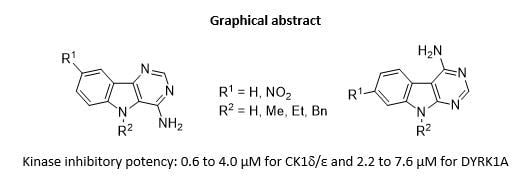
Exploring Kinase Inhibition Properties of 9H-pyrimido[5,4-b]- and [4,5-b]indol-4-amine Derivatives
Abstract
:1. Introduction
2. Results
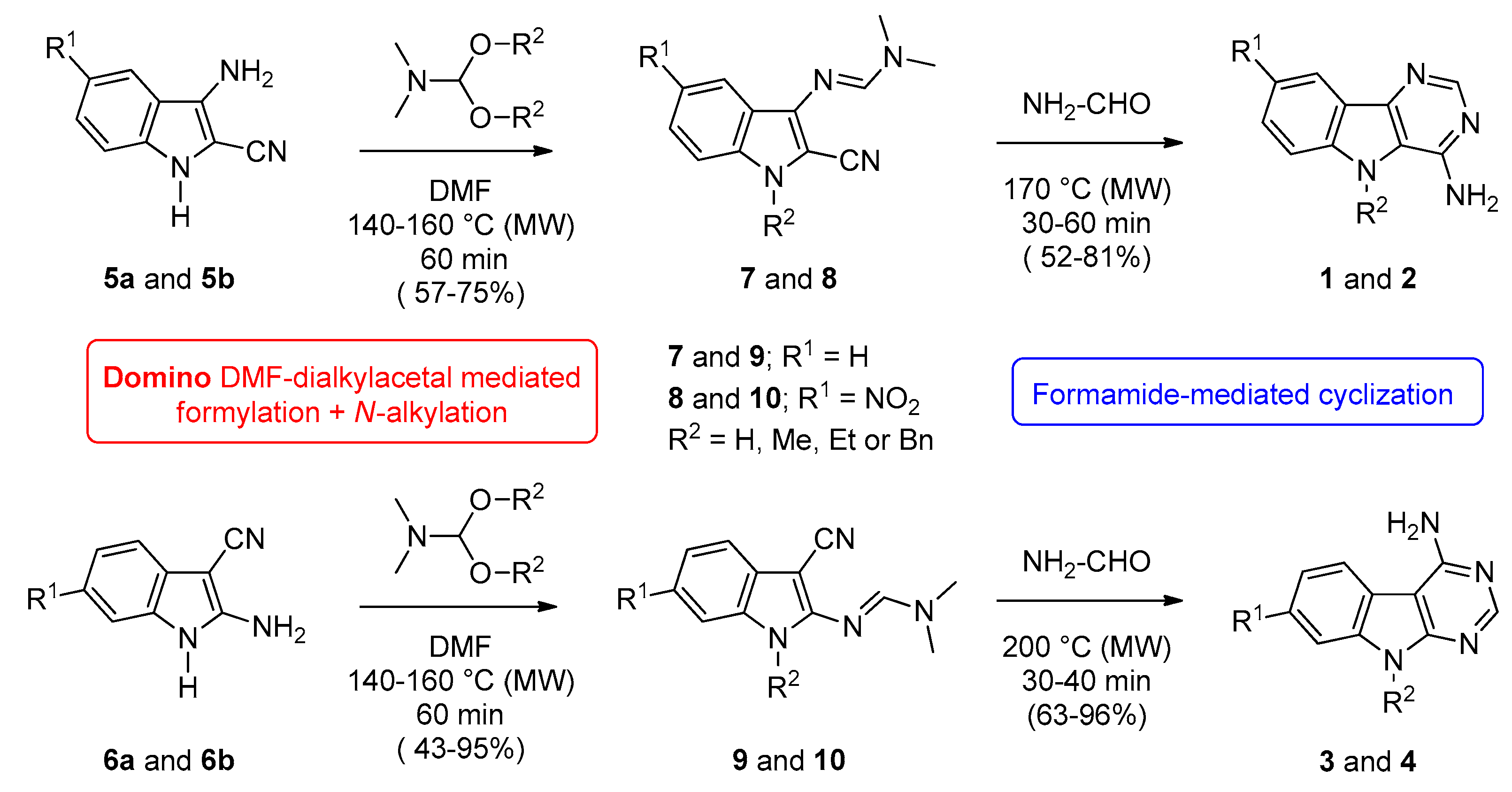
2.1. Chemistry
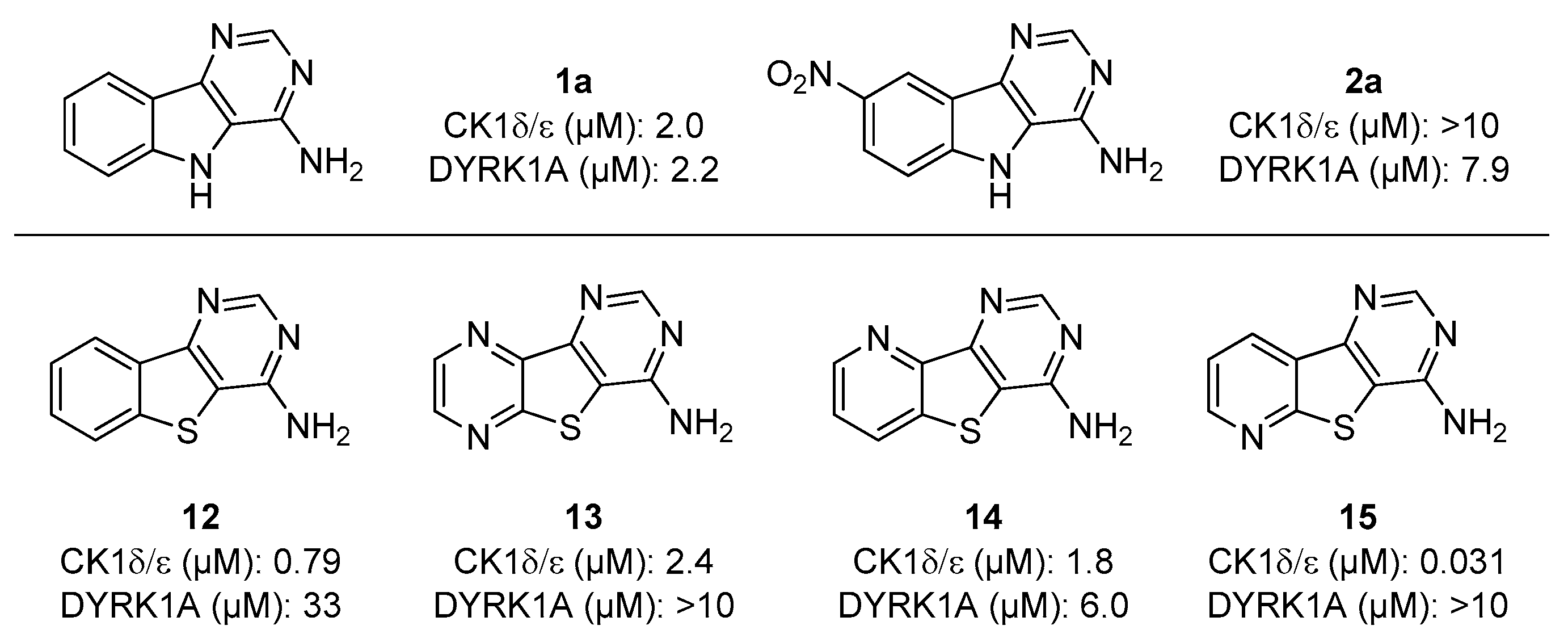
2.2. Biological Evaluation
3. Discussion
4. Materials and Methods
4.1. General Information
4.2. Chemistry
4.2.1. General Procedure for the Synthesis of 5H-Pyrimido[5,4-b]indol-4-amines (Series 1 and 2) and 5H-Pyrimido[4,5-b]indol-4-amines (Series 3 and 4).
