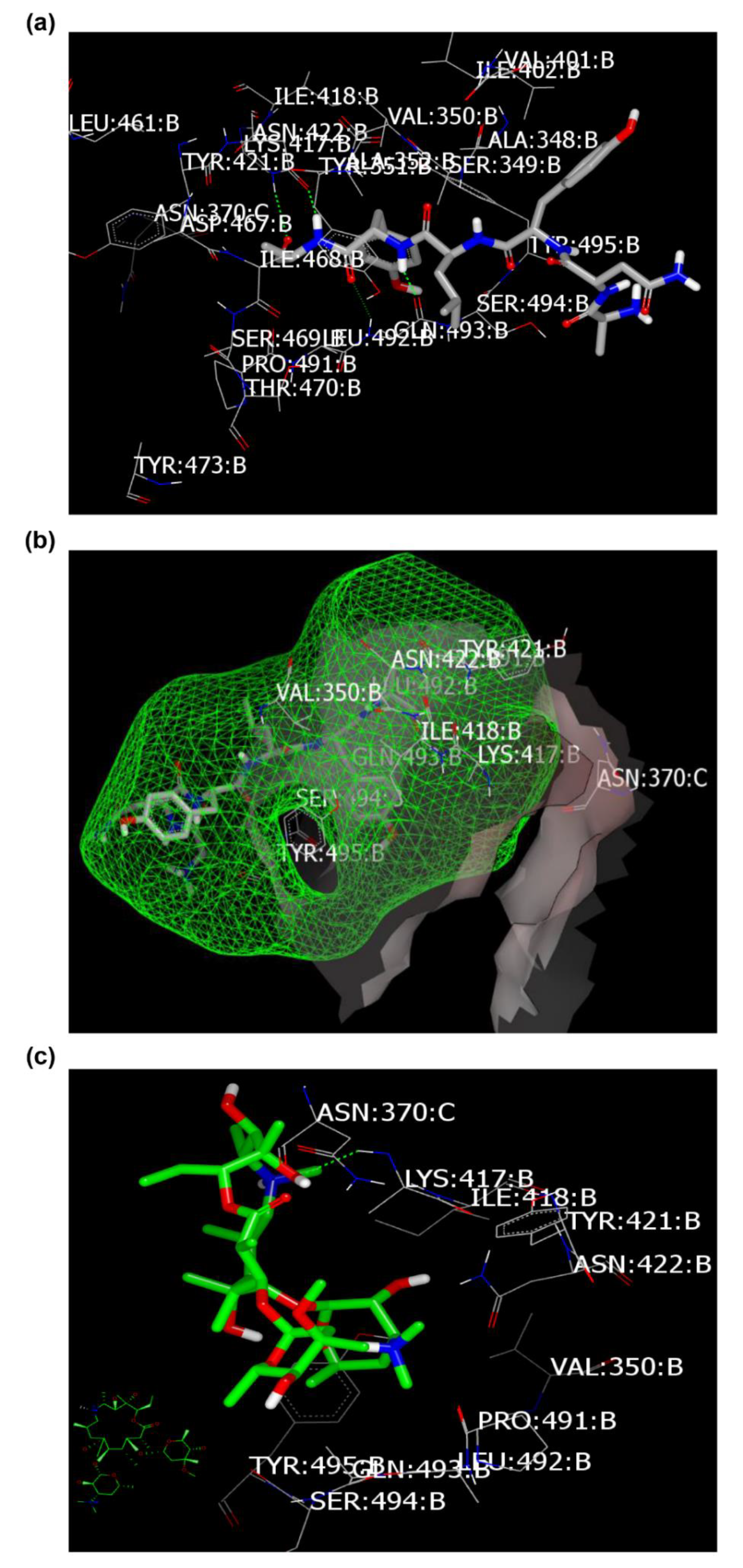
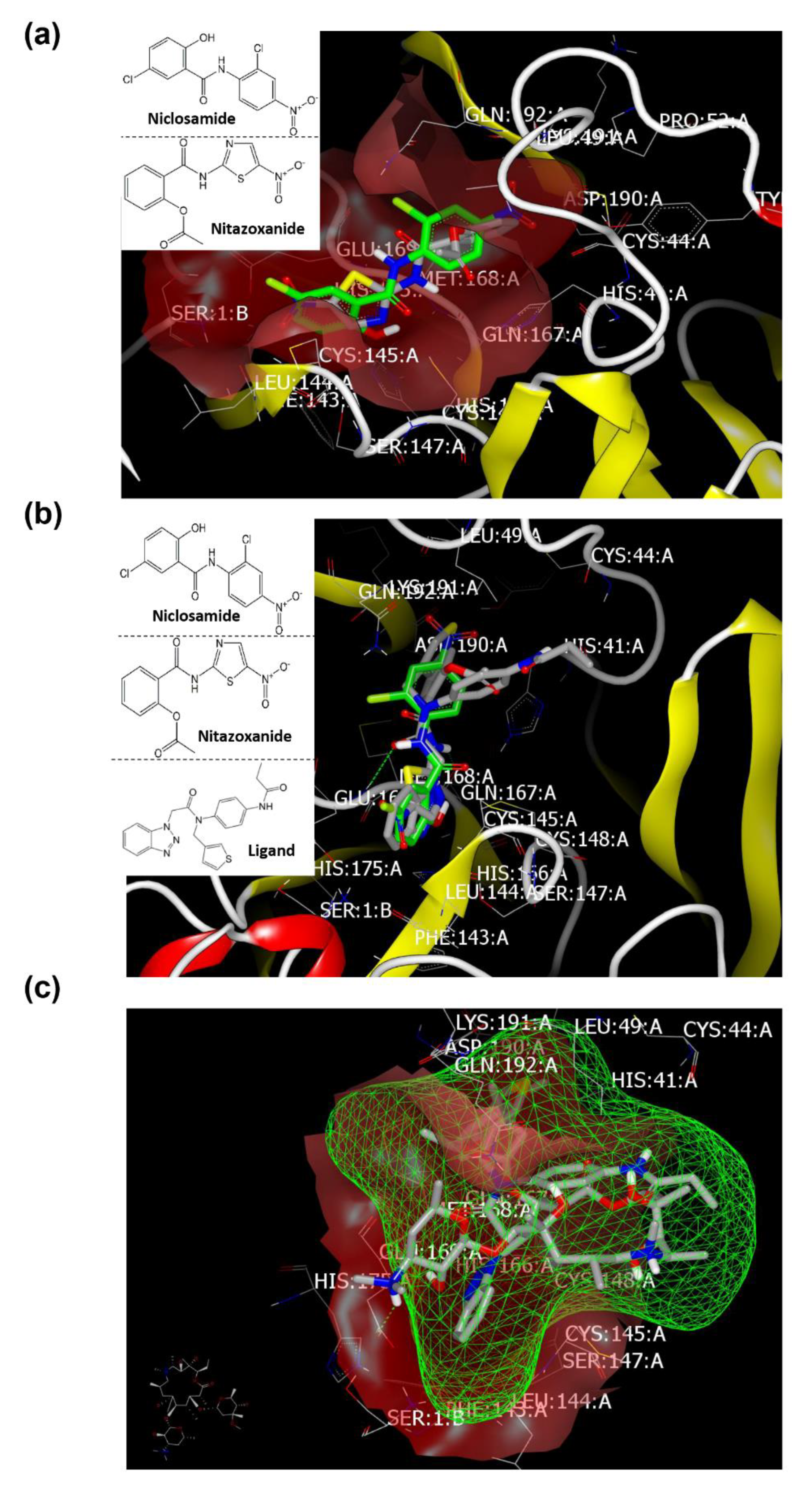
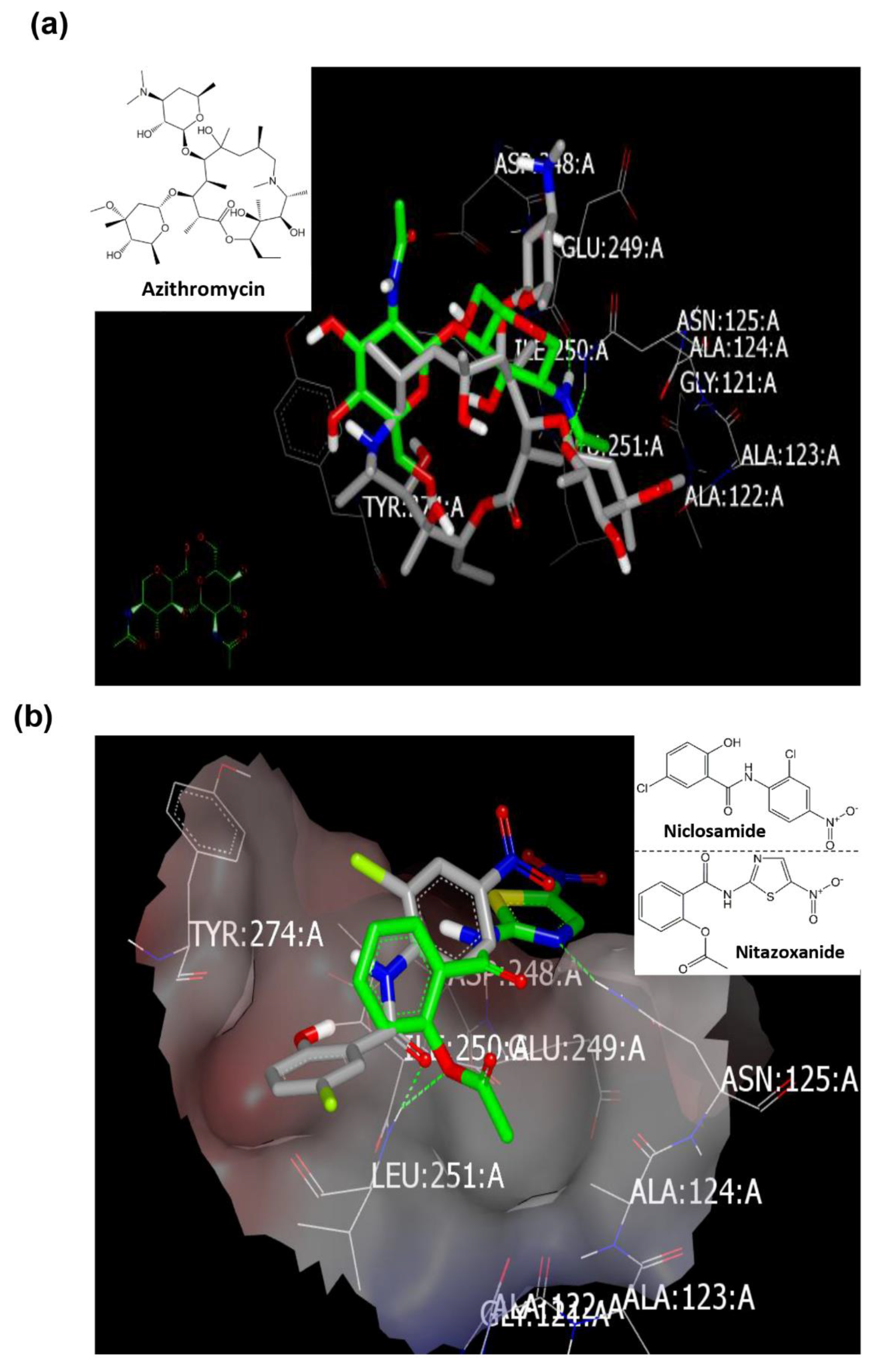
2.4. Molecular Modeling and Virtual Screening Study
2.4.1. Molecular Docking Study
Docking with Mpro (PDB ID: 6lu7) of SARS-CoV-2
Docking with Spike Glycoprotein (PDB ID: 6vsb)
2.4.2. Ligand Efficiency (LE) and Ligand lipophilic Efficiency (LLE) Scores
2.5. Azithromycin Can Selectively Inhibit the Replication of SARS-CoV-2 Virus but Not MERS-CoV
2.6. Docking Study with MERS-CoV Viral Proteins
2.6.1. Docking with the Main Protease
2.6.2. Docking with the Spike Protein (PDB ID: 5x4r)
3. Discussion
4. Materials and Methods
4.1. Virus, Cells and FDA-Approved Drugs
4.2. MTT Cytotoxicity Assay
4.3. Plaque Infectivity Assay
4.4. Plaque Reduction Assay
4.5. Inhibitory Concentration 50 (IC50) Determination
4.6. In Vitro Inhibition of Replication Efficiency at Different Virus Concentrations