4.2.2. General Procedure for the Synthesis of N-alkylated Harmine Derivatives (Compounds 11b–d).
4.3. In Vitro Kinase Preparation and Assays
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Manning, G.; Whyte, D.B.; Martinez, R.; Hunter, T.; Sudarsanam, S. The Protein Kinase Complement of the Human Genome. Science 2002, 298, 1912–1934. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ionescu, A.; Dufrasne, F.; Gelbcke, M.; Jabin, I.; Kiss, R.; Lamoral-Theys, D. DYRK1A kinase inhibitors with emphasis on cancer. Mini Rev. Med. Chem. 2012, 12, 1315–1329. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Martínez, P.; Zahonero, C.; Sánchez-Gómez, P. DYRK1A: The double-edged kinase as a protagonist in cell growth and tumorigenesis. Mol. Cell. Oncol. 2015, 2, 970048. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duchon, A.; Herault, Y. DYRK1A, a Dosage-Sensitive Gene Involved in Neurodevelopmental Disorders, Is a Target for Drug Development in Down Syndrome. Front. Behav. Neurosci. 2016, 10, 87. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Branca, C.; Shaw, D.M.; Belfiore, R.; Gokhale, V.; Shaw, A.Y.; Foley, C.; Smith, B.; Hulme, C.; Dunckley, T.; Meechoovet, B.; et al. Dyrk1 inhibition improves Alzheimer’s disease-like pathology. Aging Cell 2017, 16, 1146–1154. [Google Scholar] [CrossRef] [PubMed]
- Stotani, S.; Giordanetto, F.; Medda, F. DYRK1A inhibition as potential treatment for Alzheimer’s disease. Future Med. Chem. 2016, 8, 681–696. [Google Scholar] [CrossRef]
- Roskoski, R. Properties of FDA-approved small molecule protein kinase inhibitors: A 2020 update. Pharmacol. Res. 2020, 152, 104609. [Google Scholar] [CrossRef]
- Alexandre, F.-R.; Berecibar, A.; Wrigglesworth, R.; Besson, T. Efficient synthesis of thiazoloquinazolinone derivatives. Tetrahedron Lett. 2003, 44, 4455–4458. [Google Scholar] [CrossRef]
- Loidreau, Y.; Besson, T. Microwave-assisted thermal decomposition of formamide: A tool for coupling a pyrimidine ring with an aromatic partner. Tetrahedron 2011, 67, 4852–4857. [Google Scholar] [CrossRef]
- Foucourt, A.; Dubouilh-Benard, C.; Chosson, E.; Corbiere, C.; Buquet, C.; Iannelli, M.; Leblond, B.; Marsais, F.; Besson, T. Microwave-accelerated Dimroth rearrangement for the synthesis of 4-anilino-6-nitroquinazolines. Application to an efficient synthesis of a microtubule destabilizing agent. Tetrahedron 2010, 66, 4495–4502. [Google Scholar] [CrossRef]
- Loidreau, Y.; Dubouilh-Benard, C.; Marchand, P.; Nourrisson, M.-R.; Duflos, M.; Buquet, C.; Corbière, C.; Besson, T. Efficient New Synthesis ofN-Arylbenzo [b] furo [3,2-d] pyrimidin-4-amines and Their Benzo [b] thieno [3,2-d] pyrimidin-4-amine Analogues via a Microwave-Assisted Dimroth Rearrangement. J. Heterocycl. Chem. 2013, 50, 1187–1197. [Google Scholar] [CrossRef]
- Loidreau, Y.; Marchand, P.; Dubouilh-Benard, C.; Nourrisson, M.-R.; Duflos, M.; Loaëc, N.; Meijer, L.; Besson, T. Synthesis and biological evaluation of N-aryl-7-methoxybenzo [b] furo [3,2-d] pyrimidin-4-amines and their N-arylbenzo [b] thieno [3,2-d] pyrimidin-4-amine analogues as dual inhibitors of CLK1 and DYRK1A kinases. Eur. J. Med. Chem. 2013, 59, 283–295. [Google Scholar] [CrossRef] [PubMed]