4.7. Mechanism of Action(s)
4.7.1. Viral Adsorption Mechanism
4.7.2. Viral Replication Mechanism
4.7.3. Virucidal Mechanism
4.8. In Silico Analyses
4.8.1. Molecular Modeling
4.8.2. Physiochemical Parameter and Lipophilicity Calculations
4.9. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Peiris, J.S.M. Coronaviruses. In Clinical Virology, 4th ed.; Richman, D.D., Whitley, R.J., Hayden, F.J., Eds.; ASM Press: Washington, DC, USA, 2016; pp. 1243–1265. [Google Scholar] [CrossRef]
- Mostafa, A.; Kandeil, A.; Shehata, M.; El Shesheny, R.; Samy, A.M.; Kayali, G.; Ali, M.A. Middle east respiratory syndrome coronavirus (mers-cov): State of the science. Microorganisms 2020, 8, 991. [Google Scholar] [CrossRef]
- Wong, G.; Liu, W.; Liu, Y.; Zhou, B.; Bi, Y.; Gao, G.F. Mers, sars, and ebola: The role of super-spreaders in infectious disease. Cell Host Microbe 2015, 18, 398–401. [Google Scholar] [CrossRef] [Green Version]
- Zaki, A.M.; van Boheemen, S.; Bestebroer, T.M.; Osterhaus, A.D.; Fouchier, R.A. Isolation of a novel coronavirus from a man with pneumonia in saudi arabia. N. Engl. J. Med. 2012, 367, 1814–1820. [Google Scholar] [CrossRef]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A novel coronavirus from patients with pneumonia in china, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef]
- Mizumoto, K.; Kagaya, K.; Zarebski, A.; Chowell, G. Estimating the asymptomatic proportion of coronavirus disease 2019 (covid-19) cases on board the diamond princess cruise ship, yokohama, Japan, 2020. Eurosurveillance 2020, 25, 2000180. [Google Scholar] [CrossRef] [Green Version]
- WHO. Who Coronavirus Disease (Covid-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 8 September 2020).
- Chan, J.F.; Kok, K.H.; Zhu, Z.; Chu, H.; To, K.K.; Yuan, S.; Yuen, K.Y. Genomic characterization of the 2019 novel human-pathogenic coronavirus isolated from a patient with atypical pneumonia after visiting wuhan. Emerg. Microbes Infect. 2020, 9, 221–236. [Google Scholar] [CrossRef] [Green Version]
- Mahmoud, D.B.; Shitu, Z.; Mostafa, A. Drug repurposing of nitazoxanide: Can it be an effective therapy for covid-19? J. Genet. Eng. Biotechnol. 2020, 18, 35. [Google Scholar] [CrossRef]
- Pushpakom, S.; Iorio, F.; Eyers, P.A.; Escott, K.J.; Hopper, S.; Wells, A.; Doig, A.; Guilliams, T.; Latimer, J.; McNamee, C.; et al. Drug repurposing: Progress, challenges and recommendations. Nat. Rev. Drug Discov. 2019, 18, 41–58. [Google Scholar]
- Russell, B.; Moss, C.; Rigg, A.; Van Hemelrijck, M. Covid-19 and treatment with nsaids and corticosteroids: Should we be limiting their use in the clinical setting? Ecancermedicalscience 2020, 14, 1023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Little, P. Non-steroidal anti-inflammatory drugs and covid-19. BMJ 2020, 368, m1185. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giollo, A.; Adami, G.; Gatti, D.; Idolazzi, L.; Rossini, M. Coronavirus disease 19 (covid-19) and non-steroidal anti-inflammatory drugs (nsaid). Ann. Rheum. Dis. 2020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cox, M.J.; Loman, N.; Bogaert, D.; O’Grady, J. Co-infections: Potentially lethal and unexplored in covid-19. Lancet Microbe 2020, 1, e11. [Google Scholar] [CrossRef]
- Hsu, J. How covid-19 is accelerating the threat of antimicrobial resistance. BMJ 2020, 369, m1983. [Google Scholar] [CrossRef] [PubMed]
- Allam, A.E.; Assaf, H.K.; Hassan, H.A.; Shimizu, K.; Elshaier, Y.A.M.M. An in silico perception for newly isolated flavonoids from peach fruit as privileged avenue for a countermeasure outbreak of covid-19. RSC Adv. 2020, 10, 29983–29998. [Google Scholar] [CrossRef]
- Jin, Z.; Du, X.; Xu, Y.; Deng, Y.; Liu, M.; Zhao, Y.; Zhang, B.; Li, X.; Zhang, L.; Peng, C.; et al. Structure of mpro from sars-cov-2 and discovery of its inhibitors. Nature 2020, 582, 289–293. [Google Scholar] [CrossRef] [Green Version]
- Wrapp, D.; Wang, N.; Corbett, K.S.; Goldsmith, J.A.; Hsieh, C.-L.; Abiona, O.; Graham, B.S.; McLellan, J.S. Cryo-em structure of the 2019-ncov spike in the prefusion conformation. Science 2020, 367, 1260–1263. [Google Scholar] [CrossRef] [Green Version]
- Schultes, S.; de Graaf, C.; Haaksma, E.E.J.; de Esch, I.J.P.; Leurs, R.; Krämer, O. Ligand efficiency as a guide in fragment hit selection and optimization. Drug Discov. Today Technol. 2010, 7, e157–e162. [Google Scholar] [CrossRef] [Green Version]
- Jabeen, I.; Pleban, K.; Rinner, U.; Chiba, P.; Ecker, G.F. Structure–activity relationships, ligand efficiency, and lipophilic efficiency profiles of benzophenone-type inhibitors of the multidrug transporter p-glycoprotein. J. Med. Chem. 2012, 55, 3261–3273. [Google Scholar] [CrossRef]
- Singlas, E. clinical pharmacokinetics of azithromycin. Pathol. Biol. 1995, 43, 505–511. [Google Scholar]