- Loidreau, Y.; Marchand, P.; Dubouilh-Benard, C.; Nourrisson, M.-R.; Duflos, M.; Lozach, O.; Loaëc, N.; Meijer, L.; Besson, T. Synthesis and biological evaluation of N-arylbenzo[b]thieno[3,2-d]pyrimidin-4-amines and their pyrido and pyrazino analogues as Ser/Thr kinase inhibitors. Eur. J. Med. Chem. 2012, 58, 171–183. [Google Scholar] [CrossRef]
- Loidreau, Y.; Deau, E.; Marchand, P.; Nourrisson, M.-R.; Logé, C.; Coadou, G.; Loaëc, N.; Meijer, L.; Besson, T. Synthesis and molecular modelling studies of 8-arylpyrido[3′,2′:4,5]thieno[3,2-d]pyrimidin-4-amines as multitarget Ser/Thr kinases inhibitors. Eur. J. Med. Chem. 2015, 92, 124–134. [Google Scholar] [CrossRef]
- Jarhad, D.B.; Mashelkar, K.K.; Kim, H.-R.; Noh, M.; Jeong, L.S. Dual-Specificity Tyrosine Phosphorylation-Regulated Kinase 1A (DYRK1A) Inhibitors as Potential Therapeutics. J. Med. Chem. 2018, 61, 9791–9810. [Google Scholar] [CrossRef]
- Czarna, A.; Wang, J.; Zelencova, D.; Liu, Y.; Deng, X.; Choi, H.G.; Zhang, T.; Zhou, W.; Chang, J.W.; Kildalsen, H.; et al. Novel Scaffolds for Dual Specificity Tyrosine-Phosphorylation-Regulated Kinase (DYRK1A) Inhibitors. J. Med. Chem. 2018, 61, 7560–7572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meijer, L.; Flajolet, M.; Greengard, P. Pharmacological inhibitors of glycogen synthase kinase 3. Trends Pharmacol. Sci. 2004, 25, 471–480. [Google Scholar] [CrossRef]
- Ryoo, S.-R.; Jeong, H.K.; Radnaabazar, C.; Yoo, J.-J.; Cho, H.-J.; Lee, H.-W.; Kim, I.-S.; Cheon, Y.-H.; Ahn, Y.S.; Chung, S.-H.; et al. DYRK1A-mediated Hyperphosphorylation of Tau. J. Boil. Chem. 2007, 282, 34850–34857. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vasquez, A.A.; Aulchenko, Y.S.; Isaacs, A.; Van Oosterhout, A.; Sleegers, K.; Hofman, A.; Van Broeckhoven, C.; Oostra, B.A.; Breteler, M.; Van Duijn, C.M. Cyclin-dependent kinase 5 is associated with risk for Alzheimer’s disease in a Dutch population-based study. J. Neurol. 2008, 255, 655–662. [Google Scholar] [CrossRef]
- Cozza, G.; Pinna, L.A. Casein kinases as potential therapeutic targets. Expert Opin. Ther. Targets 2015, 20, 319–340. [Google Scholar] [CrossRef]
- Li, G.; Yin, H.; Kuret, J. Casein Kinase 1 Delta Phosphorylates Tau and Disrupts Its Binding to Microtubules. J. Boil. Chem. 2004, 279, 15938–15945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, B.; Medda, F.; Gokhale, V.; Dunckley, T.; Hulme, C. Recent Advances in the Design, Synthesis, and Biological Evaluation of Selective DYRK1A Inhibitors: A New Avenue for a Disease Modifying Treatment of Alzheimer’s? ACS Chem. Neurosci. 2012, 3, 857–872. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Becker, W.; Joost, H.-G. Structural and functional characteristics of Dyrk, a novel subfamily of protein kinases with dual specificity. Prog. Nucleic Acid Res. Mol. Biol. 1999, 62, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Aranda, S.; Laguna, A.; De La Luna, S. DYRK family of protein kinases: Evolutionary relationships, biochemical properties, and functional roles. FASEB J. 2010, 25, 449–462. [Google Scholar] [CrossRef]