- Norman, B.H. Drug induced liver injury (dili). Mechanisms and medicinal chemistry avoidance/mitigation strategies. J. Med. Chem. 2020, 63, 11397–11419. [Google Scholar] [CrossRef]
- European Association for the Study of the Liver. Easl clinical practice guidelines: Drug-induced liver injury. J. Hepatol. 2019, 70, 1222–1261. [Google Scholar] [CrossRef] [Green Version]
- Tomar, S.; Johnston, M.L.; St John, S.E.; Osswald, H.L.; Nyalapatla, P.R.; Paul, L.N.; Ghosh, A.K.; Denison, M.R.; Mesecar, A.D. Ligand-induced dimerization of middle east respiratory syndrome (mers) coronavirus nsp5 protease (3clpro): Implications for nsp5 regulation and the development of antivirals. J. Biol. Chem. 2015, 290, 19403–19422. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Y.; Cao, D.; Zhang, Y.; Ma, J.; Qi, J.; Wang, Q.; Lu, G.; Wu, Y.; Yan, J.; Shi, Y.; et al. Cryo-em structures of mers-cov and sars-cov spike glycoproteins reveal the dynamic receptor binding domains. Nat. Commun. 2017, 8, 15092. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Cao, R.; Zhang, L.; Yang, X.; Liu, J.; Xu, M.; Shi, Z.; Hu, Z.; Zhong, W.; Xiao, G. Remdesivir and chloroquine effectively inhibit the recently emerged novel coronavirus (2019-ncov) in vitro. Cell Res. 2020, 30, 269–271. [Google Scholar] [CrossRef] [PubMed]
- Pepperrell, T.; Pilkington, V.; Owen, A.; Wang, J.; Hill, A.M. Review of safety and minimum pricing of nitazoxanide for potential treatment of covid-19. J. Virus Erad. 2020, 6, 52–60. [Google Scholar] [CrossRef]
- Xu, J.; Shi, P.-Y.; Li, H.; Zhou, J. Broad spectrum antiviral agent niclosamide and its therapeutic potential. ACS Infect. Dis. 2020, 6, 909–915. [Google Scholar] [CrossRef]
- Touret, F.; Gilles, M.; Barral, K.; Nougairède, A.; van Helden, J.; Decroly, E.; de Lamballerie, X.; Coutard, B. In vitro screening of a fda approved chemical library reveals potential inhibitors of sars-cov-2 replication. Sci. Rep. 2020, 10, 13093. [Google Scholar] [CrossRef]
- Li, C.; Zu, S.; Deng, Y.Q.; Li, D.; Parvatiyar, K.; Quanquin, N.; Shang, J.; Sun, N.; Su, J.; Liu, Z.; et al. Azithromycin protects against zika virus infection by upregulating virus-induced type i and iii interferon responses. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef]
- Kouznetsova, J.; Sun, W.; Martínez-Romero, C.; Tawa, G.; Shinn, P.; Chen, C.Z.; Schimmer, A.; Sanderson, P.; McKew, J.C.; Zheng, W.; et al. Identification of 53 compounds that block ebola virus-like particle entry via a repurposing screen of approved drugs. Emerg. Microbes Infect. 2014, 3, e84. [Google Scholar] [CrossRef]
- Glatthaar-Saalmüller, B.; Mair, K.H.; Saalmüller, A. Antiviral activity of aspirin against rna viruses of the respiratory tract-an in vitro study. Influenza Respir. Viruses 2017, 11, 85–92. [Google Scholar] [CrossRef] [Green Version]
- Müller, C.; Karl, N.; Ziebuhr, J.; Pleschka, S. D, L-Lysine acetylsalicylate+ glycine impairs coronavirus replication. J. Antivir. Antiretrovir. 2016, 8, 142–150. [Google Scholar] [CrossRef]
- Amin, A.R.; Attur, M.G.; Pillinger, M.; Abramson, S.B. The pleiotropic functions of aspirin: Mechanisms of action. Cell. Mol. Life Sci. 1999, 56, 305–312. [Google Scholar] [CrossRef] [PubMed]
- Astani, A.; Albrecht, U.; Schnitzler, P. Piroxicam inhibits herpes simplex virus type 1 infection in vitro. Die Pharm. 2015, 70, 331–336. [Google Scholar]
- Westover, J.B.; Ferrer, G.; Vazquez, H.; Bethencourt-Mirabal, A.; Go, C.C. In vitro virucidal effect of intranasally delivered chlorpheniramine maleate compound against severe acute respiratory syndrome coronavirus 2. Cureus 2020, 12, e10501. [Google Scholar] [PubMed]
- Mosmann, T. Rapid colorimetric assay for cellular growth and survival: Application to proliferation and cytotoxicity assays. J. Immunol. Methods 1983, 65, 55–63. [Google Scholar] [CrossRef]
- Payne, S. Methods to study viruses. Viruses 2017, 37–52. [Google Scholar] [CrossRef]
- Hayden, F.G.; Cote, K.M.; Douglas, R.G., Jr. Plaque inhibition assay for drug susceptibility testing of influenza viruses. Antimicrob. Agents Chemother. 1980, 17, 865–870. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Zhan, B.; Yao, X.; Gao, Y.; Song, J. Antiviral activity of tannin from the pericarp of punica granatum l. Against genital herpes virus in vitro. Zhongguo Zhong Yao Za Zhi 1995, 20, 556–558. [Google Scholar]
- Kuo, Y.C.; Lin, L.C.; Tsai, W.J.; Chou, C.J.; Kung, S.H.; Ho, Y.H. Samarangenin b from limonium sinense suppresses herpes simplex virus type 1 replication in vero cells by regulation of viral macromolecular synthesis. Antimicrob. Agents Chemother. 2002, 46, 2854–2864. [Google Scholar] [CrossRef] [Green Version]
- Schuhmacher, A.; Reichling, J.; Schnitzler, P. Virucidal effect of peppermint oil on the enveloped viruses herpes simplex virus type 1 and type 2 in vitro. Phytomedicine 2003, 10, 504–510. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Lin, D.; Sun, X.; Curth, U.; Drosten, C.; Sauerhering, L.; Becker, S.; Rox, K.; Hilgenfeld, R. Crystal structure of sars-cov-2 main protease provides a basis for design of improved α-ketoamide inhibitors. Science 2020, 368, 409–412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
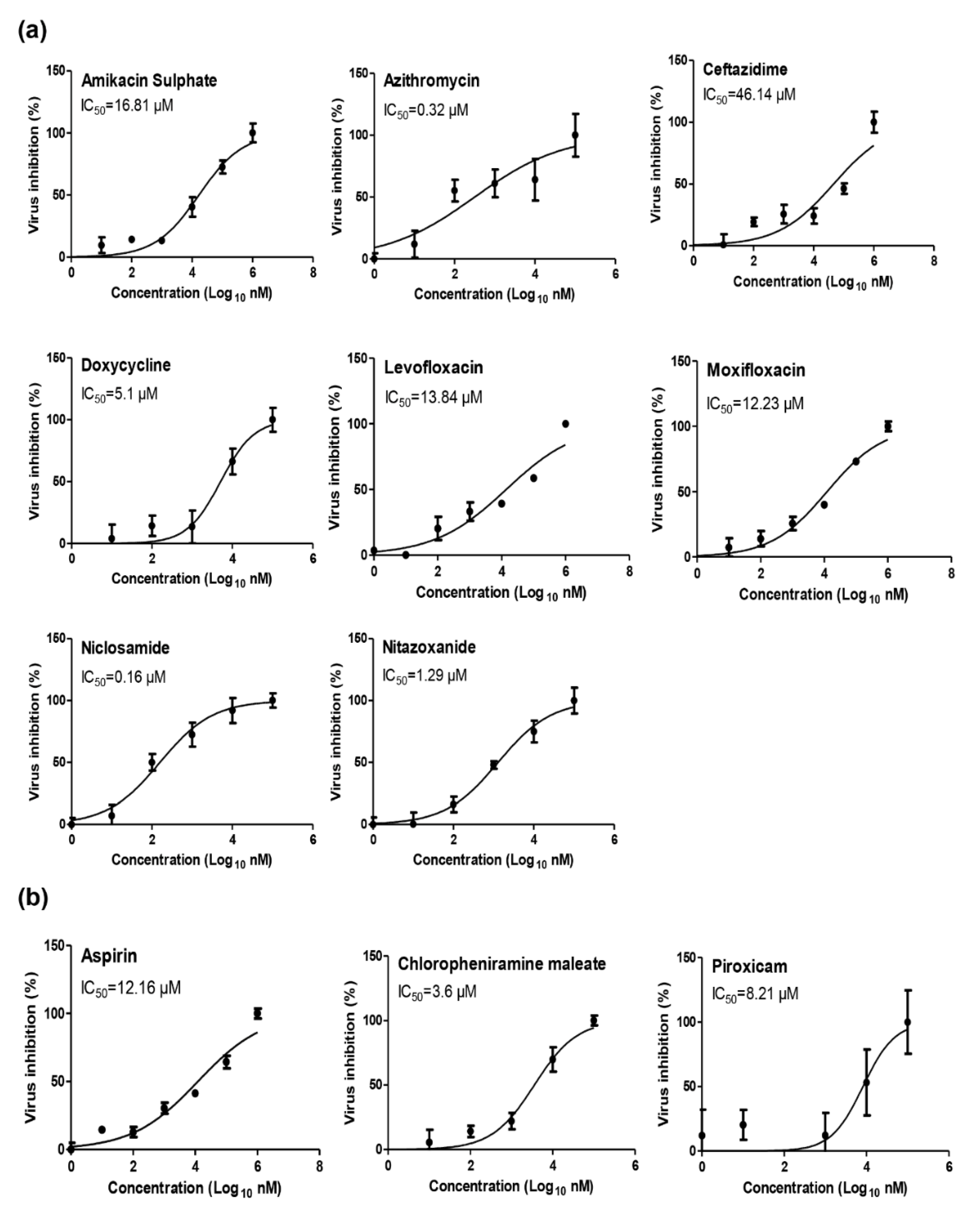



| (A) Anti-microbial FDA-approved drugs | ||||
| FDA-approved drug | Initial indication | CC50 (Vero-E6) | IC50 (NRC-03-nhCoV) | SI |
| Amikacin sulphate | Aminoglycoside antibiotic | 2456 µM | 16.81 µM | 146.10 |
| Azithromycin | Macrolide-type antibiotic | 793 µM | 0.32 µM | 2478.13 |
| Amoxicillin | Penicillin-type antibiotic | 614.57 µM | 16.12 µM | 38.12 |
| Benzathine penicillin | Long-acting penicillin antibiotic | 728.2 µM | 15.78 µM | 46.15 |
| Chloramphenicol | Broad-spectrum bacteriostatic | 33.92 µM | 16.94 µM | 2.00 |
| Cefotaxime | Third-generation cephalosporin antibiotic | 3155 µM | 42.72 µM | 73.85 |
| Cephalexin | First-generation cephalosporin antibiotic | 522.95 µM | 13.17 µM | 39.71 |
| Ceftriaxone | Third-generation cephalosporin antibiotic | 445.91 µM | 16.14 µM | 27.63 |