- Tahtouh, T.; Elkins, J.M.; Filippakopoulos, P.; Soundararajan, M.; Burgy, G.; Durieu, E.; Cochet, C.; Schmid, R.; Lo, N.C.; Delhommel, F.; et al. Selectivity, Cocrystal Structures, and Neuroprotective Properties of Leucettines, a Family of Protein Kinase Inhibitors Derived from the Marine Sponge Alkaloid Leucettamine, B. J. Med. Chem. 2012, 55, 9312–9330. [Google Scholar] [CrossRef]
- Loidreau, Y.; Melissen, S.; Levacher, V.; Logé, C.; Graton, J.; Le Questel, J.-Y.; Besson, T. Study of N1-alkylation of indoles from the reaction of 2 (or 3) -aminoindole-3- (or 2) carbonitriles with DMF-dialkylacetals. Org. Biomol. Chem. 2012, 10, 4916. [Google Scholar] [CrossRef]
- Cao, R.; Fan, W.; Guo, L.; Ma, Q.; Zhang, G.; Li, J.; Chen, X.; Ren, Z.; Qiu, L. Synthesis and structure–activity relationships of harmine derivatives as potential antitumor agents. Eur. J. Med. Chem. 2013, 60, 135–143. [Google Scholar] [CrossRef]
- Cao, R.; Chen, Q.; Hou, X.; Chen, H.; Guan, H.; Ma, Y.; Peng, W.; Xu, A. Synthesis, acute toxicities, and antitumor effects of novel 9-substituted β-carboline derivatives. Bioorg. Med. Chem. 2004, 12, 4613–4623. [Google Scholar] [CrossRef]
- Balint, B.; Weber, C.; Cruzalegui, F.; Burbridge, M.; Kotschy, A. Structure-based design and synthesis of Harmine derivatives with different selectivity profiles in kinase vs monoamine oxidase inhibition. Chem. Med. Chem. 2017, 12, 932–939. [Google Scholar] [CrossRef] [Green Version]
- Du, H.; Tian, S.; Chen, J.; Gu, H.; Li, N.; Wang, J. Synthesis and biological evaluation of N9-substituted harmine derivatives as potential anticancer agents. Bioorg. Med. Chem. Lett. 2016, 26, 4015–4019. [Google Scholar] [CrossRef]
- Patel, K.; Gadewar, M.; Tripathi, R.; Prasad, S.; Patel, D.K. A review on medicinal importance, pharmacological activity and bioanalytical aspects of beta-carboline alkaloid “Harmine”. Asian Pac. J. Trop. Biomed. 2012, 2, 660–664. [Google Scholar] [CrossRef] [Green Version]
- Adayev, T.; Wegiel, J.; Hwang, Y.-W. Harmine is an ATP-competitive inhibitor for dual-specificity tyrosine phosphorylation-regulated kinase 1A (Dyrk1A). Arch. Biochem. Biophys. 2010, 507, 212–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baell, J.B.; Street, I.P.; Sleebs, B.E. Pyrido [3’,2’:4,5] Thieno [3,2-d] Pyrimidin-4-Ylamine Derivatives and Their Therapeutical Use. Patent WO 2012/131297A1, 4 October 2012. [Google Scholar]
- Primot, A.; Baratte, B.; Gompel, M.; Borgne, A.; Liabeuf, S.; Romette, J.-L.; Jho, E.-H.; Costantini, F.; Meijer, L. Purification of GSK-3 by Affinity Chromatography on Immobilized Axin. Protein Expr. Purif. 2000, 20, 394–404. [Google Scholar] [CrossRef] [PubMed]
- Bach, S.; Knockaert, M.; Reinhardt, J.; Lozach, O.; Schmitt, S.; Baratte, B.; Koken, M.; Coburn, S.P.; Tang, L.; Jiang, T.; et al. Roscovitine Targets, Protein Kinases and Pyridoxal Kinase. J. Boil. Chem. 2005, 280, 31208–31219. [Google Scholar] [CrossRef] [Green Version]