| Cefoperazone | Third-generation cephalosporin antibiotic | 69.03 µM | 12.36 µM | 5.58 |
| Ceftazidime | Third-generation cephalosporin antibiotic | 5554 µM | 46.14 µM | 120.37 |
| Clindamycin | Lincosamide antibiotic | 436.45 µM | 15.67 µM | 27.85 |
| Ciprofloxacin | Fluoroquinolone antibiotic | 3516 µM | 61.62 µM | 57.06 |
| Doxycycline | Tetracycline antibiotic | 636.1 µM | 5.1 µM | 124.73 |
| Flucloxacillin | Narrow-spectrum penicillin-type antibiotic | 966.23 µM | 157.78 µM | 6.12 |
| Levofloxacin | Fluoroquinolone antibiotic | 2156 µM | 13.84 µM | 155.78 |
| Linezolid | Narrow-spectrum oxazolidinone antibiotic | 816.5 µM | 16.3 µM | 50.1 |
| Moxifloxacin | Fluoroquinolone antibiotic | 2242 µM | 12.23 µM | 183.32 |
| Nitrofurantoin | Narrow-spectrum antibiotic | 599.113 µM | 16.22 µM | 36.94 |
| Neomycin | Aminoglycoside antibiotic | 833.1 µM | 18.12 µM | 45.98 |
| Niclosamide | Anthelminthic and antibacterial drug | 204.61 µM | 0.16 µM | 1278.81 |
| Nitazoxanide | Broad-spectrum anti-infective drug | 665.15 µM | 1.29 µM | 515.62 |
| Nystatin | Antifungal medication | 182.64 µM | 160.85 µM | 1.14 |
| (B) Analgesics and antipyretics | ||||
| FDA-approved drug | Initial indication | CC50 (Vero-E6) | IC50 (NRC-03-nhCoV) | SI |
| Acetyl Salicylic acid “Aspirin” | Anti-inflammatory and antipyretic | 1255 µM | 12.16 µM | 103.21 |
| Paracetamol | Analgesic and antipyretic | 4980 µM | ≥IC50 | <1 |
| Celecoxib | Nonsteroidal anti-inflammatory drug (NSAID) | 140.37 µM | 13.02 µM | 10.78 |
| Ciclesonide | Glucocorticoid used to treat asthma and rhinitis | 119.5 µM | 4.2 µM | 28.73 |
| Chlorpheniramine maleate | Antihistamine used to treat allergic rhinitis | 465.65 µM | 3.6 µM | 129.35 |
| Dexamethasone | Anti-inflammatory corticosteroid medication | 1901 µM | 122.55 µM | 15.51 |
| Diclofenac sodium | Nonsteroidal anti-inflammatory drug (NSAID) | 138.31 µM | 96.24 µM | 1.44 |
| Fluticasone Propionate | Synthetic glucocorticoid to treat asthma and COPD | 32.04 µM | 1.71 µM | 18.74 |
| Formoterol Fumarate | Long-acting bronchodilator | 568.63 µM | 71.8 µM | 7.92 |
| Hydrocortisone | Anti-inflammatory glucocorticoid | 614 µM | 7.1 µM | 87.10 |
| Indomethacin | Nonsteroidal anti-inflammatory drug | 671.7 µM | 8.51 µM | 78.93 |
| Ibuprofen | Nonsteroidal anti-inflammatory drug | 1166 µM | 88.71 µM | 13.14 |
| Ketoprofen | Nonsteroidal anti-inflammatory drug | 822.62 µM | 21.5 µM | 38.31 |
| Ketorolac Tromethamine | Nonsteroidal anti-inflammatory drug | 2042 µM | 153.42 µM | 13.31 |
| Metamizole sodium | Analgesic and antipyretic | 947.5 µM | 14.97 µM | 63.29 |
| Montelukast | Leukotriene receptor antagonist to treat asthma | 9.86 µM | 2.7 µM | 3.65 |
| Meloxicam | Nonsteroidal anti-inflammatory drug | 262.16 µM | 12.4 µM | 21.21 |
| Methylprednisolone | Glucocorticoid anti-inflammatory medication | 3344 µM | 90.44 µM | 36.97 |
| Naphazoline | Decongestant | 636.1 µM | 9.52 µM | 66.82 |
| Piroxicam | Nonsteroidal anti-inflammatory drug | 1795 µM | 8.21 µM | 218.64 |
| Salmeterol | Long-acting bronchodilator | 4.1 µM | 1.5 µM | 2.73 |
| Name of Compound | Conc. (µM) | Mode of Action * | ||
|---|---|---|---|---|
| Viral Adsorption | Viral Replication | Virucidal | ||
| Azithromycin | 1.3 | 31% | 4% | 51% |
| 0.64 | 27% | 2% | 51% | |
| 0.322 | 2% | 0% | 34% | |
| 0.16 | 0% | 0% | 12% | |
| Niclosamide | 10.4 | 0% | 70% | 37% |
| 5.2 | 0% | 68% | 21% | |
| 2.6 | 0% | 55% | 21% | |
| 1.302 | 0% | 23% | 16% | |
| Nitazoxanide | 10.4 | 11% | Toxic | 78% |
| 5.2 | 11% | Toxic | 75% | |
| 2.6 | 1% | 40% | 61% | |
| 1.302 | 0% | 35% | 39% | |
| Name of Compound | Spike Glycoprotein | Main Protease | ||||
|---|---|---|---|---|---|---|
| 6vsb | Binding Interaction | 6y2f | Binding Interaction | 6lu7 | Binding Interaction | |
| Azithromycin | 173 | HB with LYS:417A | 153 | No HB formations | 197 | HBs with GLU:166A and GLN:189A. Fully occupied receptor domains with two terminal HBs formation. |
| Niclosamide | 153 | HB with GLN:493A | 153 | HB with GLN:192A | 113 | No HB formation. The phenolic moiety oriented deeply in the pocket domain. |
| Nitazoxanide | 150 | HB with ASN:422A | 96 | HB with MET:165A | 134 | No HB formation. The salicyloyl moiety oriented deeply in the pocket domain. |
| Rule of Five (RO5) | cLogP | Experimental Data | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Mwt | NHA | HBA | HBD | IC50 (µM) | pIC50 | LE | LLE | ||
| Azithromycin | 749.00 | 52 | 14 | 5 | 2.44 | 0.32 | 6.49 | 0.17 | 4.05 |
| Niclosamide | 327.12 | 21 | 4 | 2 | 2.95 | 0.16 | 6.79 | 0.44 | 3.84 |
| Nitazoxanide | 307.28 | 21 | 7 | 1 | 2.12 | 1.29 | 5.89 | 0.38 | 3.77 |
| Celecoxib | 381.38 | 26 | 4 | 1 | 4.01 | 13.02 | 4.89 | 0.26 | 0.88 |
| Piroxicam | 331.35 | 23 | 5 | 2 | 3.1 | 8.21 | 5.09 | 0.30 | 1.99 |
| Doxycycline | 444.44 | 32 | 9 | 6 | −0.7 | 5.1 | 5.29 | 0.22 | 5.99 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mostafa, A.; Kandeil, A.; A. M. M. Elshaier, Y.; Kutkat, O.; Moatasim, Y.; Rashad, A.A.; Shehata, M.; Gomaa, M.R.; Mahrous, N.; Mahmoud, S.H.; et al. FDA-Approved Drugs with Potent In Vitro Antiviral Activity against Severe Acute Respiratory Syndrome Coronavirus 2. Pharmaceuticals 2020, 13, 443. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13120443
Mostafa A, Kandeil A, A. M. M. Elshaier Y, Kutkat O, Moatasim Y, Rashad AA, Shehata M, Gomaa MR, Mahrous N, Mahmoud SH, et al. FDA-Approved Drugs with Potent In Vitro Antiviral Activity against Severe Acute Respiratory Syndrome Coronavirus 2. Pharmaceuticals. 2020; 13(12):443. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13120443
Chicago/Turabian StyleMostafa, Ahmed, Ahmed Kandeil, Yaseen A. M. M. Elshaier, Omnia Kutkat, Yassmin Moatasim, Adel A. Rashad, Mahmoud Shehata, Mokhtar R. Gomaa, Noura Mahrous, Sara H. Mahmoud, and et al. 2020. "FDA-Approved Drugs with Potent In Vitro Antiviral Activity against Severe Acute Respiratory Syndrome Coronavirus 2" Pharmaceuticals 13, no. 12: 443. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13120443