- Reinhardt, J.; Ferandin, Y.; Meijer, L. Purification of CK1 by affinity chromatography on immobilised axin. Protein Expr. Purif. 2007, 54, 101–109. [Google Scholar] [CrossRef] [PubMed]



| Starting Indole | Temperature (°C) | Time (min) | Product | R1 | R2 | Yield (%) |
|---|---|---|---|---|---|---|
| 7a | 170 | 30 | 1a | H | H | 81 |
| 7b | 170 | 30 | 1b | H | Me | 58 |
| 7c | 170 | 30 | 1c | H | Et | 64 |
| 7d | 170 | 40 | 1d | H | Bn | 52 |
| 8a | 170 | 60 | 2a | NO2 | H | 65 |
| 8b | 170 | 40 | 2b | NO2 | Me | 75 |
| 8c | 170 | 30 | 2c | NO2 | Et | 72 |
| 8d | 170 | 30 | 2d | NO2 | Bn | 79 |
| 9a | 200 | 40 | 3a | H | H | 71 |
| 9b | 200 | 30 | 3b | H | Me | 85 |
| 9c | 200 | 30 | 3c | H | Et | 96 |
| 9d | 200 | 30 | 3d | H | Bn | 67 |
| 10a | 200 | 30 | 4a | NO2 | H | 67 |
| 10b | 200 | 30 | 4b | NO2 | Me | 80 |
| 10c | 200 | 30 | 4c | NO2 | Et | 63 |
| 10d | 200 | 30 | 4d | NO2 | Bn | 67 |
| N,N-dimethylformamide (DMF)-dialkylacetal (R) | Temperature (°C) | Product | Yield (%) |
|---|---|---|---|
| DMF-DMA (Me) | 140 | 11b | 79 |
| DMF-DEA (Et) | 160 | 11c | 80 |
| DMF-DBA (Bn) | 160 | 11d | 62 |

| Compound | R1 | R2 | CDK5/p25 | CK1δ/ε | DYRK1A | GSK-3α/β |
|---|---|---|---|---|---|---|
| 1a | H | H | >10 | 2.0 | 2.2 | >10 |
| 1b | H | Me | >10 | 4.0 | 5.8 | >10 |
| 1c | H | Et | >10 | 2.8 | 4.1 | >10 |
| 1d | H | Bn | >10 | 0.6 | >10 | >10 |
| 2a | NO2 | H | >10 | >10 | 7.6 | >10 |
| 2b | NO2 | Me | >10 | >10 | >10 | >10 |
| 2c | NO2 | Et | >10 | >10 | >10 | >10 |
| 2d | NO2 | Bn | >10 | >10 | >10 | >10 |
| 3a | H | H | 6 | 0.7 | 3.1 | >10 |
| 3b | H | Me | >10 | 2.5 | >10 | >10 |
| 3c | H | Et | >10 | 1.6 | 9.8 | >10 |
| 3d | H | Bn | >10 | 2.7 | >10 | >10 |
| 4a | NO2 | H | >10 | 3.5 | 7.6 | >10 |
| 4b | NO2 | Me | >10 | 2.8 | >10 | >10 |
| 4c | NO2 | Et | >10 | 1.6 | 5.9 | >10 |
| 4d | NO2 | Bn | >10 | >10 | >10 | >10 |
| 11a (Harmine) | R = H | >10 | 1.5 | 0.029 | >10 | |
| 11b | R = Me | >10 | >10 | 0.13 | >10 | |
| 11c | R = Et | >10 | >10 | 0.037 | >10 | |
| 11d | R = Bn | >10 | >10 | 0.059 | >10 | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Loidreau, Y.; Dubouilh-Benard, C.; Nourrisson, M.-R.; Loaëc, N.; Meijer, L.; Besson, T.; Marchand, P. Exploring Kinase Inhibition Properties of 9H-pyrimido[5,4-b]- and [4,5-b]indol-4-amine Derivatives. Pharmaceuticals 2020, 13, 89. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13050089
Loidreau Y, Dubouilh-Benard C, Nourrisson M-R, Loaëc N, Meijer L, Besson T, Marchand P. Exploring Kinase Inhibition Properties of 9H-pyrimido[5,4-b]- and [4,5-b]indol-4-amine Derivatives. Pharmaceuticals. 2020; 13(5):89. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13050089
Chicago/Turabian StyleLoidreau, Yvonnick, Carole Dubouilh-Benard, Marie-Renée Nourrisson, Nadège Loaëc, Laurent Meijer, Thierry Besson, and Pascal Marchand. 2020. "Exploring Kinase Inhibition Properties of 9H-pyrimido[5,4-b]- and [4,5-b]indol-4-amine Derivatives" Pharmaceuticals 13, no. 5: 89. https://0-doi-org.brum.beds.ac.uk/10.3390/ph13050089